Evaluation of Lactic Acid Bacteria on the Inhibition of Vibrio parahaemolyticus Infection and Its Application to Food Systems
Abstract
:1. Introduction
2. Results
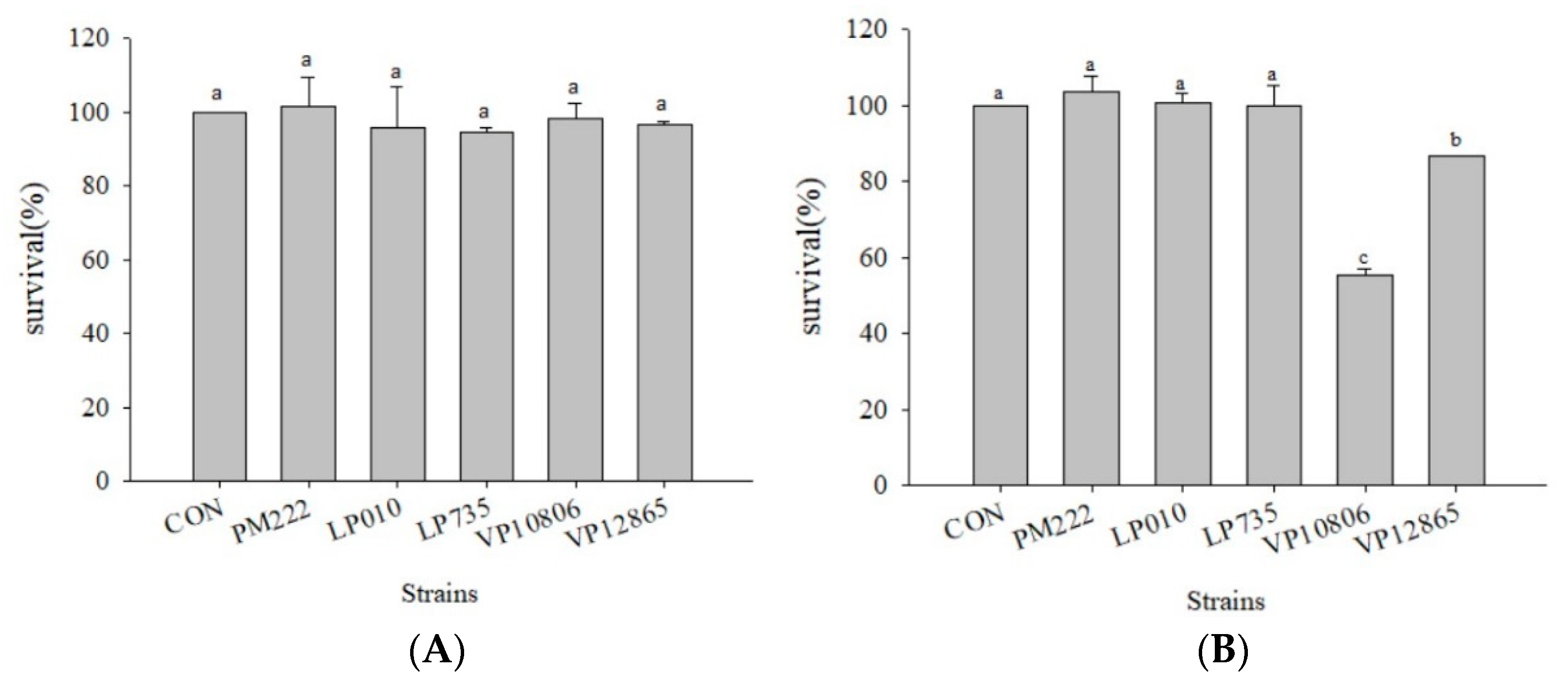
2.1. Survival Rate of Caco-2 Cells Co-Cultured with V. parahaemolyticus and LAB
2.2. LAB Suppresses V. parahaemolyticus in Food Mode
2.3. LAB Inhibit V. parahaemolyticus-Induced Secretion of Inflammatory Cytokines by HT-29 Intestinal Epithelial Cells or RAW 264.7 Macrophages
2.4. Effects of Adding LAB and V. parahaemolyticus on the Expression of Inflammatory-Related Genes in Different Cell Lines
2.5. Adsorption Experiments on Mouse Epithelial Cells and Intestinal Mucus
2.6. Effect of LAB on Serum Immunity of V. parahaemolyticus in Mice
2.7. Gut Histology Changes after Treatment with V. parahaemolyticus
3. Materials and Methods
3.1. LAB Strain and Cell Line Culture
3.2. Cell Viability Analysis
3.3. LAB Inhibits V. parahaemolyticus in Food Mode
3.4. Levels of IL-1β, IL-6, IL-8, and TNF-α Cytokines Measured by ELISA
3.5. Total RNA Extraction
3.6. Real-Time Quantitative Polymerase Chain Reaction
3.7. LAB Treatment to Inhibit V. parahaemolyticus Infection in Animal Model
3.8. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shimohata, T.; Takahashi, A. Diarrhea induced by infection of Vibrio parahaemolyticus. J. Med. Investig. 2010, 57, 179–182. [Google Scholar] [CrossRef]
- Shirazinejad, A.; Ismail, N.; Bhat, R. Lactic acid as a potential decontaminant of selected foodborne pathogenic bacteria in shrimp (Penaeus merguiensis de Man). Foodborne Pathog. Dis. 2010, 7, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Terzi, G.; Gucukoglu, A. Effects of lactic acid and chitosan on the survival of V. parahaemolyticus in mussel samples. J. Anim. Vet. 2010, 9, 990–994. [Google Scholar] [CrossRef]
- Hwanhlem, N.; Watthanasakphuban, N.; Riebroy, S.; Benjakul, S.; Maneerat, S. Probiotic lactic acid bacteria from Kung-Som: Isolation, screening, inhibition of pathogenic bacteria. Int. J. Food Sci. 2010, 45, 594–601. [Google Scholar] [CrossRef]
- Xi, D. Application of Probiotics and Green Tea Extract in Post-Harvest Processes of Pacific Oysters (Crassostrea gigas) for Reducing Vibrio parahaemolyticus and Extending Shelf Life. Oregon State University, 2011. Available online: https://ir.library.oregonstate.edu/concern/graduate_thesis_or_dissertations/3j333476m (accessed on 2 August 2017).
- Liu, C.H.; Chiu, C.H.; Wang, S.W.; Cheng, W. Dietary administration of the probiotic, Bacillus subtilis E20, enhances the growth, innate immune responses, and disease resistance of the grouper, Epinephelus coioides. Fish Shellfish Immunol. 2012, 33, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.H.; Rahimnejad, S.; Yang, S.Y.; Kim, K.W.; Lee, K.J. Evaluations of Bacillus spp. as dietary additives on growth performance, innate immunity and disease resistance of olive flounder (Paralichthys olivaceus) against Streptococcus iniae and as water additives. Aquaculture 2013, 402, 50–57. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Raja, R.; Ravikumar, R.; Carvalho, I.S. Mechanism of action of probiotics. Braz. Arch. Biol. Technol. 2013, 56, 113–119. [Google Scholar] [CrossRef]
