Isolation of High Purity Anthocyanin Monomers from Red Cabbage with Recycling Preparative Liquid Chromatography and Their Photostability
Abstract
:1. Introduction
2. Results
2.1. HPLC Analysis of Red Cabbage Anthocyanins and Preparative Scale Isolation
2.2. Isolation of Anthocyanin Monomers with Recycling Preparative Chromatography
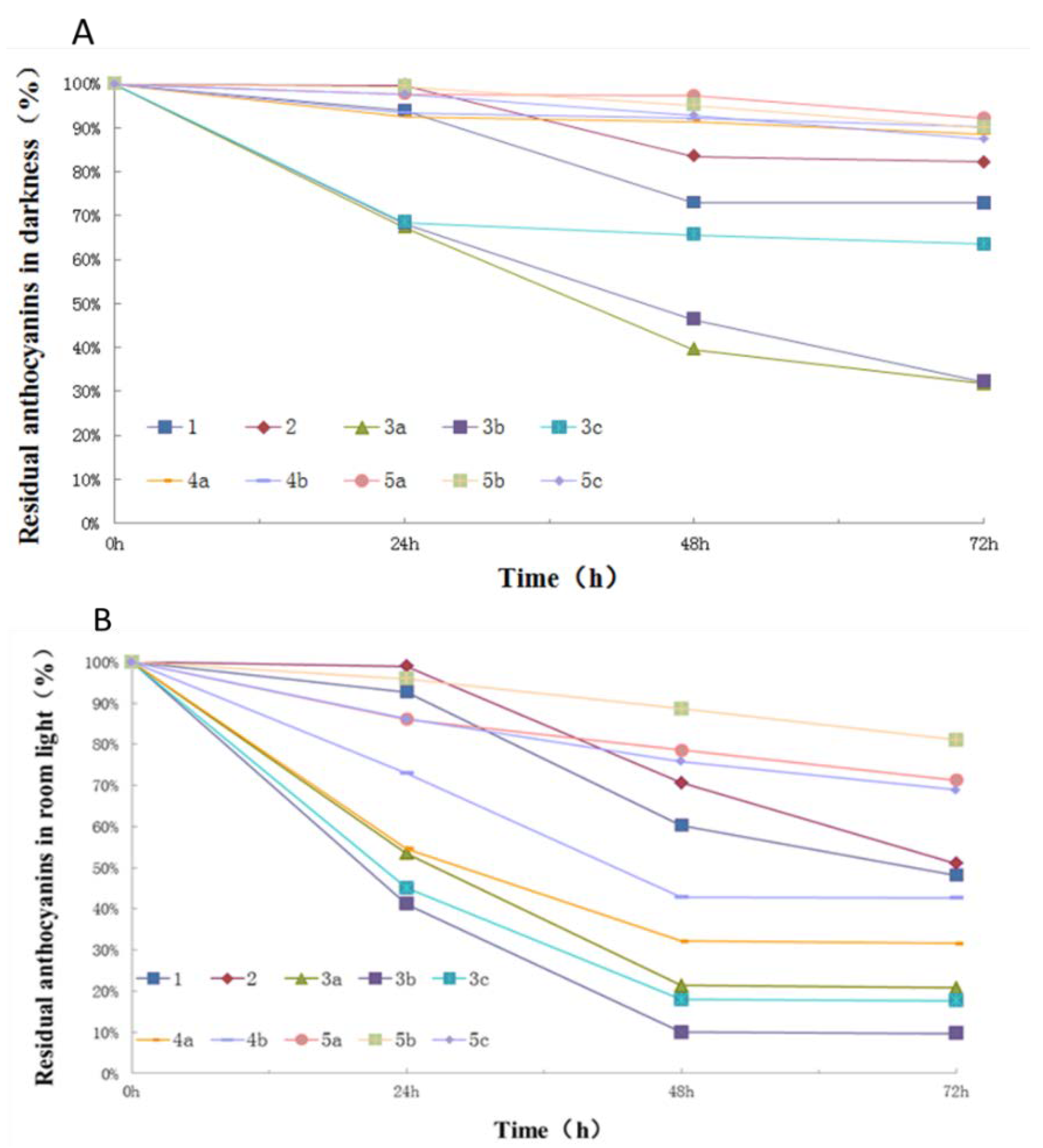
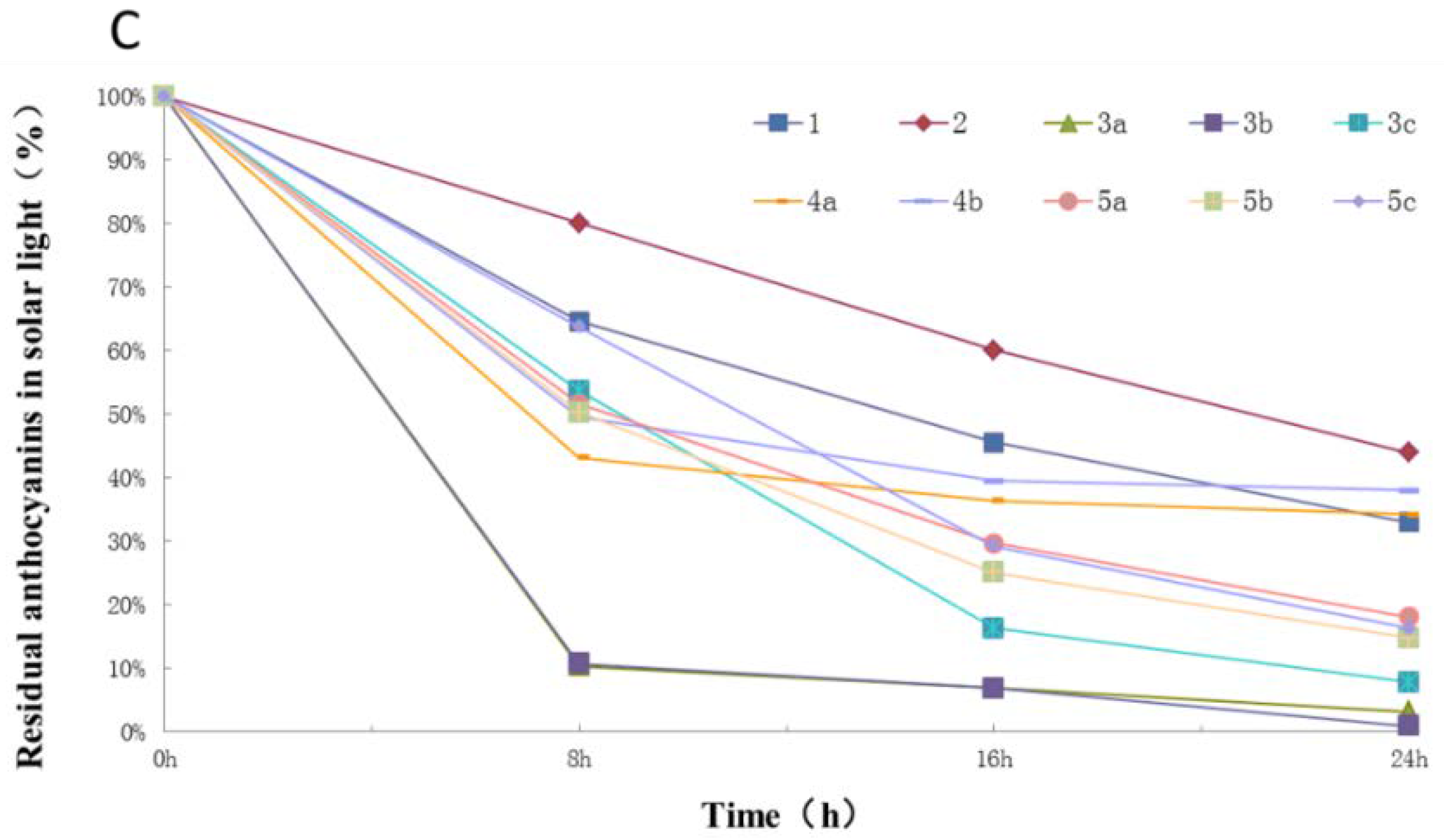
2.3. Photostability of Isolated Anthocyanins from Red Cabbage
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Sample Preparation
4.3. HPLC Analysis
4.4. Preparative HPLC
4.5. The Structural Identification of Anthocyanin Monomers
4.6. The Photostabilities of Various Isolated Anthocyanin Monomers
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Yu, X.Q. The nutritional value of cabbage and the technique of spring cultivation. Shanghai Veg. 2011, 5, 27–28. [Google Scholar]
- Song, Y.; Yang, J. Research progress on functional components of brassica vegetables. J. Zhejiang Agric. Sci. 2014, 6, 837–840. [Google Scholar]
- Song, X.Q.; Ye, L.; Yang, X.B. Comparative study of three methods for extraction of purple cabbage pigment. Food Sci. 2011, 32, 74–77. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 2, 933–956. [Google Scholar] [CrossRef]
- Sichel, G.; Corsaro, C.; Scalla, M.; di Bilio, A.J.; Bonomo, R.P. In vitro scavenger activity of some flavonoids and melanin agsinst O2−. Free Radic. Biol. Med. 1991, 11, 1–8. [Google Scholar] [CrossRef]
