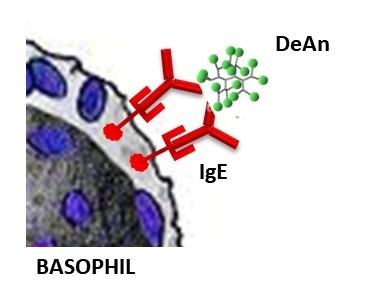
Dendrimeric Antigens for Drug Allergy Diagnosis: A New Approach for Basophil Activation Tests
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Characterization of DeAn
2.2. Clinical Evaluation
3. Discussion
4. Materials and Methods
4.1. Molecular Dynamic Simulation (MDS)
4.2. DOSY Nuclear Magnetic Resonance (NMR) Experiments
4.3. Patients
4.4. Basophil Activation Tests
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers Designed for Functions: From Physical, Photophysical, and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics, and Nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, M.A.; Grinstaff, M.W.; Paetsch, I.; Hunold, P.; Mahler, M.; Shamsi, K.; Nagel, E.; Price, C.F.; Clark, L.J.; Paull, J.R.A.; et al. Biomedical applications of dendrimers: a tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; Rodrigues, J.; Tomas, H.; Zablocka, M.; Shi, X.; Caminade, A.-M.; Majoral, J.-P. Dendrimers in combination with natural products and analogues as anti-cancer agents. Chem. Soc. Rev. 2018, 47, 514–532. [Google Scholar] [CrossRef] [PubMed]
- Uetrecht, J. Idiosyncratic Drug Reactions: Past, Present, and Future. Chem. Res. Toxicol. 2008, 21, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Uetrecht, J. Immune-Mediated Adverse Drug Reactions. Chem. Res. Toxicol. 2009, 22, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Ebo, D.G.; Leysen, J.; Mayorga, C.; Rozieres, A.; Knol, E.F.; Terreehorst, I. The in vitro diagnosis of drug allergy: status and perspectives. Allergy 2011, 66, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Moggs, J.G.; Terranova, R.; Kammüller, M.E.; Chibout, S.-D.; Chapman, V.; Dearman, R.J.; Kimber, I. Regulation of Allergic Responses to Chemicals and Drugs: Possible Roles of Epigenetic Mechanisms. Toxicol. Sci. 2012, 130, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A. Allergy and hypersensitivity: Mechanisms of allergic disease. Curr. Opin. Immunol. 2006, 18, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Antunez, C.; Martin, E.; Cornejo-Garcia, J.; Blanca-Lopez, N.; R-Pena, R.; Mayorga, C.; Torres, M.; Blanca, M. Immediate Hypersensitivity Reactions to Penicillins and Other Betalactams. Curr. Pharm. Des. 2006, 12, 3327–3333. [Google Scholar] [CrossRef] [PubMed]
- Ariza, A.; Barrionuevo, E.; Mayorga, C.; Montañez, M.I.; Perez-Inestrosa, E.; Ruiz-Sánchez, A.; Rodríguez-Guéant, R.M.; Fernández, T.D.; Guéant, J.L.; Torres, M.J.; et al. IgE to penicillins with different specificities can be identified by a multiepitope macromolecule: Bihaptenic penicillin structures and IgE specificities. J. Immunol. Methods 2014, 406, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Montañez, M.I.; Ariza, A.; Mayorga, C.; Fernandez, T.D.; Torres, M.J. Cross-Reactivity in Betalactam Allergy: Alternative Treatments. Curr. Treat. Opt. Allergy 2015, 2, 141–154. [Google Scholar] [CrossRef]
- Fernandez, T.D.; Mayorga, C.; Salas, M.; Barrionuevo, E.; Posadas, T.; Ariza, A.; Laguna, J.J.; Moreno, E.; Torres, M.J.; Doña, I.; et al. Evolution of diagnostic approaches in betalactam hypersensitivity. Expert Rev. Clin. Pharmacol. 2017, 10, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Doña, I.; Torres, M.J.; Montañez, M.I.; Fernández, T.D. In vitro diagnostic testing for antibiotic allergy. Allergy Asthma Immunol. Res. 2017, 9, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Blanca, M.; Mayorga, C.; Perez, E.; Suau, R.; Juarez, C.; Vega, J.M.; Carmona, M.J.; Perez-Estrada, M.; Garcia, J. Determination of IgE antibodies to the benzyl penicilloyl determinant. A comparison between poly-l-lysine and human serum albumin as carriers. J. Immunol. Methods 1992, 153, 99–105. [Google Scholar] [CrossRef]