- Nayak, S. Probiotics and immunity: A fish perspective. Fish Shellfish Immunol. 2010, 29, 2–14. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, Y.; Xu, L.; Yang, Y.; Marubashi, T.; Zhou, Z.; Yao, B. Effects of dietary Bacillus subtilis C-3102 on the production, intestinal cytokine expression and autochthonous bacteria of hybrid tilapia Oreochromis niloticus ♀ × Oreochromis aureus ♂. Aquaculture 2013, 412, 125–130. [Google Scholar] [CrossRef]
- Fernández, M.F.; Boris, S.; Barbes, C. Probiotic properties of human lactobacilli strains to be used in the gastrointestinal tract. J. Appl. Microbiol. 2003, 94, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Montiel, R.; Martín-Cabrejas, I.; Langa, S.; El Aouad, N.; Arqués, J.; Reyes, F.; Medina, M. Antimicrobial activity of reuterin produced by Lactobacillus reuteri on Listeria monocytogenes in cold-smoked salmon. Food Microbiol. 2014, 44, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gueimonde, M.; Margolles, A.; de los Reyes-Gavilan, C.G.; Salminen, S. Competitive exclusion of enteropathogens from human intestinal mucus by Bifidobacterium strains with acquired resistance to bile—A preliminary study. Int. J. Food Microbiol. 2007, 113, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Satish Kumar, R.; Kanmani, P.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Lactobacillus plantarum AS1 binds to cultured human intestinal cell line HT-29 and inhibits cell attachment by enterovirulent bacterium Vibrio parahaemolyticus. Lett. Appl. Microbiol. 2011, 53, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Jin, N.; Li, X.; Mi, Z.; Zhang, J.; Sun, L.; Li, X.; Zheng, H.; Li, P. Induction of an effective anti-tumor immune response and tumor regression by combined administration of IL-18 and Apoptin. Cancer Immunol. Immunother. 2007, 56, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Matlawska-Wasowska, K.; Finn, R.; Mustel, A.; O’Byrne, C.P.; Baird, A.W.; Coffey, E.T.; Boyd, A. The Vibrio parahaemolyticus type III secretion systems manipulate host cell MAPK for critical steps in pathogenesis. BMC Microbiol. 2010, 10, 329. [Google Scholar] [CrossRef] [PubMed]
- Brisbin, J.T.; Zhou, H.; Gong, J.; Sabour, P.; Akbari, M.R.; Haghighi, H.R.; Yu, H.; Clarke, A.; Sarson, A.J.; Sharif, S. Gene expression profiling of chicken lymphoid cells after treatment with Lactobacillus acidophilus cellular components. Dev. Comp. Immunol. 2008, 32, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Q.; Jin, C.J.; Gao, L.; Fang, W.M.; Gu, R.X.; Qian, J.Y.; Jiao, X.A. Alleviating effects of Lactobacillus strains on pathogenic Vibrio parahaemolyticus-induced intestinal fluid accumulation in the mouse model. FEMS Microbiol. Lett. 2013, 339, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, J.M.; Rui, H.; Zhou, X.; Iida, T.; Kodoma, T.; Ito, S.; Davis, B.M.; Bronson, R.T.; Waldor, M.K. Inflammation and disintegration of intestinal villi in an experimental model for Vibrio parahaemolyticus-induced diarrhea. PLoS Pathog. 2012, 8, e1002593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.K.; Lim, C.Y.; Teng, W.L.; Ouwehand, A.C.; Tuomola, E.M.; Salminen, S. Quantitative approach in the study of adhesion of lactic acid bacteria to intestinal cells and their competition with enterobacteria. Appl. Environ. Microbiol. 