- Xu, Y.M.; Zhao, X.Y.; Ma, Y.; Meng, X.J.; Li, D.H. Study on antioxidant activities of purple cabbage pigment. Food Res. Dev. 2006, 127, 59–61. [Google Scholar]
- Li, Y.C.; Meng, X.J.; Sun, J.J.; Yu, N. Effects of anthocyanins from blueberry on lowing the cholesterol and antioxidation. Food Ferment. Ind. 2018, 34, 44–48. [Google Scholar]
- Yang, X.L.; Yang, L.; Zheng, H.Y. Hypolipidemic and antioxidant effects of mulberry (Morus alba L.) fruit in hyperlipidaemia rats. Food Chem. Toxicol. 2010, 48, 2374–2379. [Google Scholar] [CrossRef] [PubMed]
- Mladěnka, P.; Zatloukalová, L.; Filipský, T.; Hrdina, R. Cardiovascular effects of flaconoids are not caused only by direct antioxidant activity. Free Radic. Biol. Med. 2010, 49, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Hassellund, S.S.; Flaa, A.; Kjeldsen, S.E.; Seljeflot, I.; Karlsen, A.; Erlund, I.; Rostrup, M. Effects of anthocyanins on cardiovascular risk factors and inflammation in pre-hypertensive men: A double-blind randomized placebo-controlled crossover study. J. Hum. Hypertens. 2013, 27, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.H.; Qin, P.Y.; Ren, G.X. Effect of anthocyanin-rich extract from black rice (Oryza sativa L. Japonica) on chronically alcoholinduced liver damage in rats. J. Agric. Food Chem. 2010, 58, 3191–3196. