Synthesis and Insecticidal Activity of Mesoionic Pyrido[1,2-α]pyrimidinone Derivatives Containing a Neonicotinoid Moiety
Abstract
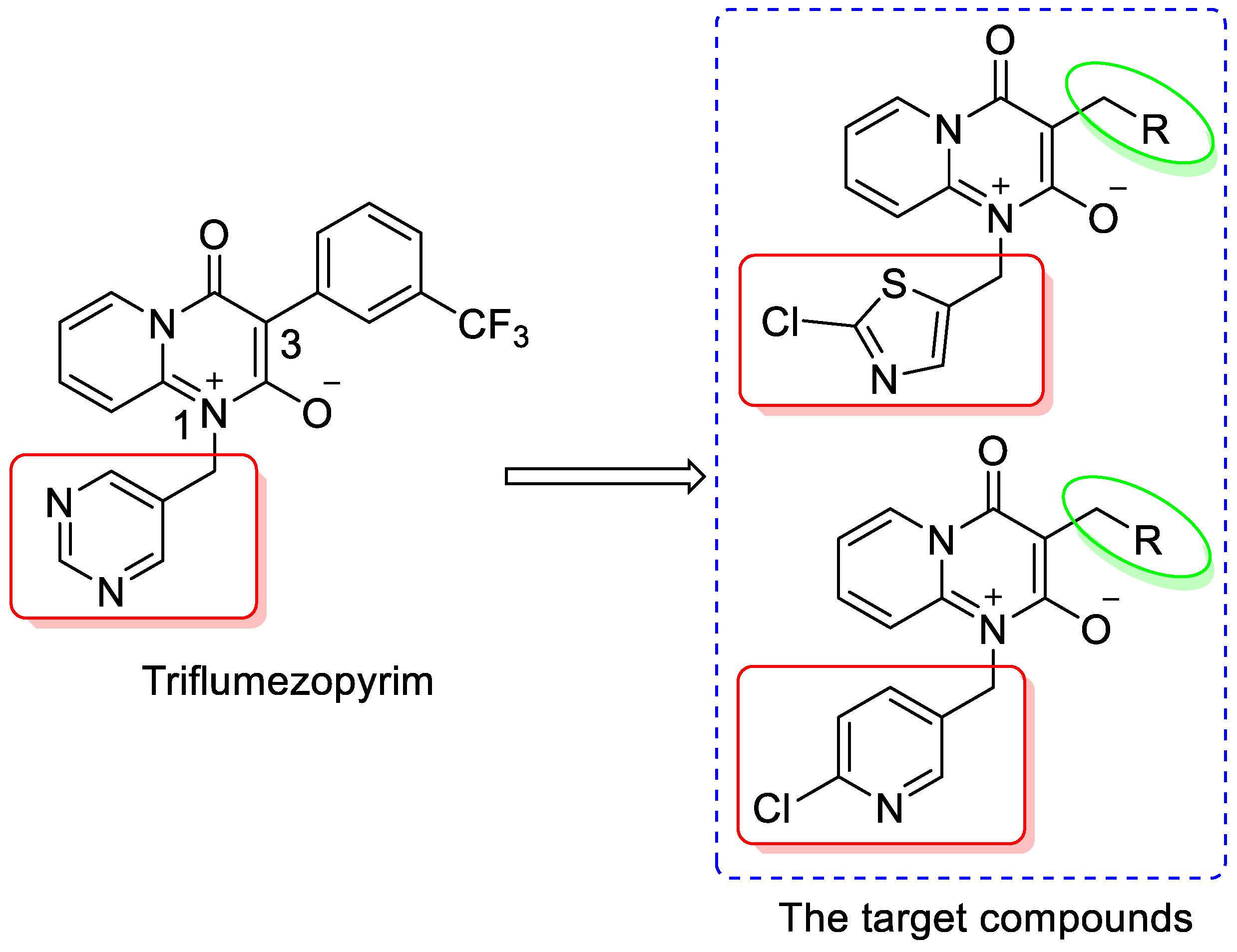
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Evaluation
2.3. Label-Free proteomics Comparative Analysis
2.3.1. Analysis of Protein between Control and Treatment Groups
2.3.2. Bioinformatics Analysis
4. Materials and Methods
4.1. Synthesis
4.2. Biological Assay
4.3. Proteomics
4.3.1. Sample Preparation
4.3.2. Proteins Extraction for LC−MS/MS Analysis
4.3.3. LC-MS/MS Analysis, Database Searching, and Bioinformatics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, S.; Zhou, S.; Xie, Y.T.; Jin, R.Y.; Meng, X.D.; Zhang, D.K.; Hua, X.W.; Liu, M.; Wu, C.C.; Xiong, L.X.; et al. The exploration of chiral N-cyano sulfiliminyl dicarboxamides on insecticidal activities. Chin. Chem. Lett. 2017, 28, 1499–1504. [Google Scholar] [CrossRef]
- Wang, B.L.; Zhu, H.W.; Ma, Y.; Xiong, L.X.; Li, Y.Q.; Zhao, Y.; Zhang, J.F.; Chen, Y.W.; Zhou, S.; Li, Z.M. Synthesis, insecticidal activities, and SAR studies of novel pyridylpyrazole acid derivatives based on amide bridge modification of anthranilic diamide insecticides. J. Agric. Food Chem. 2013, 61, 5483–5493. [Google Scholar] [CrossRef] [PubMed]
- Stewart, F.H.C. The chemistry of the sydnones. Chem. Rev. 1964, 64, 129–147. [Google Scholar] [CrossRef]
- Latthe, P.R.; Shinge, P.S.; Badami, B.V.; Patil, P.B.; Holihosur, S.N. Curtius rearrangement reactions of 3-(4-azidocarbonyl) phenylsydnone. Synthesis of 4-(sydnon-3-yl) phenyl carbamates, N-aryl-N′-[4-(sydnon-3-yl)] phenyl ureas and their antimicrobial and insecticidal activities. J. Chem. Sci. 2006, 118, 249–256. [Google Scholar] [CrossRef]
- Hill, J.B. 3-Arylthioalkyl-4-optionally substituted sydnones. U.S. Patent 3,883,548, 13 May 1975. [Google Scholar]
- Regnier, G.; Canevari, R.; Laubie, M. Sydnone Imine Compounds. U.S. Patent 3,898,230, 5 August 1975. [Google Scholar]
- Ray, R.E.; Wagner, H.A. Anti-Inflammatory Sydnones. U.S. Patent 4,020,079, 26 April 1977. [Google Scholar]
- Imashiro, Y.; Masuda, K. 3-Tertiary amino-4-tertiary amino methyl sydnones. U.S. Patent 3,591,586, 6 July 1971. [Google Scholar]
- Imashiro, Y.; Masuda, K. 3-Hydrocarbon-4-tertiary amino methyl sydnones. U.S. Patent 3,642,793, 15 February 1972. [Google Scholar]