- Torres, M.J.; Mayorga, C.; Pamies, R.; Juarez, C.; Blanca, M.; Romano, A. Immunologic response to different determinants of benzylpenicillin, amoxicillin, and ampicillin. Comparison between urticaria and anaphylactic shock. Allergy 1999, 54, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Fréchet, J.M.J.; Tomalia, D.A. Dendrimers and Other Dendritic Polymers; Wiley: Hoboken, NJ, USA, 2001; ISBN 9780470845820. [Google Scholar]
- Montañez, M.I.; Najera, F.; Mayorga, C.; Ruiz-Sanchez, A.J.; Vida, Y.; Collado, D.; Blanca, M.; Torres, M.J.; Perez-Inestrosa, E. Recognition of multiepitope dendrimeric antigens by human immunoglobulin E. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sancho, F.; Pérez-Inestrosa, E.; Suau, R.; Mayorga, C.; Torres, M.J.A.; Blanca, M. Dendrimers as Carrier Protein Mimetics for IgE Antibody Recognition. Synthesis and Characterization of Densely Penicilloylated Dendrimers. Bioconjug. Chem. 2002, 13, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Montañez, M.I.; Perez-Inestrosa, E.; Suau, R.; Mayorga, C.; Torres, M.J.; Blanca, M. Dendrimerized Cellulose as a Scaffold for Artificial Antigens with Applications in Drug Allergy Diagnosis. Biomacromolecules 2008, 9, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Montañez, M.I.; Mayorga, C.; Torres, M.J.; Blanca, M.; Perez-Inestrosa, E. Methodologies to anchor dendrimeric nanoconjugates to solid phase: toward an efficient in vitro detection of allergy to β-lactam antibiotics. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Montañez, M.I.; Najera, F.; Perez-Inestrosa, E. NMR Studies and Molecular Dynamic Simulation of Synthetic Dendritic Antigens. Polymers 2011, 3, 1533–1553. [Google Scholar] [CrossRef]
- Ruiz-Sanchez, A.J.; Montanez, M.I.; Mayorga, C.; Torres, M.J.; Kehr, N.S.; Vida, Y.; Collado, D.; Najera, F.; De Cola, L.; Perez-Inestrosa, E. Dendrimer-modified solid supports: nanostructured materials with potential drug allergy diagnostic applications. Curr. Med. Chem. 2012, 19, 4942–4954. [Google Scholar] [CrossRef] [PubMed]
- Vida, Y.; Montañez, M.I.; Collado, D.; Najera, F.; Ariza, A.; Blanca, M.; Torres, M.J.; Mayorga, C.; Perez-Inestrosa, E. Dendrimeric antigen–silica particle composites: an innovative approach for IgE quantification. J. Mater. Chem. B 2013, 1, 3044–3050. [Google Scholar] [CrossRef]
- Montañez, M.I.; Ruiz-Sanchez, A.J.; Perez-Inestrosa, E. A perspective of nanotechnology in hypersensitivity reactions including drug allergy. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, C.; Perez-Inestrosa, E.; Molina, N.; Montañez, M.I. Development of nanostructures in the diagnosis of drug hypersensitivity reactions. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.J.; Padial, A.; Mayorga, C.; Fernández, T.; Sanchez-Sabate, E.; Cornejo-Garcia, J.A.; Antúnez, C.; Blanca, M. The diagnostic interpretation of basophil activation test in immediate allergic reactions to betalactams. Clin. Exp. Allergy 2004, 34, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- De Weck, A.L.; Sanz, M.L.; Gamboa, P.M.; Aberer, W.; Sturm, G.; Bilo, M.B.; Montroni, M.; Blanca, M.; Torres, M.J.; Mayorga, L.; et al. Diagnosis of Immediate-Type ß-Lactam Allergy In Vitro by Flow-Cytometric Basophil Activation Test and Sulfidoleukotriene Production: A Multicenter Study. J. Investig. Allergol. Clin. Immunol. 2009, 19, 91. [Google Scholar]
