Characterization of Cyclodextrin/Volatile Inclusion Complexes: A Review
Abstract
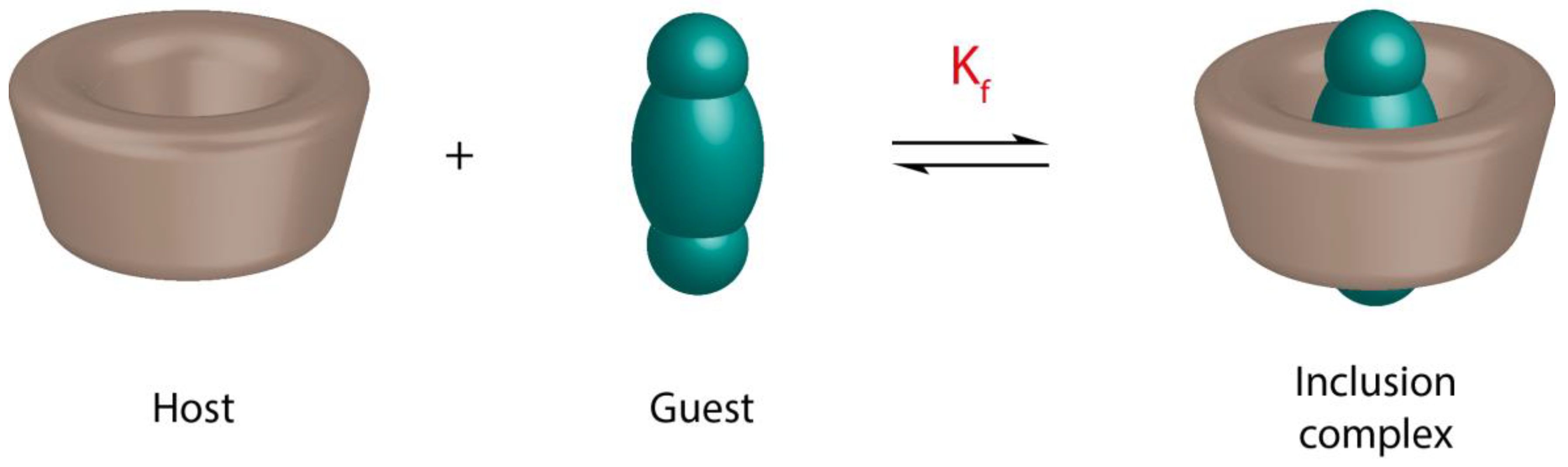
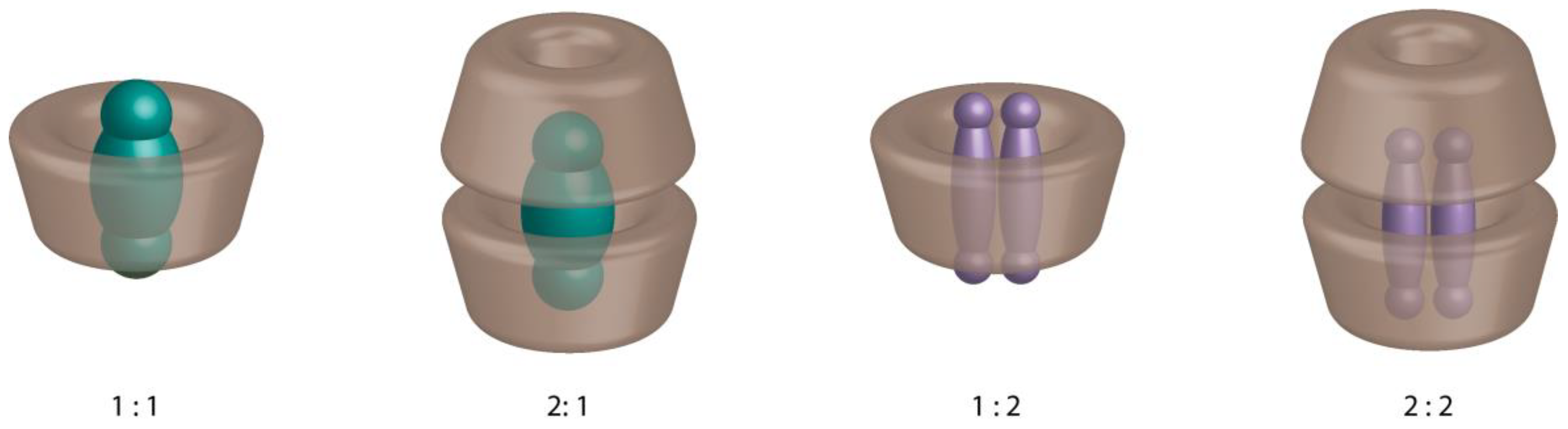
:1. Introduction
2. Characterization of Cyclodextrin Inclusion Complexes
2.1. Volatilization Method
2.2. Chromatographic Methods
