Antibodies towards Tyrosine Amyloid-Like Fibrils Allow Toxicity Modulation and Cellular Imaging of the Assemblies
Abstract
:1. Introduction
2. Results and Discussion
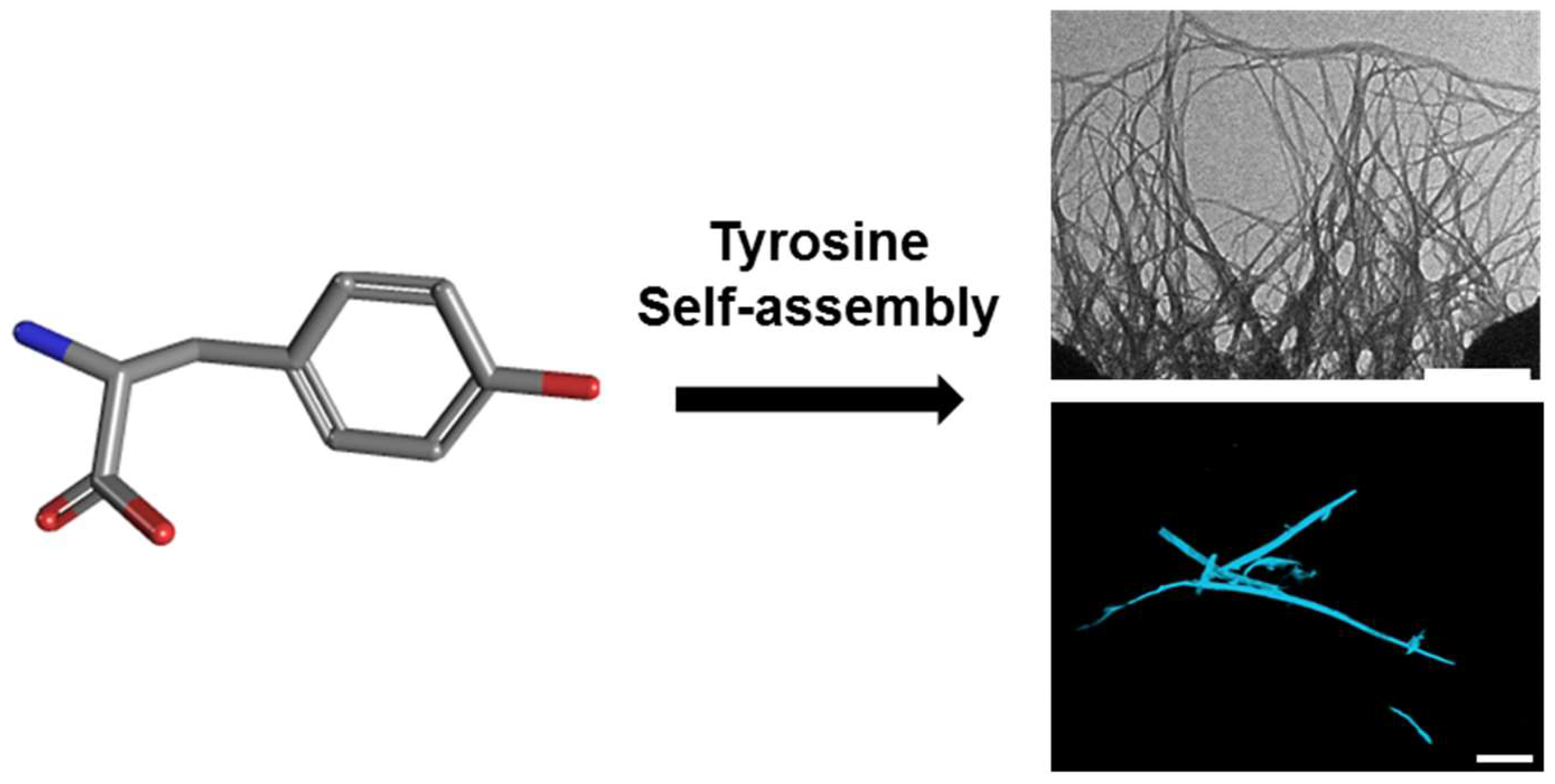
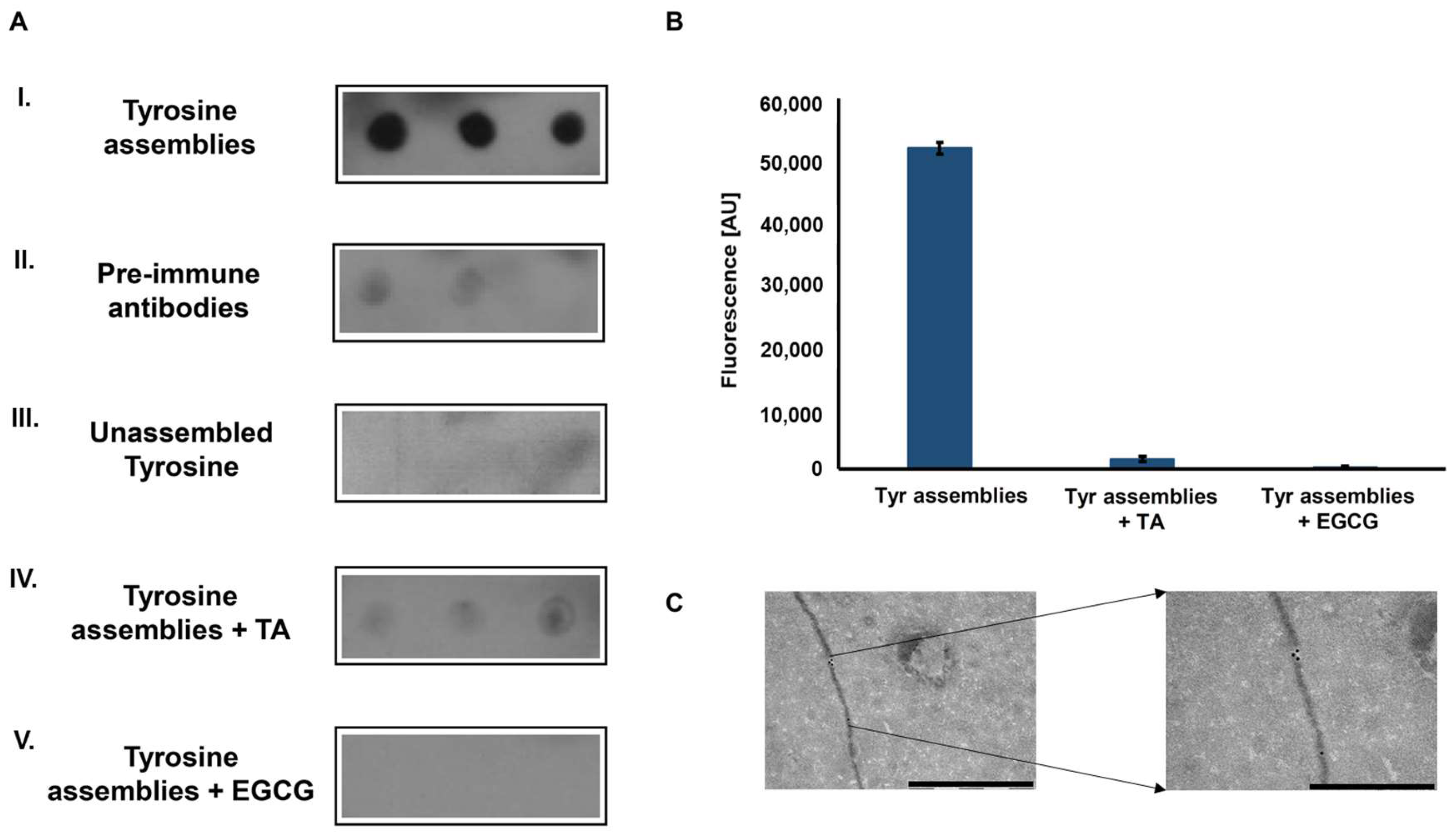
2.1. Tyrosine Self-Assembly, Antibody Production and Characterization
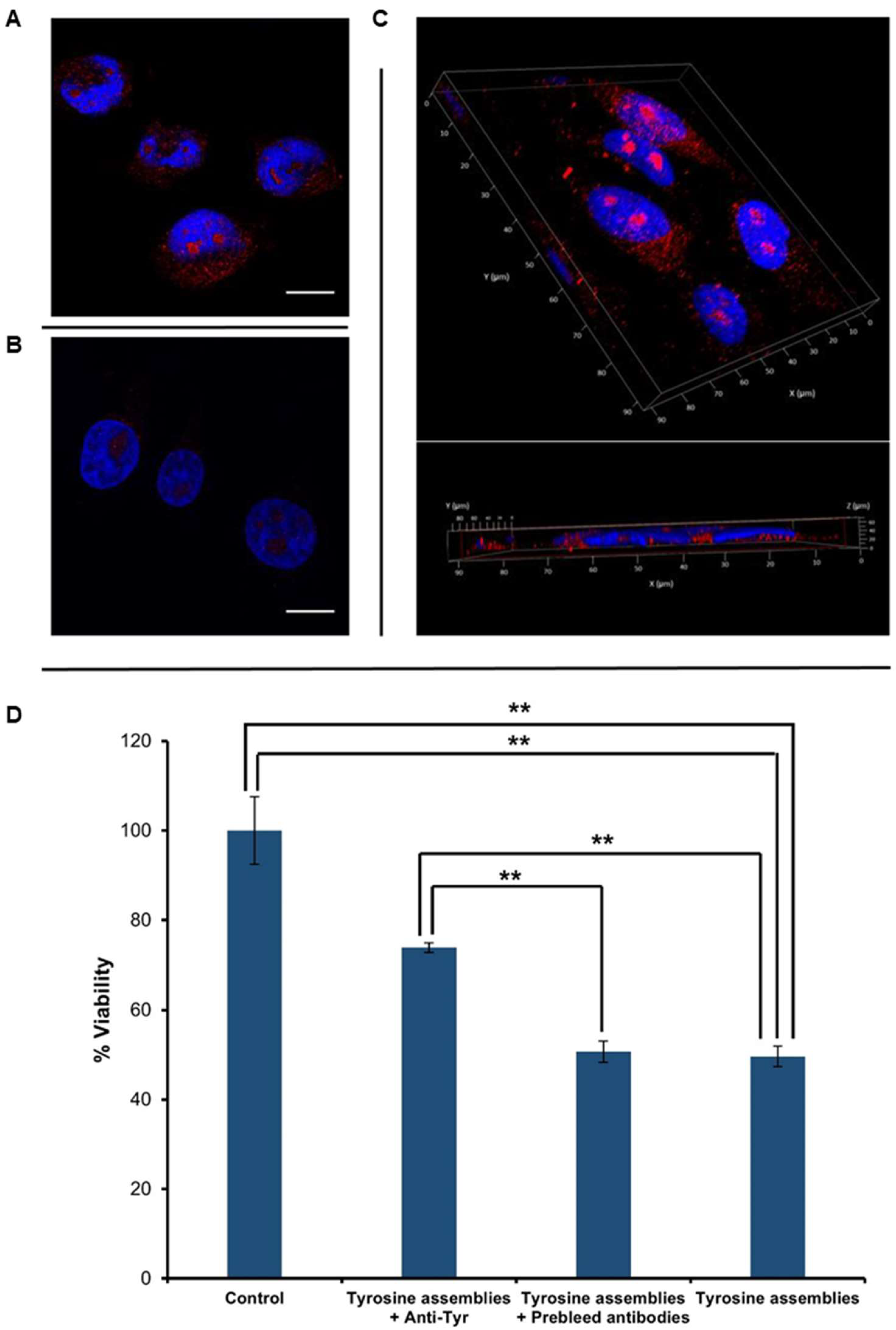
2.2. Cellular Internalization and Cytotoxicity of Tyr Assemblies
3. Materials and Methods
3.1. Materials
3.1.1. Anti-Tyrosine Antibodies Production
3.1.2. Secondary Antibodies
3.2. Experimental Methods
3.2.1. Tyrosine Fibril Formation
3.2.2. Dot-Blot Assay
3.2.3. ThT Endpoint Fluorescence assay
3.2.4. Rabbit Antibody Immuno-Gold Assay
3.2.5. Immuno-Staining
3.2.6. Cytotoxicity Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Knowles, T.P.J.; Vendruscolo, M.; Dobson, C.M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014, 15, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Buell, A.K.; Galvagnion, C.; Gaspar, R.; Sparr, E.; Vendruscolo, M.; Knowles, T.P.J.; Linse, S.; Dobson, C.M. Solution conditions determine the relative importance of nucleation and growth processes in -synuclein aggregation. Proc. Natl. Acad. Sci. USA 2014, 111, 7671–7676. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Functional Amyloid, and Human Disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Eichner, T.; Radford, S.E. A Diversity of Assembly Mechanisms of a Generic Amyloid Fold. Mol. Cell 2011, 43, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Gazit, E. A possible role for p-stacking in the self-assembly of amyloid fibrils. FASEB J. 2002, 16, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Dobson, C.M. Amyloid formation by globular proteins under native conditions. Nat. Chem. Biol. 2009, 5, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Makin, O.S.; Serpell, L.C. Structures for amyloid fibrils. FEBS J. 2005, 272, 5950–5961. [Google Scholar] [CrossRef] [PubMed]