- Torres, M.J.; Ariza, A.; Mayorga, C.; Doña, I.; Blanca-Lopez, N.; Rondon, C.; Blanca, M. Clavulanic acid can be the component in amoxicillin-clavulanic acid responsible for immediate hypersensitivity reactions. J. Allergy Clin. Immunol. 2010, 125, 502–505.e2. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, M.A.; Orr, B.G.; Banaszak Holl, M.M. Diffusion NMR Study of Generation-Five PAMAM Dendrimer Materials. J. Phys. Chem. B 2014, 118, 7195–7202. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, V.A.; Gavín, J.A.; Alderete, J.B. Scaling trend in diffusion coefficients of low generation G0–G3 PAMAM dendrimers in aqueous solution at high and neutral pH. Struct. Chem. 2012, 23, 123–128. [Google Scholar] [CrossRef]
- De Weck, A.L.; Jeunet, F.; Schulz, K.H.; Louis, P.; Girard, J.P.; Grilliat, J.P.; Moneret-Vautrin, D.; Storck, H.; Wuthrich, B.; Spengler, H.; et al. Clinical trial of Ro 6-0787, a monovalent specific hapten inhibitor of penicillin allergy. Z. Immunitatsforsch. Exp. Klin. Immunol. 1975, 150, 138–160. [Google Scholar] [PubMed]
- Paar, J.M.; Harris, N.T.; Holowka, D.; Baird, B. Bivalent ligands with rigid double-stranded DNA spacers reveal structural constraints on signaling by Fc epsilon RI. J. Immunol. 2002, 169, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Posner, R.G.; Subramanian, K.; Goldstein, B.; Thomas, J.; Feder, T.; Holowka, D.; Baird, B. Simultaneous cross-linking by two nontriggering bivalent ligands causes synergistic signaling of IgE Fc epsilon RI complexes. J. Immunol. 1995, 155, 3601–3609. [Google Scholar] [PubMed]
- Ariza, A.; Mayorga, C.; Salas, M.; Doña, I.; Martín-Serrano, Á.; Pérez-Inestrosa, E.; Pérez-Sala, D.; Guzmán, A.E.; Montañez, M.I.; Torres, M.J. The influence of the carrier molecule on amoxicillin recognition by specific IgE in patients with immediate hypersensitivity reactions to betalactams. Sci. Rep. 2016, 6, 35113. [Google Scholar] [CrossRef] [PubMed]
- Ariza, A.; Mayorga, C.; Fernández, T.D.; Barbero, N.; Martín-Serrano, A.; Pérez-Sala, D.; Sánchez-Gómez, F.J.; Blanca, M.; Torres, M.J.; Montanez, M.I. Hypersensitivity Reactions to β-Lactams: Relevance of Hapten-Protein Conjugates. J. Investig. Allergol. Clin. Immunol. 2015, 25, 12–25. [Google Scholar] [PubMed]
- Ariza, A.; Collado, D.; Vida, Y.; Montañez, M.I.; Pérez-Inestrosa, E.; Blanca, M.; Torres, M.J.; Cañada, F.J.; Pérez-Sala, D. Study of protein haptenation by amoxicillin through the use of a biotinylated antibiotic. PLoS ONE 2014, 9, e90891. [Google Scholar] [CrossRef] [PubMed]
- Gieras, A.; Linhart, B.; Roux, K.H.; Dutta, M.; Khodoun, M.; Zafred, D.; Cabauatan, C.R.; Lupinek, C.; Weber, M.; Focke-Tejkl, M.; et al. IgE epitope proximity determines immune complex shape and effector cell activation capacity. J. Allergy Clin. Immunol. 2016, 137, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Sil, D.; Lee, J.B.; Luo, D.; Holowka, D.; Baird, B. Trivalent Ligands with Rigid DNA Spacers Reveal Structural Requirements For IgE Receptor Signaling in RBL Mast Cells. ACS Chem. Biol. 2007, 2, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Baird, E.J.; Holowka, D.; Geoffrey, W.; Coates, A.; Baird, B. Highly Effective Poly(Ethylene Glycol) Architectures for Specific Inhibition of Immune Receptor Activation. Biochemistry 2003, 42, 12739–12748. