Effects of UV-B Radiation on the Content of Bioactive Components and the Antioxidant Activity of Prunella vulgaris L. Spica during Development
Abstract
:1. Introduction
2. Results
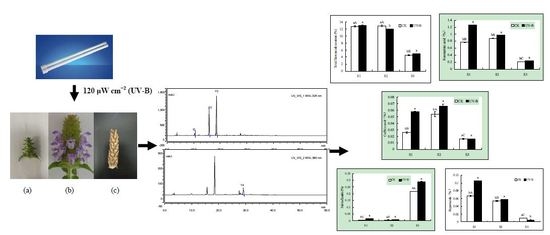
2.1. Effects of UV-B Radiation on the Contents of Total Flavonoids, Rosmarinic Acid and Caffeic Acid
2.2. Effects of UV-B Radiation on the Contents of Salviaflaside and Hyperoside
2.3. Effects of UV-B Radiation on the Antioxidant Activities
2.4. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. UV-B Treatments
4.3. Determination of Total Flavonoids Content
4.4. Determination of the Caffeic Acid, Salviaflaside, Rosmarinic Acid and Hyperoside Contents
4.5. DPPH Free Radical Scavenging Assay
4.6. Trolox Equivalent Antioxidant Capacity Assay (TEAC Assay)
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bjorn, L.O. Effects of ozone depletion and increased UV-B on terrestrial ecosystems. Int. J. Environ. Stud. 1996, 51, 217–243. [Google Scholar] [CrossRef]
- McKenzie, R.L.; Bjorn, L.O.; Bais, A.; Ilyasd, M. Changes in biologically active UV radiation reaching the earth’s surface. Photochem. Photobiol. Sci. 2003, 2, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.R.; Tarpley, L. Morphological and physiological response of nine southern U.S. rice cultivars differing in their tolerance to enhanced ultraviolet-B radiation. Environ. Exp. Bot. 2011, 70, 174–184. [Google Scholar] [CrossRef]
- Kumari, R.; Agrawal, S.B.; Singh, S.; Dubey, N.K. Supplemental ultraviolet-B induced changes in essential oil composition and total phenolics of Acorus calamus L. (sweet flag). Ecotoxicol. Environ. Saf. 2009, 72, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.R.; Chen, Y.H.; Guo, Q.S.; Wang, W.M.; Liu, L.; Fan, J.; Cao, L.P.; Li, C. Short-term UV-B radiation effects on morphology, physiological traits and accumulation of bioactive compounds in Prunella vulgaris L. J. Plant. Interact. 2017, 12, 348–354. [Google Scholar] [CrossRef]
- Nishimura, T.; Ohyama, K.; Goto, E.; Inagaki, N.; Morota, T. Ultraviolet-B radiation suppressed the growth and anthocyanin production of perilla plants grown under controlled environments with artificial light. Acta Horticult. 2008, 797, 425–429. [Google Scholar] [CrossRef]
- Lee, M.J.; Son, J.E.; Oh, M.M. Growth and phenolic content of sow thistle grown in a closed-type plant production system with a UV-A or UV-B lamp. Hortic. Environ. Biotechnol. 2013, 54, 492–500. [Google Scholar] [CrossRef]
- Indrajith, A.; Ravindran, K.C. Antioxidant potential of Indian medicinal plant in Phyllanthus amarus L. under supplementary UV-B radiation. Recent Res. Sci. Technol. 2009, 1, 34–39. [Google Scholar]
- Gu, X.D.; Sun, M.Y.; Zhang, L.; Fu, H.W.; Cui, L.; Chen, R.Z.; Zhang, D.W.; Tian, J.K. UV-B induced changes in the secondary metabolites of Morus alba L. leaves. Molecules 2010, 15, 2980–2993. [Google Scholar] [CrossRef] [PubMed]
- Ning, W.; Peng, X.; Ma, L.Y.; Cui, L.; Lu, X.P.; Wang, J.; Tian, J.K.; Li, X.M.; Wang, W.; Zhang, L. Enhanced secondary metabolites production and antioxidant activity in postharvest Lonicera japonica Thunb. in response to UV radiation. Innov. Food. Sci. Emerg. 2012, 13, 231–243. [Google Scholar] [CrossRef]
- Sun, M.Y.; Gu, X.D.; Fu, H.W.; Zhang, L.; Chen, R.Z.; Cui, L.; Zheng, L.H.; Zhang, D.W.; Tian, J.K. Change of secondary metabolites in leaves of Ginkgo biloba L. in response to UV-B induction. Innov. Food Sci. Emerg. 2010, 11, 672–676. [Google Scholar] [CrossRef]