- Gazit, E. Global analysis of tandem aromatic octapeptide repeats: The significance of the aromatic-glycine motif. Bioinformatics 2002, 18, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.P.J.; Oppenheim, T.W.; Buell, A.K.; Chirgadze, D.Y.; Welland, M.E. Nanostructured films from hierarchical self-assembly of amyloidogenic proteins. Nat. Nanotechnol. 2010, 5, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Khurana, R.; Coleman, C.; Ionescu-Zanetti, C.; Carter, S.A.; Krishna, V.; Grover, R.K.; Roy, R.; Singh, S. Mechanism of thioflavin T binding to amyloid fibrils. J. Struct. Biol. 2005, 151, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Frid, P.; Anisimov, S.V.; Popovic, N. Congo red and protein aggregation in neurodegenerative diseases. Brain Res. Rev. 2007, 53, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Shaham-Niv, S.; Rehak, P.; Vuković, L.; Adler-Abramovich, L.; Král, P.; Gazit, E. Formation of Apoptosis-Inducing Amyloid Fibrils by Tryptophan. Isr. J. Chem. 2017, 57, 729–737. [Google Scholar] [CrossRef]
- Shaham-Niv, S.; Adler-abramovich, L.; Schnaider, L.; Gazit, E. Extension of the generic amyloid hypothesis to nonproteinaceous metabolite assemblies. Sci. Adv. 2015, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Adler-Abramovich, L.; Vaks, L.; Carny, O.; Trudler, D.; Magno, A.; Caflisch, A.; Frenkel, D.; Gazit, E. Phenylalanine assembly into toxic fibrils suggests amyloid etiology in phenylketonuria. Nat. Chem. Biol. 2012, 8, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.A.; Ferrier, D.R. Lippincott’s Illustrated Reviews: Biochemistry Fifth Edition; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; ISBN 978-1-60831-412-6. [Google Scholar]