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.B. N(alpha-d-penicilloyl) amines as univalent hapten inhibitors of antibodydependent allergic reactions to penicillin. J. Med. Pharm. Chem. 1962, 91, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- De Weck, A.L.; Girard, J.P. Specific Inhibition of Allergic Reactions to Penicillin in Man by a Monovalent Hapten. Int. Arch. Allergy Immunol. 1972, 42, 798–815. [Google Scholar] [CrossRef]
- Case, D.A.; Darden, T.A.; Cheatham, T.E., III; Simmerling, C.L.; Wang, J.; Duke, R.E.; Luo, R.; Walker, R.C.; Zhang, W.; Merz, K.M.; et al. AMBER 12; University of California: San Francisco, CA, USA, 2012. [Google Scholar]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Maingi, V.; Jain, V.; Bharatam, P.V.; Maiti, P.K. Dendrimer building toolkit: Model building and characterization of various dendrimer architectures. J. Comput. Chem. 2012, 33, 1997–2011. [Google Scholar] [CrossRef] [PubMed]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J. Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Torres, M.J.; Blanca, M.; Fernandez, J.; Romano, A.; Weck, A.; Aberer, W.; Brockow, K.; Pichler, W.J.; Demoly, P. Diagnosis of immediate allergic reactions to beta-lactam antibiotics. Allergy 2003, 58, 961–972. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds not are available from the authors. |


| Dendrimer | MDS | NMR Experiments | ||||
|---|---|---|---|---|---|---|
| Rg (Å) | Iz/Ix | Iz/Iy | δ | D (m2s−1) | RH (Å) | |
| BPO-DeAn-G2 | 14.20 | 2.40 | 2.40 | 0.054 | 1.38 × 10−10 | 14.50 |
| BPO-DeAn-G4 | 21.90 | 1.25 | 1.17 | 0.005 | 1.00 × 10−10 | 20.04 |
| AXO-DeAn-G2 | 13.65 | 1.74 | 1.20 | 0.024 | 1.40 × 10−10 | 14.32 |
| AXO-DeAn-G4 | 22.03 | 1.61 | 1.07 | 0.019 | 1.00 × 10−10 | 20.04 |
| Subject | Sex | Age (Years) | Reaction | Responsible Drug | Int R-S | Skin Test | RAST | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BP-OL | MD | AX | +to Other Drug | BPO-PLL | AXO-PLL | ||||||
| Contr 1 | F | 43 | Urticaria/AE | Cefaclor | 5 | − | − | − | Cefaclor | − | − |
| Contr 2 | F | 48 | Anaphylaxis | Cefur | 3 | − | − | − | Cefur | − | − |
| Pat 1 | M | 28 | Anaphylaxis | AX | 5 | − | − | + | nd | − | − |
| Pat 2 | M | 30 | Anaphylaxis | AX | 5 | nd | nd | nd | nd | + | + |
| Pat 3 | F | 18 | Anaphylaxis | BP | 6 | + | - | + | nd | + | + |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, N.; Martin-Serrano, A.; Fernandez, T.D.; Tesfaye, A.; Najera, F.; Torres, M.J.; Mayorga, C.; Vida, Y.; Montañez, M.I.; Perez-Inestrosa, E. Dendrimeric Antigens for Drug Allergy Diagnosis: A New Approach for Basophil Activation Tests. Molecules 2018, 23, 997. https://doi.org/10.3390/molecules23050997
Molina N, Martin-Serrano A, Fernandez TD, Tesfaye A, Najera F, Torres MJ, Mayorga C, Vida Y, Montañez MI, Perez-Inestrosa E. Dendrimeric Antigens for Drug Allergy Diagnosis: A New Approach for Basophil Activation Tests. Molecules. 2018; 23(5):997. https://doi.org/10.3390/molecules23050997
Chicago/Turabian StyleMolina, Noemi, Angela Martin-Serrano, Tahia D. Fernandez, Amene Tesfaye, Francisco Najera, María J. Torres, Cristobalina Mayorga, Yolanda Vida, Maria I. Montañez, and Ezequiel Perez-Inestrosa. 2018. "Dendrimeric Antigens for Drug Allergy Diagnosis: A New Approach for Basophil Activation Tests" Molecules 23, no. 5: 997. https://doi.org/10.3390/molecules23050997