- Kappe, C.O.; Kappe, T. Cross-conjugated and pseudo-cross-conjugated mesomeric betaines, XVIII: Bicyclic mesoionic pyrimidines with cardiovascular activity. Arch. Pharm. 1991, 324, 863–866. [Google Scholar] [CrossRef]
- Kamble, R.R.; Sudha, B.S. Synthesis, spectral characterization and antihaemostatic activity of 1,2,4-triazoles incorporating 1,2,4-triazine rings. J. Chem. Sci. 2006, 118, 191–194. [Google Scholar] [CrossRef]
- Glennon, R.A.; Rogers, M.E.; Bass, R.G.; Ryan, S.B. Mesoionic xanthine analogs as inhibitors of cyclic AMP phosphodiesterase. J. Pharm. Sci. 1978, 67, 1762–1765. [Google Scholar] [CrossRef] [PubMed]
- Glennon, R.A.; Tejani-Butt, S.M.; Padgett, W.; Daly, J.W. Mesoionic xanthine analogues: Antagonists of adenosine receptors. J. Med. Chem. 1984, 27, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- Coburn, R.A.; Glennon, R.A. Mesoionic purinone analogs IV: Synthesis and in vitro antibacterial properties of mesoionic thiazolo [3,2-a] pyrimidin-5,7-diones and mesoionic 1,3,4-thiadiazolo [3,2-a] pyrimidin-5,7-diones. J. Pharm. Sci. 1973, 62, 1785–1789. [Google Scholar] [CrossRef] [PubMed]
- Coburn, R.A.; Carapellotti, R.A. Synthesis and properties of mesoionic pyrimido [1,2-b] pyridazine-2,4-diones and mesoionic pyridazino [2,3-a]-s-triazine-2,4-diones: Mesoionic analogs structurally related to fervenulin. J. Pharm. Sci. 1976, 65, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- White, E.H.; Egger, N. Reaction of sydnones with ozone as a method of deamination: On the mechanism of inhibition of monoamine oxidase by sydnones. J. Am. Chem. Soc. 1984, 106, 3701–3703. [Google Scholar] [CrossRef]
- Jogul, J.J.; Badami, B.V. Sydnone derivatives as synthons for novel bismesoionic compounds. Synthesis of 3-(2-sulphido-1,3,4-thiadiazolium-4-carbonylphenyl) sydnones and 4-[4-(2-sulphido-1,3,4-thiadiazolium) benzoyl]-1,3,4-thiadiazolium-2-thiolates from 3-[4/3-(hydrazinocarbonyl)phenyl]sydnones, and their antimicrobial and antitubercular activitie. J. Serb. Chem. Soc. 2006, 71, 851–860. [Google Scholar]
- Holyoke, C.W.; Zhang, W. Mesoionic Pesticides. U.S. Patent 8,552,007, 8 October 2013. [Google Scholar]
- Holyoke Jr, C.W.; Zhang, W.; Pahutski, T.F., Jr.; Lahm, G.P.; Tong, M.H.T.; Cordova, D.; Leighty, R.M. Triflumezopyrim: Discovery and optimization of a mesoionic insecticide for rice. In Discovery and Synthesis of Crop Protection Products; American Chemical Society: Washington, DC, USA, 2015. [Google Scholar]