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Hertog, M.G.L.; Katan, M.B. Analysis and health effects of flavonoids. Food Chem. 1996, 57, 43–46. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Topolska, J.; Honke, J. Anthocyanins profile and antioxidant capacity of red cabbage are influenced by genotype and vegetation period. J. Funct. Foods 2014, 7, 201–211. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Xu, Y.J.; Du, Q.Z. Review on anthocyanins bioactivities. Food Mach. 2006, 26, 154–157. [Google Scholar]
- Marianne, D.; Wesergaard, N.; Stapelfeldt, H. Light and heat sensitivity of red cabbage extract in soft drink model systems. Food Chem. 2001, 72, 431–437. [Google Scholar]
- Li, H.M. Extraction and characterization of red cabbage colour. China Food Addit. 1999, 3, 12–18. [Google Scholar]
- Zhu, Z.B.; Wu, Y.F.; Yi, J.H. Purification of purple cabbage anthocyanins. Food Sci. Technol. 2012, 37, 239–243. [Google Scholar]
- Liu, J.B.; Chen, J.J.; Wang, E.L.; Liu, Y.J. Separation of anthocyanin monomers from blueberry fruit through chromatographic techniques. Food Sci. 2017, 38, 206–213. [Google Scholar]
- Wang, E.; Yin, Y.; Xu, C.; Liu, J. Isolation of high-purity anthocyanin mixtures and monomers from blueberries using combined chromatographic techniques. J. Chromatogr. A 2014, 1327, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.; Jorgenson, J.W. Pressure-induced retention variations in reversed-phased alternate pumping recycle chromatography. Anal. Chem. 1998, 70, 2773–2782. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.L.; Xing, H.B.; Bao, Z.B.; Su, B.G.; Yang, Q.W.; Yang, Y.W.; Zhang, Z.G. Recent advances in separation of bioactive natural products. Chin. J. Chem. Eng. 2013, 21, 937–952. [Google Scholar] [CrossRef]
- Panagiotis, A.; Per, J.R.S.; Charlotta, T. Characterisation of anthocyanins in red cabbage using high resolution liquid chromatography coupled with photodiode array detection and electrospray ionization-linear ion trap mass spectrometry. Food Chem. 2008, 109, 219–226. [Google Scholar]
- Buraidah, M.H.; Teo, L.P.; Yusuf, S.N.F.; Noor, M.M.; Kufian, M.Z.; Careem, M.A.; Majid, S.R.; Taha, R.M.; Arof, A.K. TiO2/Chitosan-NH4I (+I2)-BMII-Based Dye-Sensitized Solar Cells with Anthocyanin Dyes Extracted from Black Rice and Red Cabbage. Int. J. Photoenergy 2011, 2011, 273683. [Google Scholar] [CrossRef]
- Gachovska, T.; Cassada, D.; Subbiah, J.; Hanna, M.; Thippareddi, H.; Snow, D. Enhanced Anthocyanin Extraction from Red Cabbage Using Pulsed Electric Field Processing. J. Food Sci. 2010, 75, E323–E329. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Fyffe, S.; Dobson, P.; Stewart, D. Anthocyanins from red cabbage-stability to simulated gastrointestinal digestion. Phytochemistry 2007, 68, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Wiczkowski, W.; Szawara-Nowak, D.; Romaszko, J. The