- Levy, P.A. Inborn Errors of Metabolism: Part 1: Overview. Pediatr. Rev. 2009, 30, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Levy, P.A. Inborn Errors of Metabolism: Part 2: Specific Disorders. Pediatr. Rev. 2009, 30, e22–e28. [Google Scholar] [CrossRef] [PubMed]
- Ménard-Moyon, C.; Venkatesh, V.; Krishna, K.V.; Bonachera, F.; Verma, S.; Bianco, A. Self-Assembly of Tyrosine into Controlled Supramolecular Nanostructures. Chem. A Eur. J. 2015, 21, 11681–11686. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.A.; Mitchell, G.A.; Tanguay, R.M. Tyrosinemia: A review. Pediatr. Dev. Pathol. 2001, 4, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Kvittingen, E.A. Hereditary tyrosinemia type I—An overview. Scand. J. Clin. Lab. Investig. Suppl. 1986, 184, 27–34. [Google Scholar]
- Macsai, M.S.; Schwartz, T.L.; Hinkle, D.; Hummel, M.B.; Mulhern, M.G.; Rootman, D. Tyrosinemia type II: Nine cases of ocular signs and symptoms. Am. J. Ophthalmol. 2001, 132, 522–527. [Google Scholar] [CrossRef]
- Heylen, E.; Scherer, G.; Vincent, M.-F.; Marie, S.; Fischer, J.; Nassogne, M.-C. Tyrosinemia Type III detected via neonatal screening: Management and outcome. Mol. Genet. Metab. 2012, 107, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Najafi, R.; Mostofizadeh, N.; Hashemipour, M. A Case of Tyrosinemia Type III with Status Epilepticus and Mental Retardation. Adv. Biomed. Res. 2018, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Arn, P.H. Phenylketonuria (PKU). Encycl. Neurol. Sci. 2014, 887–889. [Google Scholar] [CrossRef]