- Maienfisch, P.; Stevenson, T.M.E. Discovery and Synthesis of Crop Protection Products; American Chemical Society: Washington, DC, USA, 2015. [Google Scholar]
- Zhang, W.; Holyoke, C.W.; Barry, J.; Leighty, R.M.; Cordova, D.; Vincent, D.R.; Briddell, T.A. Mesoionic pyrido [1,2-a] pyrimidinones: A novel class of insecticides inhibiting nicotinic acetylcholine receptors. Bioorg. Med. Chem. Lett. 2016, 26, 5444–5449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W. Mesoionic pyrido[1,2-a]pyrimidinone insecticides: From discovery to triflumezopyrim and dicloromezotiaz. Acc. Chem. Res. 2017, 50, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.K.; Deshpande, S.R.; Wagh, R.D. Mesoionic sydnone derivatives: An overview. J. Chem. Pharm. Res. 2012, 4, 1185–1199. [Google Scholar]
- Zhang, W.; Holyoke, C.W.; Pahutski, T.F.; Lahm, G.P.; Barry, J.D.; Cordova, D.; Hughes, K.A. Mesoionic pyrido [1,2-a] pyrimidinones: Discovery of triflumezopyrim as a potent hopper insecticide. Bioorg. Med. Chem. Lett. 2017, 27, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Cordova, D.; Benner, E.A.; Schroeder, M.E.; Holyoke, C.W.; Zhang, W.; Pahutski, T.F.; Hamm, J.C. Mode of action of triflumezopyrim: A novel mesoionic insecticide which inhibits the nicotinic acetylcholine receptor. Insect Biochem. Mol. Biol. 2016, 74, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Lee, P.W.; Liu, Z.; Xu, X.; Li, Z.; Qian, X. cis-Configuration: A new tactic/rationale for neonicotinoid molecular design. J. Agric. Food Chem. 2010, 59, 2943–2949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Cui, S.X.; Xu, Z.P.; Li, D.M.; Tian, Z.Z. Synthesis and insecticidal activities of novel bridged-neonicotinoids. Chin. Chem. Lett. 2017, 28, 1743–1745. [Google Scholar] [CrossRef]
- Elbert, A.; Erdelen, C.; Kuhnhold, J.; Nauen, R.; Schmidt, H.W.; Hattori, Y. Thiacloprid, a novel neonicotinoid insecticide for foliar application. In The BCPC Conference: Pests and Diseases, Proceedings of an International Conference held at the Brighton Hilton Metropole Hotel, Brighton, UK, 13–16 November 2000; British Crop Protection Council: Hampshire, UK, 2000; Volume 1, pp. 21–26. [Google Scholar]
- Aoki, I.; Tabuchi, T.; Minamida, I. Preparation of Pyridine Derivatives and Other Heterocycles as Insecticides. European Patent Application EP 381,130, 8 August 1990. [Google Scholar]