impact of red cabbage fermentation on bioavailability of anthocyanins and antioxidant capacity of human plasma. Food Chem. 2016, 190, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.L.; Prior, R.L. Identification and characterization of anthocyanins by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry in common foods in the United States: Vegetables, nuts, and grains. J. Agric. Food Chem. 2005, 53, 3101–3113. [Google Scholar] [CrossRef] [PubMed]
- Podsędek, A.; Redzynia, M.; Klewicka, E.; Koziołkiewicz, M. Matrix Effects on the Stability and Antioxidant Activity of Red Cabbage Anthocyanins under Simulated Gastrointestinal Digestion. BioMed Res. Int. 2014, 2014, 365738. [Google Scholar] [CrossRef] [PubMed]
- Tierno, R.; Ruiz de Galarreata, J.-I. Influence of Selected Factors on Anthocyanin Stability in Colored Potato Extracts. J. Food Process. Preserv. 2016, 40, 1020–1026. [Google Scholar] [CrossRef]
- Wang, L.; Sun, S.X.; Shao, Y.D.; Jun-Li, Y.E.; Yang, S.Z. Extraction and stability of the anthocyanin from blood-flesh peach. Sci. Technol. Food Ind. 2014, 35, 113–122. [Google Scholar]
- Kocic, B.; Filipovic, S.; Nikolic, M.; Petrovic, B. Effects of anthocyanins and anthocyanin-rich extracts on the risk for cancers of the gastrointestinal tract. J. BUON 2011, 16, 602–608. [Google Scholar] [PubMed]