- Blau, N.; Van Spronsen, F.J.; Levy, H.L. Phenylketonuria. Lancet 2010, 376, 1417–1427. [Google Scholar] [CrossRef]
- Singh, V.; Rai, R.K.; Arora, A.; Sinha, N.; Thakur, A.K. Therapeutic implication of l-phenylalanine aggregation mechanism and its modulation by D-phenylalanine in phenylketonuria. Sci. Rep. 2015, 4, 3875. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.F.M.; Vandenakker, C.C.; Schleeger, M.; Velikov, K.P.; Koenderink, G.H.; Bonn, M. The polyphenol EGCG inhibits amyloid formation less efficiently at phospholipid interfaces than in bulk solution. J. Am. Chem. Soc. 2012, 134, 14781–14788. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Anti-amyloidogenic activity of tannic acid and its activity to destabilize Alzheimer’s β-amyloid fibrils in vitro. Biochim. Biophys. Acta Mol. Basis Dis. 2004, 1690, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Shaham-Niv, S.; Rehak, P.; Zaguri, D.; Levin, A.; Adler-Abramovich, L.; Vuković, L.; Král, P.; Gazit, E. Differential Inhibition of Metabolite Amyloid Formation by Generic Fibrillation-Modifying Polyphenols. Commun. Chem. 2018, 1–11, in press. [Google Scholar] [CrossRef]
- Lesné, S.; Koh, M.T.; Kotilinek, L.; Kayed, R.; Glabe, C.G.; Yang, A.; Gallagher, M.; Ashe, K.H. A specific amyloid-β protein assembly in the brain impairs memory. Nature 2006, 440, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Muñoz, M.J.; Castillo-Carranza, D.L.; Kayed, R. Therapeutic approaches against common structural features of toxic oligomers shared by multiple amyloidogenic proteins. Biochem. Pharmacol. 2014, 88, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Kayed, R.; Lasagna-Reeves, C.A. Molecular mechanisms of amyloid oligomers toxicity. In Alzheimer’s Disease: Advances for a New Century; IOS Press: Amsterdam, The Netherlands, 2013; pp. 67–78. ISBN 9781614991540. [Google Scholar]