- Ma, C.H.; Chu, J.Z.; Shi, X.F.; Liu, C.Q.; Yao, X.Q. Effects of enhanced UV-B radiation on the nutritional and active ingredient contents during the floral development of medicinal chrysanthemum. J. Photochem. Photobiol. B 2016, 158, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Liu, L.; Guo, Q.S.; Zhu, Z.B.; Zhang, L.X. Effects of different water management options and fertilizer supply on photosynthesis, fluorescence parameters and water use efficiency of Prunella vulgaris seedlings. Biol. Res. 2016, 49, 12. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.B.; Xia, B.H.; Xie, W.J.; Zhou, Y.M.; Xie, J.C.; Li, H.Q.; Liao, D.F.; Lin, L.M.; Li, C. Phytochemistry and pharmacological activities of the genus Prunella. Food Chem. 2016, 204, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Zhao, Y.Y.; Wang, B.; Ai, T.M.; Chen, Y.Y. Depsides from Prunella vulgaris. Chin. Chem. Lett. 2000, 11, 997–1000. [Google Scholar]
- Xu, D.C.; Liu, S.J.; Yu, N.J.; Fang, C.W. Study of chemical composition of Prunella vulgaris L. ear. Mod. Chin. Med. 2010, 1, 21–22. [Google Scholar]
- Wu, Y.; Fang, M.F.; Yue, M.; Chai, Y.F.; Wang, H.; Li, Y.F. Advances in influence of UV-B radiation on medicinal plant secondary metabolism. China J. Chin. Mater. Med. 2012, 37, 2248–2251. (In Chinese) [Google Scholar]
- Chen, Y.H.; Yu, M.M.; Zhu, Z.B.; Zhang, L.X.; Guo, Q.S. Optimisation of potassium chloride nutrition for proper growth, physiological development and bioactive component production in Prunella vulgaris L. PLoS ONE 2013, 8, e66259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bai, Y.M.; Fang, M.; Le, Z. Accumulation of total flavonoids at reproductive stage and antioxidant activity of Prunella vulgaris Linn. Acta Agric. Boreali-Sin. 2012, 27, 170–174. (In Chinese) [Google Scholar]
- Chen, Y.H.; Guo, Q.S.; Zhu, Z.B.; Zhang, L.X. Changes in bioactive components related to the harvest time from the spicas of Prunella vulgaris. Pharm. Biol. 2012, 9, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Y, B.G.; Yin, D.D.; Miao, F. Accumulation laws of main medicinal ingredients in different part of Prunella vulgaris L. Acta Agric. Boreali-Sin. 2010, 19, 137–140. (In Chinese) [Google Scholar]
- Özkan, E.E.; Özsoy, N.; Özhan, G.; Çelik, B.Ö.; Mat, A. Chemical composition and biological activities of Hypericum pamphylicum. Ind. Crop Prod. 2013, 50, 182–189. [Google Scholar] [CrossRef]
- Qiu, J.L.; Liu, P.; Xiong, W.Z.; Liu, Y.Z. Determination of the content of hyperin in Jinhuakui by HPLC method. China J. Chin. Med. 2015, 30, 1793–1794. [Google Scholar]
- Hosni, K.; Msaada, K.; Taârit, M.; Marzouk, B. Phenological variations of secondary metabolites from Hypericum triquetrifolium Turra. Biochem. Syst. Ecol. 2011, 39, 43–50. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Marinova, E.; Pokorny, J. Natural antioxidants from herbs and spices. Eur. J. Lipid. Sci. Technol. 2006, 108, 776–793. [Google Scholar] [CrossRef]
- Karp, S.M.; Koch, T.R. Oxidative stress and antioxidants in inflammatory bowel disease. DM-Dis. Mon. 2006, 52, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Polidori, M.C.; Cherubini, A.; Mecocci, P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: An overview. J. Chromatogr. B 2005, 827, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Barontini, M.; Bernini, R.; Carastro, R.; Gentili, P.; Romani, A. Synthesis and DPPH radical scavenging activity of novel compounds obtained from tyrosol and cinnamic acid derivatives. New J. Chem. 2014, 38, 809–816. [Google Scholar] [CrossRef]
- Bernini, R.; Barontini, M.; Cis, V.; Carastro, I.; Tofani, D.; Chiodo, R.A.; Lupattelli, P.; Incerpi, S. Synthesis and evaluation of the antioxidant activity of lipophilic phenethyl trifluoroacetate esters by in vitro ABTS, DPPH and in cell-culture DCF assays. Molecules 2018, 23, 208. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Y.; Feng, B.L.; Deng, T.; An, S.Q.; Gao, J.F.; Chai, Y. Antioxidant activity of ethanol extracts of different Buckwheat. Acta. Agric. Boreali-sin. 2007, 16, 76–79, 84. (In Chinese) [Google Scholar]