- Zheng, Y.; Dong, Q. Research progress in pharmacological activity and its mechanism of anthocyanins in vivo. Sci. Technol. Food Ind. 2014, 10, 396–400. [Google Scholar]
- Nizamutdinova, I.T.; Kim, Y.M.; Chung, J.I.; Shin, S.C.; Jeong, Y.K.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. Anthocyanins from black soybean seed coats stimulate wound healing in fibroblasts and keratinocytes and prevent inflammation in endothelial cells. Food Chem. Toxicol. 2009, 47, 2806–2818. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Takeda, K.; Ohnish, T. Light-induced anthocyanin reduces the extent of damage to DNA in UV irradiated centaurea Cyanus cells in culture. Plant Cell Physiol. 1991, 32, 541–547. [Google Scholar]
- Yi, J.H.; Pan, M.T.; Zhu, Z.B. Isolation and purification of anthocyanins by high-speed counter-current chromatography from red cabbage. Food Mach. 2012, 28, 129–133. [Google Scholar]
- Yu, Z.Y.; Zhao, J.H.; Li, X.G.; Xu, Y.Q.; Tang, X.; Yang, Y. Purification of anthocyanin from blueberry by sequential medium pressure column chromatography on macroporous resin and sephadex LH-20. Food Sci. 2018, 39, 118–123. [Google Scholar]
- Chen, Y.; Zhang, W.J.; Zhao, T.; Li, F.; Zhang, M.; Li, J.; Zou, Y.; Wang, W.; Cobbina, S.J.; Wu, X.; et al. Absorption properties of macroporous absorbent resins for separation of anthocyanins from mulberry. Food Chem. 2016, 194, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Sidana, J.; Joshi, L.K. Recycle HPLC: A powerful tool for the purification of natural products. Chromatogr. Res. Int. 2013, 2013, 509812. [Google Scholar] [CrossRef]