- Ishimitsu, K.; Suzuki, J.; Ohishi, H.; Yamada, T.; Hatano, R.; Takakusa, N.; Mitsui, J. Preparation of Pyridylalkylamine Derivatives as Insecticides. PCT International Application WO 9,104,965, 25 September 1991. [Google Scholar]
- Moriie, K.; Ootsu, J.; Hatsutori, Y.; Watanabe, A.; Ito, A. Preparation of Nitroiminotetrahydrooxadiazines as Insecticides. Japanese Patent JP 7,224,062, 22 August 1995. [Google Scholar]
- Uneme, H.; Iwanaga, K.; Higuchi, N.; Kando, Y.; Okauchi, T.; Akayama, A.; Minamida, I. Synthesis and insecticidal activity of nitroguanidine derivatives. Pestic. Sci. 1999, 55, 202–205. [Google Scholar] [CrossRef]
- Elbert, A.; Overbeck, H.; Iwaya, K.; Tsuboi, S. Imidacloprid, a novel systemic nitromethylene analogue insecticide for crop protection. In Brighton Crop Protection Conference, Pests and Diseases; British Crop Protection Council: Hampshire, UK, 1990. [Google Scholar]
- Kuhlmann, A.U.; Hoffmann, T.; Bursy, J.; Jebbar, M.; Bremer, E. Ectoine and hydroxyectoine as protectants against osmotic and cold stress: Uptake through the SigB-controlled betaine-choline-carnitine transporter-type carrier EctT from Virgibacillus pantothenticus. J. Bacteriol. 2011, 193, 4699–4708. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, S.K.; Kutzer, M.; Procek, J.; Gröbner, G.; Harryson, P. Tunable membrane binding of the intrinsically disordered dehydrin Lti30, a cold-induced plant stress protein. Plant Cell 2011, 23, 2391–2404. [Google Scholar] [CrossRef] [PubMed]
- Laksanalamai, P.; Narayan, S.; Luo, H.; Robb, F.T. Chaperone action of a versatile small heat shock protein from Methanococcoides burtonii, a cold adapted archaeon. Proteins 2009, 75, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Carter, E.A.; Bonab, A.A.; Hamrahi, V.; Pitman, J.; Winter, D.; Macintosh, L.J.; Tompkins, R.G. Effects of burn injury, cold stress and cutaneous wound injury on the morphology and energy metabolism of murine brown adipose tissue (BAT) in vivo. Life Sci. 2011, 89, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, W.; Badawey, E.S.; Hojas, G.; Roschger, P.; Kappe, T. Malonates in cyclocondensation reactions. Molecules 2001, 6, 338–352. [Google Scholar] [CrossRef]