2000, 66, 3692–3697. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Lu, Y.C.; Ou, C.C.; Lin, S.L.; Tsai, C.C.; Huang, C.T.; Lin, M.Y. Lactobacillus plantarum MYL26 induces endotoxin tolerance phenotype in Caco-2 cells. BMC Microbiol. 2013, 13, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, B.S.M. The efficacy of grape seed extract, citric acid and lactic acid on the inactivation of Vibrio parahaemolyticus in shucked oysters. Food Control 2014, 41, 13–16. [Google Scholar] [CrossRef]
- Yun, B.; Oh, S.; Griffiths, M.W. Lactobacillus acidophilus modulates the virulence of Clostridium difficile. J. Dairy Sci. 2014, 97, 4745–4758. [Google Scholar] [CrossRef] [PubMed]
- Boonma, P.; Spinler, J.K.; Venable, S.F.; Versalovic, J.; Tumwasorn, S. Lactobacillus rhamnosus L34 and Lactobacillus casei L39 suppress Clostridium difficile-induced IL-8 production by colonic epithelial cells. BMC Microbiol. 2014, 14, 177. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Yoon, Y.S.; Seo, J.G.; Lee, H.G.; Chung, M.J.; Yum, D.Y. A study on the prevention of salmonella infection by using the aggregation characteristics of lactic Acid bacteria. Toxicol. Res. 2013, 29, 129. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yue, L.; Guan, X.; Qiao, S. The adhesion of putative probiotic lactobacilli to cultured epithelial cells and porcine intestinal mucus. J. Appl. Microbiol. 2008, 104, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.; Tannock, G. Colonization of the porcine gastrointestinal tract by lactobacilli. Appl. Environ. Microbiol. 1989, 55, 279–283. [Google Scholar] [PubMed]
- Blum, S.; Reniero, R.; Schiffrin, E.; Crittenden, R.; Mattila-Sandholm, T.; Ouwehand, A.; Salminen, S.; Von Wright, A.; Saarela, M.; Saxelin, M. Adhesion studies for probiotics: Need for validation and refinement. Trends Food Sci. Technol. 1999, 10, 405–410. [Google Scholar] [CrossRef]
- Kim, H.G.; Gim, M.G.; Kim, J.Y.; Jin Hwang, H.; Ham, M.S.; Lee, J.M.; Hartung, T.; Park, J.W.; Han, S.H.; Chung, D.K. Lipoteichoic acid from Lactobacillus plantarum elicits both the production of interleukin-23p19 and suppression of pathogen-mediated interleukin-10 in THP-1 cells. FEMS Immunol. Med. Microbiol. 2006, 49, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Gao, Q.; Min, M.; Zhang, C.; Peng, S.; Shi, Z. Ability of Lactobacillus plantarum lipoteichoic acid to inhibit Vibrio anguillarum-induced inflammation and apoptosis in silvery pomfret (Pampus argenteus) intestinal epithelial cells. Fish Shellfish Immunol. 2016, 54, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Zhang, H.; Liu, X.; Gong, Y.; Chen, Y.; Cao, Y.; Hu, C. Pathogenic analysis of Vibrio alginolyticus infection in a mouse model. Folia Microbiol. 2014, 59, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Candela, M.; Perna, F.; Carnevali, P.; Vitali, B.; Ciati, R.; Gionchetti, P.; Rizzello, F.; Campieri, M.; Brigidi, P. Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: Adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int. J. Food Microbiol. 2008, 125, 286–292. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |















| Strains | LAB | Survival of Vibrio parahaemolyticus (Log CFU/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 °C | Room Temperature | ||||||||
| 1 h | 4 h | 1 h | 4 h | ||||||
| Culture | Supernatant | Culture | Supernatant | Culture | Supernatant | Culture | Supernatant | ||
| 10806 | Control | 3.69 ± 0.08 a | 3.74 ± 0.05 a | <1 | <1 | 5.20 ± 0.01 a | 5.21 ± 0.01 a | 5.70 ± 0.03 a | 5.82 ± 0.04 a |
| Lactobacillus plantarum PM 222 | <1 b | <1 b | <1 | <1 | 3.81 ± 0.03 b | 4.52 ± 0.04 b | <1 b | <1 b | |
| Lactobacillus plantarum LP 735 | <1 b | <1 b | <1 | <1 | 2.62 ± 0.02 d | 3.54 ± 0.04 d | <1 b | <1 b | |
| Lactobacillus plantarum LP 010 | <1 b | <1 b | <1 | <1 | 2.79 ± 0.04 c | 3.66 ± 0.05 c | <1 b | <1 b | |
| 12865 | Control | 4.28 ± 0.09 a | 4.23 ± 0.01 a | 3.73 ± 0.10 a | 3.74 ± 0.04 a | 5.95 ± 0.02 a | 6.01 ± 0.03 a | 6.55 ± 0.05 a | 6.61 ± 0.06 a |
| Lactobacillus plantarum PM 222 | <1 b | <1 b | <1 b | <1 b | 4.84 ± 0.03 b | 5.00 ± 0.03 b | <1 b | <1 b | |
| Lactobacillus plantarum LP 735 | <1 b | <1 b | <1 b | <1 b | 3.83 ± 0.03 c | 3.90 ± 0.04 c | <1 b | <1 b | |
| Lactobacillus plantarum LP 010 | <1 b | <1 b | <1 b | <1 b | 2.74 ± 0.06 d | 3.22 ± 0.11 d | <1 b | <1 b | |
| Strains | Survival of Lactic Acid Bacteria (Log CFU/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| BCRC 10806 | BCRC 12865 | |||||||
| 4 °C | Room Temperature | 4 °C | Room Temperature | |||||
| 1 h | 4 h | 1 h | 4 h | 1 h | 4 h | 1 h | 4 h | |
| Lactobacillus plantarum PM 222 | 6.16 ± 0.02 Ab | 6.11 ± 0.01 Ac | 6.10 ± 0.02 Ac | 6.02 ± 0.02 Ac | 6.14 ± 0.04 Ab | 6.00 ± 0.01 Bc | 6.06 ± 0.06 Ab | 6.07 ± 0.00 Ac |
| Lactobacillus plantarum LP 735 | 6.38 ± 0.01 Aa | 6.37 ± 0.01 Ab | 6.35 ± 0.02 Ab | 6.46 ± 0.09 Ab | 6.43 ± 0.00 Aa | 6.33 ± 0.01 Bb | 6.37 ± 0.02 Aa | 6.38 ± 0.02 Ab |
| Lactobacillus plantarum LP 010 | 6.43 ± 0.02 Aa | 6.46 ± 0.00 Aa | 6.52 ± 0.00 Aa | 6.71 ± 0.03 Ba | 6.45 ± 0.02 Aa | 6.41 ± 0.01 Aa | 6.46 ± 0.02 Aa | 6.72 ± 0.01 Ba |
| Species | Strain | Adhesive Ability | Intestinal Mucus (OD570 nm) § |
|---|---|---|---|
| Epithelial Cell (Bacteria per Cell) ‡ | |||
| Lactobacillus plantarum | PM 222 | 26.4 ± 9.6 a | 0.12 ± 0.03 a |
| Lactobacillus plantarum | LP 010 | 18.7 ± 9.0 ab | 0.09 ± 0.01 b |
| Lactobacillus plantarum | LP 735 | 14 ± 9.0 c | 0.05 ± 0.00 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-C.; Hung, Y.-H.; Chou, L.-C. Evaluation of Lactic Acid Bacteria on the Inhibition of Vibrio parahaemolyticus Infection and Its Application to Food Systems. Molecules 2018, 23, 1238. https://doi.org/10.3390/molecules23051238
Tsai C-C, Hung Y-H, Chou L-C. Evaluation of Lactic Acid Bacteria on the Inhibition of Vibrio parahaemolyticus Infection and Its Application to Food Systems. Molecules. 2018; 23(5):1238. https://doi.org/10.3390/molecules23051238
Chicago/Turabian StyleTsai, Cheng-Chih, Yung-Hsien Hung, and Lan-Chun Chou. 2018. "Evaluation of Lactic Acid Bacteria on the Inhibition of Vibrio parahaemolyticus Infection and Its Application to Food Systems" Molecules 23, no. 5: 1238. https://doi.org/10.3390/molecules23051238