- Alley, W.R., Jr.; Mann, B.F.; Hruska, V.; Novotny, M.V. Isolation and purification of glycoconjugates from complex biological sources by recycling high-performance liquid chromatography. Anal. Chem. 2013, 85, 10408–10416. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds isolated from the red cabbage are available from the authors. |
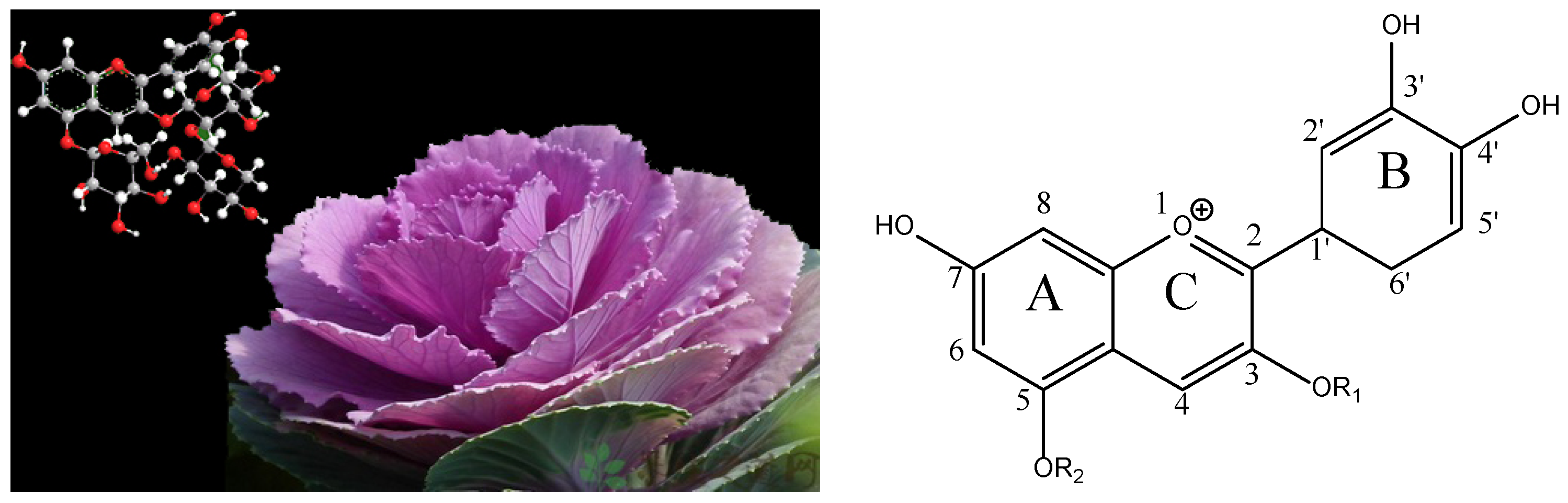
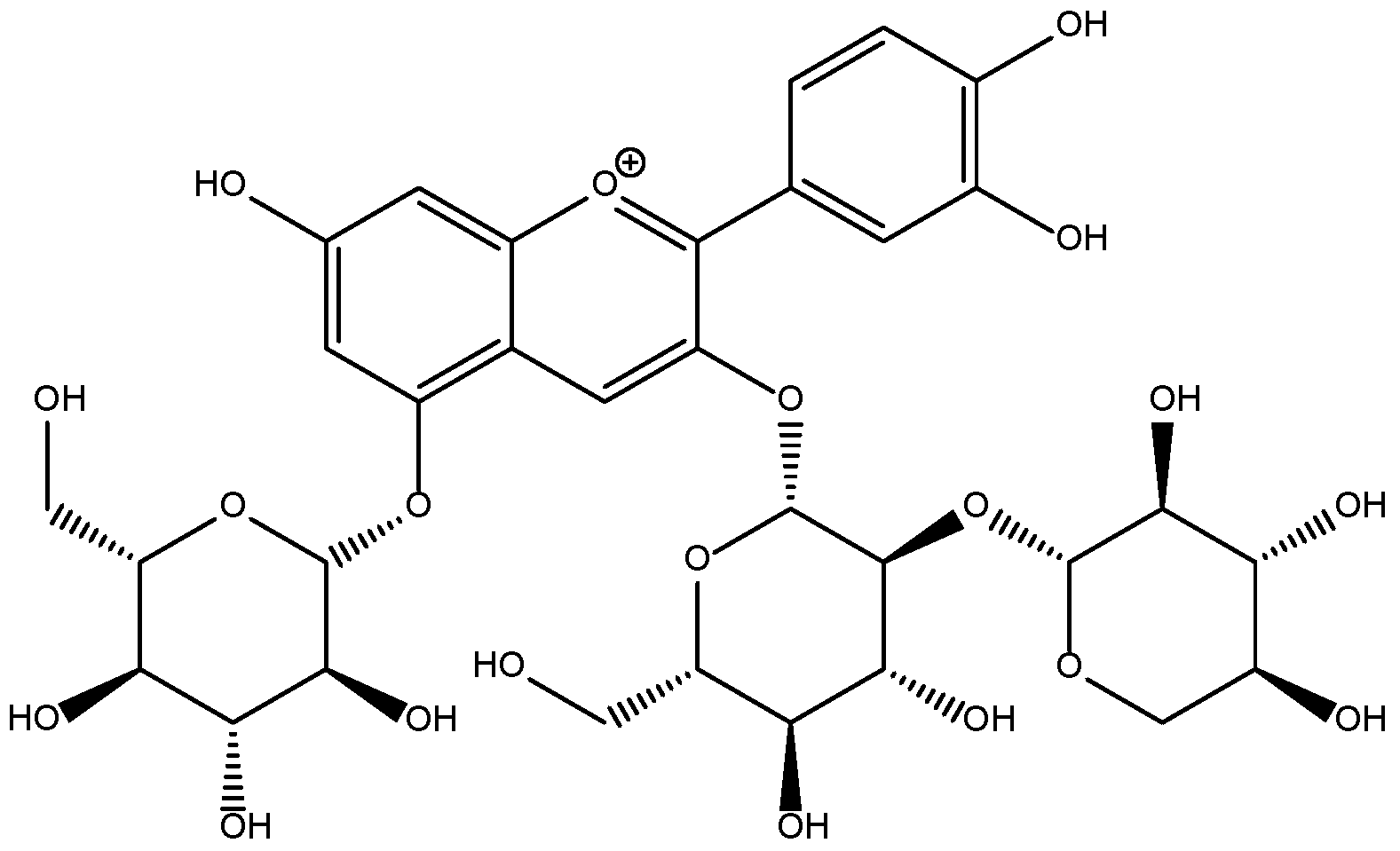

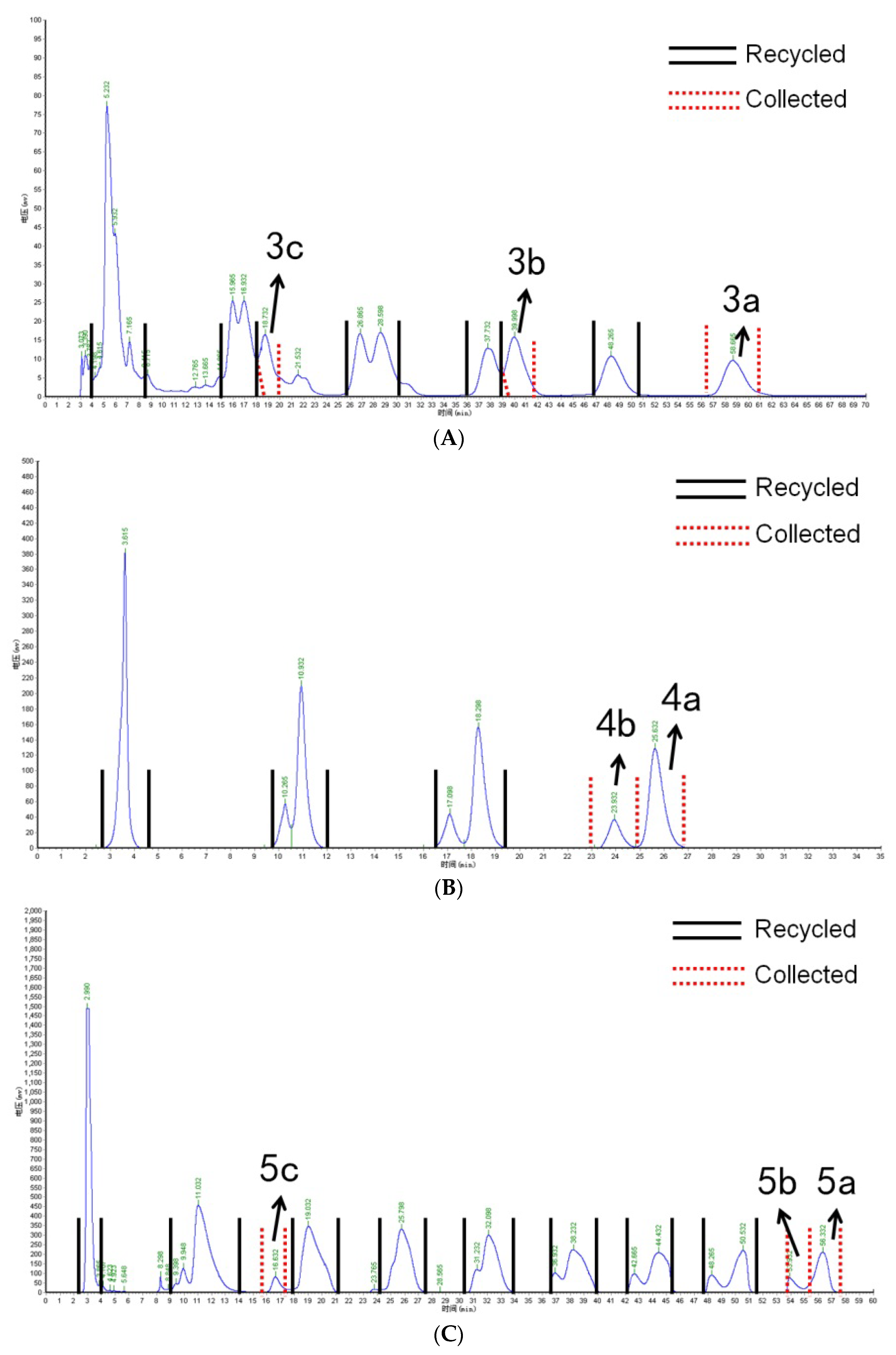
 ” represents the parent ion peak for each anthocyanin ([M + H]+).
” represents the parent ion peak for each anthocyanin ([M + H]+).
 ” represents the parent ion peak for each anthocyanin ([M + H]+).
” represents the parent ion peak for each anthocyanin ([M + H]+).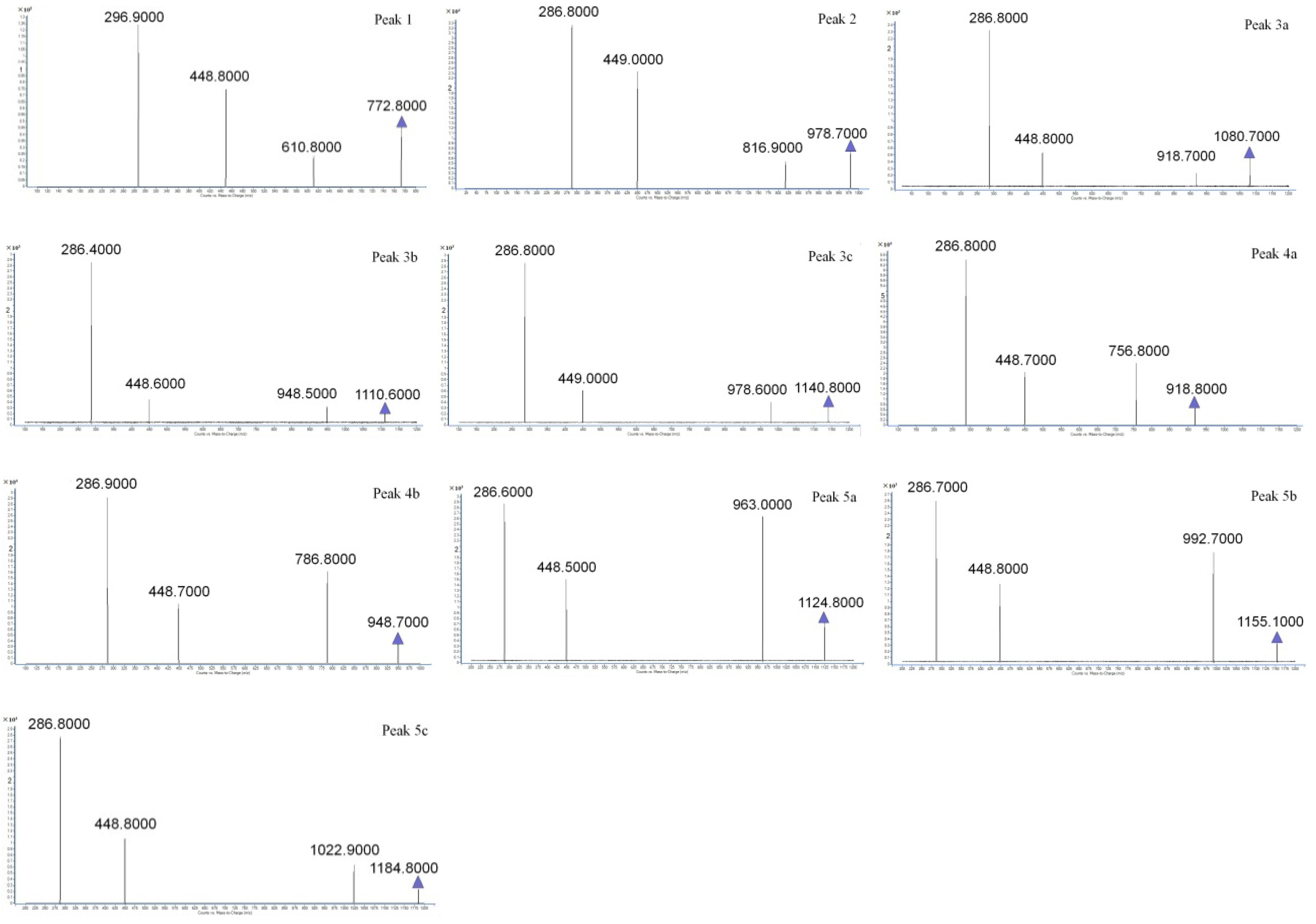

| Peak | ts (min) | PDA | M (m/z) | Fragment Ions (m/z) | Identified Anthocyanin |
|---|---|---|---|---|---|
| 1 | 8.936 | 510, 280 | 772.8 | 610.8, 488.8, 286.9 | Cy-3-soph-5-Glc [24] |
| 2 | 11.674 | 525, 330, 280 | 978.7 | 816.9, 449.0, 286.8 | Cy-3(sin)-diGlc-5-Glc [25] |
| 3a | 15.928 | 525, 325, 280 | 1080.7 | 918.7, 448.8, 286.8 | Cy-3-(caff-pC)-diGlc-5-Glc [26] |
| 3b | 16.283 | 525, NR | 1110.6 | 948.8, 448.5, 280.7 | Cy-3-(glucofer)-diGlc-5-Glc [27] |