- Böhme, T.M.; Keim, C.; Kreutzmann, K.; Linder, M.; Dingermann, T.; Dannhardt, G.; Lambrecht, G. Structure-activity relationships of dimethindene derivatives as new M2-selective muscarinic receptor antagonists. J. Med. Chem. 2003, 46, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, V.; Skobridis, K.; Tzakos, A.G.; Ragoussis, V. A simple method for the alkaline hydrolysis of esters. Tetrahedron Lett. 2007, 48, 8230–8233. [Google Scholar] [CrossRef]
- Zettl, H.; Ness, J.; Hähnke, V.; Beher, D.; Jumpertz, T.; Saric, A.; Weggen, S. Discovery of γ-secretase modulators with a novel activity profile by text-based virtual screening. ACS Chem. Biol. 2012, 7, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.B.; Lambert, R.E.; Fronczek, F.R.; Cragg, P.J.; Wallace, K.J. An activated coumarin-enamine Michael acceptor for CN−. New J. Chem. 2014, 38, 4678–4683. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, Y.L.; Zhao, C.Y.; Li, H.H.; Wang, M.J.; Morris-Natschke, S.L.; Liu, Y.Q. Design, synthesis, crystal structure, bioactivity, and molecular docking studies of novel sulfonylamidine-derived neonicotinoid analogs. Med. Chem. Res. 2014, 23, 5043–5057. [Google Scholar] [CrossRef]
- Tian, Z.; Jiang, Z.; Li, Z.; Song, G.; Huang, Q. Syntheses and biological activities of octahydro-1H-cyclopenta[d] pyrimidine derivatives. J. Agric. Food Chem. 2007, 55, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Shao, X.; Li, Z.; Qian, X.; Huang, Q. Synthesis, insecticidal activity, and QSAR of novel nitromethylene neonicotinoids with tetrahydropyridine fixed cis configuration and exo-ring ether modification. J. Agric. Food Chem. 2007, 55, 2288–2292. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Komatsu, S.; Noda, H. Proteomic analysis of brown planthopper: Application to the study of carbamate toxicity. Insect Biochem. Mol. Biol. 2004, 34, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.J. Uncovering the complex metabolic network underlying diterpenoid phytoalexin biosynthesis in rice and other cereal crop plants. Phytochemistry 2006, 67, 2307–2317. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sengupta, D.; Kannan, M.; Reddy, A.R. A root proteomics-based insight reveals dynamic regulation of root proteins under progressive drought stress and recovery in Vigna radiata (L.) Wilczek. Planta 2011, 233, 1111–1127. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.M.; Yu, L.; Chen, Z.; Zhang, S.X.; Shi, J.; Zhao, X.Z.; Yang, Y.Y.; Hu, D.Y.; Song, B.A. Label-free quantitative proteomic analysis of chitosan oligosaccharide-treated rice infected with southern rice black-streaked dwarf virus. Viruses 2017, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized ppb-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Gene Ontology Consortium. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 2004, 32, 258–261. [Google Scholar]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic. Acids Res. 2008, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Thissen, D.; Steinberg, L.; Kuang, D. Quick and easy implementation of the Benjamini-Hochberg procedure for controlling the false positive rate in multiple comparisons. J. Educ. Behav. Stat. 2002, 27, 77–83. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar]
Sample Availability: Samples of the compounds I1–I28 are available from the authors. |
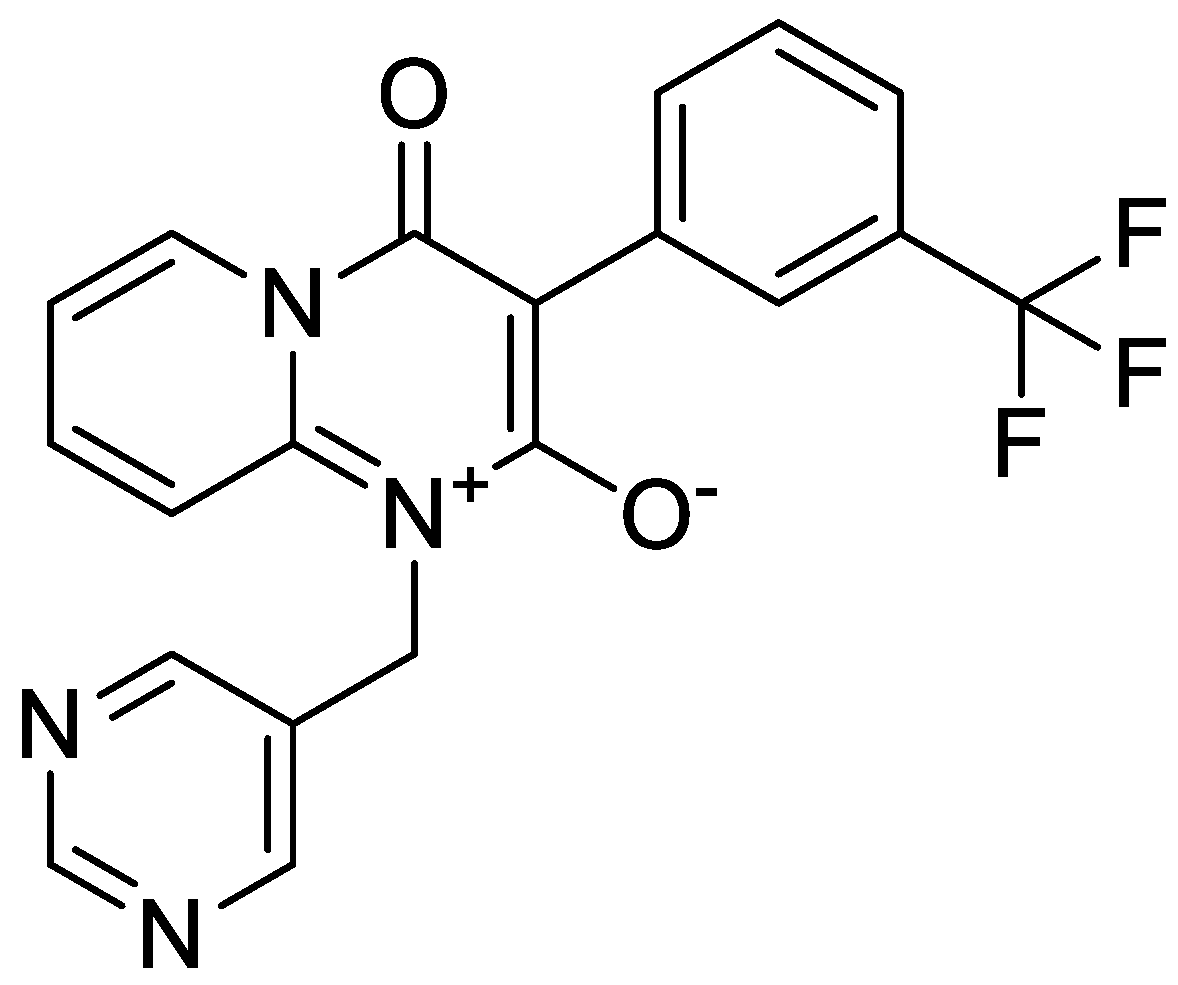



| Compounds | Concentration (μg/mL) | Mortality Rate (%) |
|---|---|---|
| I1 | 500 | 85 |
| I2 | 500 | 69 |
| I3 | 500 | 31 |
| I4 | 500 | 23 |
| I5 | 500 | 0 |
| I6 | 500 | 51 |
| I7 | 500 | 77 |
| I8 | 500 | 51 |
| I9 | 500 | 28 |
| I10 | 500 | 46 |
| I11 | 500 | 35 |
| I12 | 500 | 43 |
| I13 | 500 | 100 |
| 200 | 100 | |
| 100 | 92 | |
| 50 | 30 | |
| I14 | 500 | 16 |
| I15 | 500 | 0 |
| I16 | 500 | 23 |
| I17 | 500 | 49 |
| I18 | 500 | 0 |
| I19 | 500 | 58 |
| I20 | 500 | 62 |
| I21 | 500 | 39 |
| I22 | 500 | 62 |
| I23 | 500 | 58 |
| I24 | 500 | 0 |
| I25 | 500 | 62 |
| I26 | 500 | 27 |
| I27 | 500 | 46 |
| I28 | 500 | 62 |
| Imidacloprid | 500 | 100 |
| 200 | 100 | |
| 100 | 100 | |
| 50 | 100 | |
| Triflumezopyrim | 500 | 100 |
| 200 | 100 | |
| 100 | 100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, J.; Yu, L.; Liu, D.; Hu, D. Synthesis and Insecticidal Activity of Mesoionic Pyrido[1,2-α]pyrimidinone Derivatives Containing a Neonicotinoid Moiety. Molecules 2018, 23, 1217. https://doi.org/10.3390/molecules23051217
Pan J, Yu L, Liu D, Hu D. Synthesis and Insecticidal Activity of Mesoionic Pyrido[1,2-α]pyrimidinone Derivatives Containing a Neonicotinoid Moiety. Molecules. 2018; 23(5):1217. https://doi.org/10.3390/molecules23051217
Chicago/Turabian StylePan, Jianke, Lu Yu, Dengyue Liu, and Deyu Hu. 2018. "Synthesis and Insecticidal Activity of Mesoionic Pyrido[1,2-α]pyrimidinone Derivatives Containing a Neonicotinoid Moiety" Molecules 23, no. 5: 1217. https://doi.org/10.3390/molecules23051217