- Barreca, D.; Bellocco, E.; Leuzzi, U.; Gattuso, G. First evidence of C- and O-glycosyl flavone in blood orange (Citrus sinensis (L.) Osbeck) juice and their influence on antioxidant properties. Food Chem. 2014, 149, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Optimisation of aqueous extraction conditions for the recovery of phenolic compounds and antioxidants from lemon pomace. Int. J. Food. Sci. Technol. 2016, 51, 2009–2018. [Google Scholar] [CrossRef]
- Feng, W.H.; Li, C.; Xin, W.M.; Lin, L.M.; Xia, B.H.; Rong, L.X.; Yang, L.X.; Yi, H.; Zhang, Y.X.; Chen, L.M.; et al. Exploration on feasibility of introducing bioassay method into quality evaluation of Chinese herbal medicines by studying on the correlation between antioxidant activity of Prunella vulgaris and its total phenolic acids content for example. China J. Chin. Mater. Med. 2016, 41, 2660–2668. (In Chinese) [Google Scholar]
- Bellocco, E.; Barreca, D.; Laganà, G.; Leuzzi, U.; Tellone, E.; Ficarra, S.; Kotyk, A.; Galtieri, A. Influence of l-rhamnosyl-Dglucosyl derivatives on properties and biological interaction of flavonoids. Mol. Cell. Biochem. 2009, 321, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.J.; Ou, B.X.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the total flavonoids, rosmarinic acid, caffeic acid, salviaflaside and hyperoside are available from the authors. |




| Antioxidant Index | UV-B Dosage (μW cm−2 nm−1) | Bud Stage | Full-Flowering | Mature-Fruiting |
|---|---|---|---|---|
| DPPH● (%) | 0 | 90.41 ± 0.23 aA | 91.24 ± 0.10 aA | 74.36 ± 2.90 aB |
| 120 | 90.35 ± 0.83 aA | 88.61 ± 0.34 bA | 70.49 ± 2.62 aB | |
| TEAC (mmol L−1 Trolox) | 0 | 1.31 ± 0.01 aA | 1.35 ± 0.02 aA | 0.80 ± 0.05 bB |
| 120 | 1.13 ± 0.05 bB | 1.33 ± 0.05 aA | 1.00 ± 0.02 aC |
| Antioxidant Index | UV-B Dose (μW cm−2 nm−1) | Rosmarinic Acid (%) | Caffeic Acid (%) | Hyperoside (%) | Salviaflaside (%) | Total Flavonoids (%) |
|---|---|---|---|---|---|---|
| DPPH● (%) | 0 | 0.993 * | 0.727 | 0.967 | −0.999 * | 0.999 * |
| 120 | 0.187 | 0.189 | 0.229 | 0.312 | 0.999 * | |
| TEAC (mmol L−1 Trolox) | 0 | 0.191 | 0.202 | 0.244 | 0.334 | 0.999 * |
| 120 | 0.132 | 0.136 | 0.163 | 0.224 | 0.720 |
| Standard Chemicals | Regression Equation | R2 | Linear Range |
|---|---|---|---|
| Total flavonoids | y = 0.5899x + 0.0199 | 0.9999 | 0.00–2.00 |
| Rosmarinic acid | y = 39999x + 0.0031 | 0.9999 | 0.000209–0.001045 |
| Caffeic acid | y = 49130x − 0.0422 | 0.9999 | 0.000108–0.00054 |
| Salviaflaside | y = 23282x − 0.044 | 0.9999 | 0.00022–0.0011 |
| Hyperoside | y = 33699x − 0.0094 | 0.9999 | 0.00011–0.00055 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhang, X.; Guo, Q.; Liu, L.; Li, C.; Cao, L.; Qin, Q.; Zhao, M.; Wang, W. Effects of UV-B Radiation on the Content of Bioactive Components and the Antioxidant Activity of Prunella vulgaris L. Spica during Development. Molecules 2018, 23, 989. https://doi.org/10.3390/molecules23050989
Chen Y, Zhang X, Guo Q, Liu L, Li C, Cao L, Qin Q, Zhao M, Wang W. Effects of UV-B Radiation on the Content of Bioactive Components and the Antioxidant Activity of Prunella vulgaris L. Spica during Development. Molecules. 2018; 23(5):989. https://doi.org/10.3390/molecules23050989
Chicago/Turabian StyleChen, Yuhang, Xuerong Zhang, Qiaosheng Guo, Li Liu, Chen Li, Liping Cao, Qin Qin, Miao Zhao, and Wenming Wang. 2018. "Effects of UV-B Radiation on the Content of Bioactive Components and the Antioxidant Activity of Prunella vulgaris L. Spica during Development" Molecules 23, no. 5: 989. https://doi.org/10.3390/molecules23050989