| 3c | 16.607 | 525, NR | 1140.8 | 978.6, 449.0, 286.8 | Cy-3-(glucosin)-diGlc-5-Glc [26] |
| 4a | 21.853 | 525, 325, 280 | 918.9 | 765.9, 448.9, 286.8 | Cy-3-(pC)-diGlc-5-Glc [28] |
| 4b | 22.046 | 525, NR | 948.8 | 786.9, 448.7, 286.8 | Cy-3-(fer)-diGlc-5-Glc [28] |
| 5a | 24.032 | 535, 320, 285 | 1124.8 | 963.0, 448.5, 286.8 | Cy-3-(fer)(fer)-diGlc-5-Glc [24] |
| 5b | 24.189 | 535, NR | 1155.1 | 992.7, 448.8, 286.7 | Cy-3-(sin)(fer)-diGlc-5-Glc [29] |
| 5c | 24.458 | 535, NR | 1184.8 | 1022.9, 448.8, 286.8 | Cy-3-(sin)(sin)-diGlc-5-Glc [30] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, Z.; Zhang, H.; Liu, Y.; Zhang, S.; Meng, Q.; Liu, W. Isolation of High Purity Anthocyanin Monomers from Red Cabbage with Recycling Preparative Liquid Chromatography and Their Photostability. Molecules 2018, 23, 991. https://doi.org/10.3390/molecules23050991
Chen Y, Wang Z, Zhang H, Liu Y, Zhang S, Meng Q, Liu W. Isolation of High Purity Anthocyanin Monomers from Red Cabbage with Recycling Preparative Liquid Chromatography and Their Photostability. Molecules. 2018; 23(5):991. https://doi.org/10.3390/molecules23050991
Chicago/Turabian StyleChen, Yijun, Zikun Wang, Hanghang Zhang, Yuan Liu, Shuai Zhang, Qingyan Meng, and Wenjie Liu. 2018. "Isolation of High Purity Anthocyanin Monomers from Red Cabbage with Recycling Preparative Liquid Chromatography and Their Photostability" Molecules 23, no. 5: 991. https://doi.org/10.3390/molecules23050991