- Kayed, R.; Glabe, C.G. Conformation-Dependent Anti-Amyloid Oligomer Antibodies. Methods Enzymol. 2006, 413, 326–344. [Google Scholar] [PubMed]
- Stravalaci, M.; Tapella, L.; Beeg, M.; Rossi, A.; Joshi, P.; Pizzi, E.; Mazzanti, M.; Balducci, C.; Forloni, G.; Biasini, E.; et al. The Anti-Prion Antibody 15B3 Detects Toxic Amyloid-β Oligomers. J. Alzheimer’s Dis. 2016, 53, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- Shaham-Niv, S.; Rehak, P.; Zaguri, D.; Kolusheva, S.; Král, P.; Gazit, E. Metabolite amyloid-like fibrils interact with model membranes. Chem. Commun. 2018, 54, 4561–4564. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: The authors declare that all of the data supporting the findings of this study are available within the article. Correspondence and requests for materials should be addressed to [email protected]; Tel: + 972-3-640-9030. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaguri, D.; Kreiser, T.; Shaham-Niv, S.; Gazit, E. Antibodies towards Tyrosine Amyloid-Like Fibrils Allow Toxicity Modulation and Cellular Imaging of the Assemblies. Molecules 2018, 23, 1273. https://doi.org/10.3390/molecules23061273
Zaguri D, Kreiser T, Shaham-Niv S, Gazit E. Antibodies towards Tyrosine Amyloid-Like Fibrils Allow Toxicity Modulation and Cellular Imaging of the Assemblies. Molecules. 2018; 23(6):1273. https://doi.org/10.3390/molecules23061273
Chicago/Turabian StyleZaguri, Dor, Topaz Kreiser, Shira Shaham-Niv, and Ehud Gazit. 2018. "Antibodies towards Tyrosine Amyloid-Like Fibrils Allow Toxicity Modulation and Cellular Imaging of the Assemblies" Molecules 23, no. 6: 1273. https://doi.org/10.3390/molecules23061273