2.2.1. Static Headspace-Gas Chromatography
2.2.2. High-Performance Liquid Chromatography
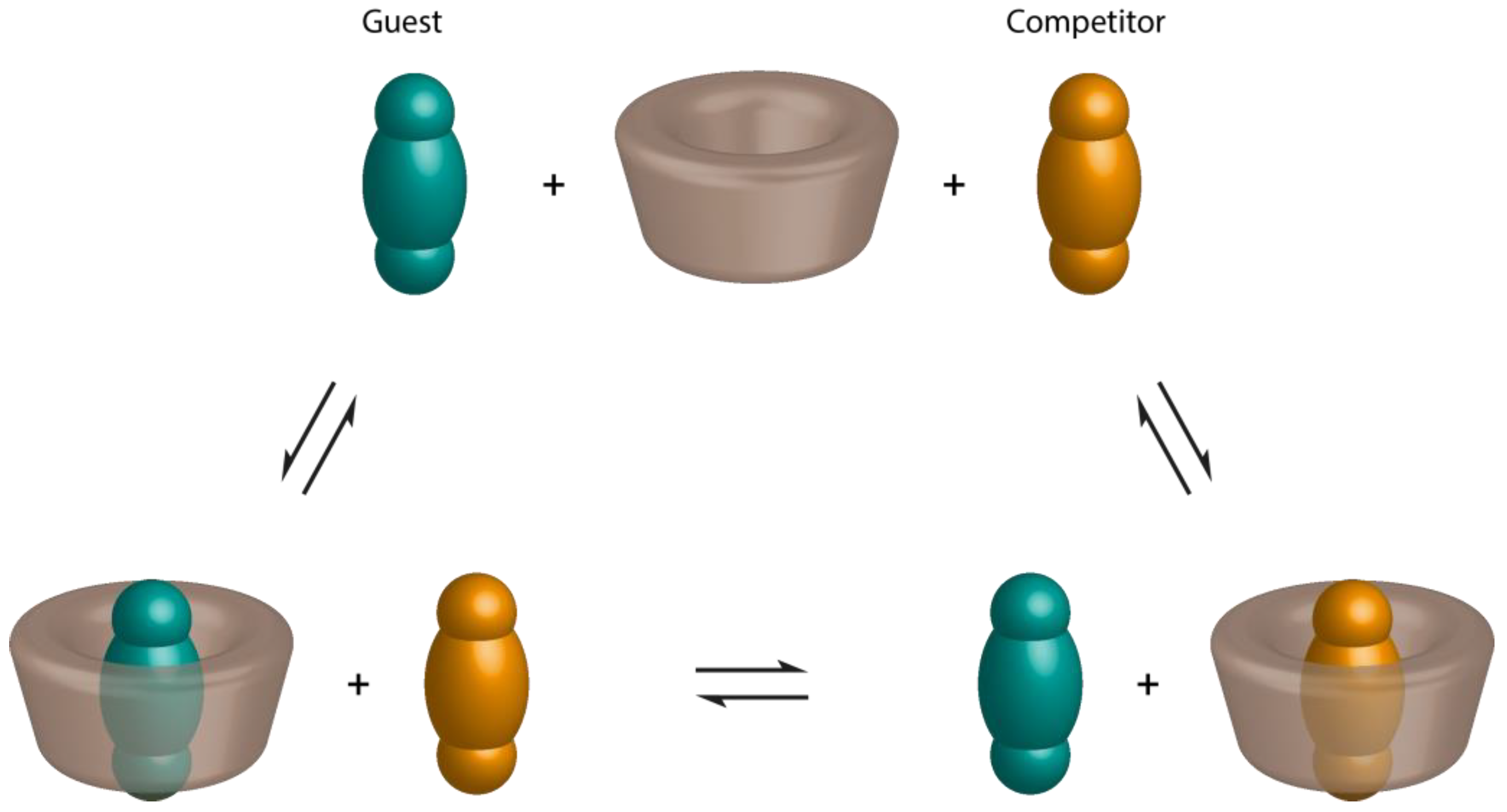
2.3. Spectroscopic Methods
2.3.1. UV-Visible Spectroscopy
2.3.2. Fluorescence Spectroscopy
2.3.3. Nuclear Magnetic Resonance
2.4. Isothermal Titration Calorimetry
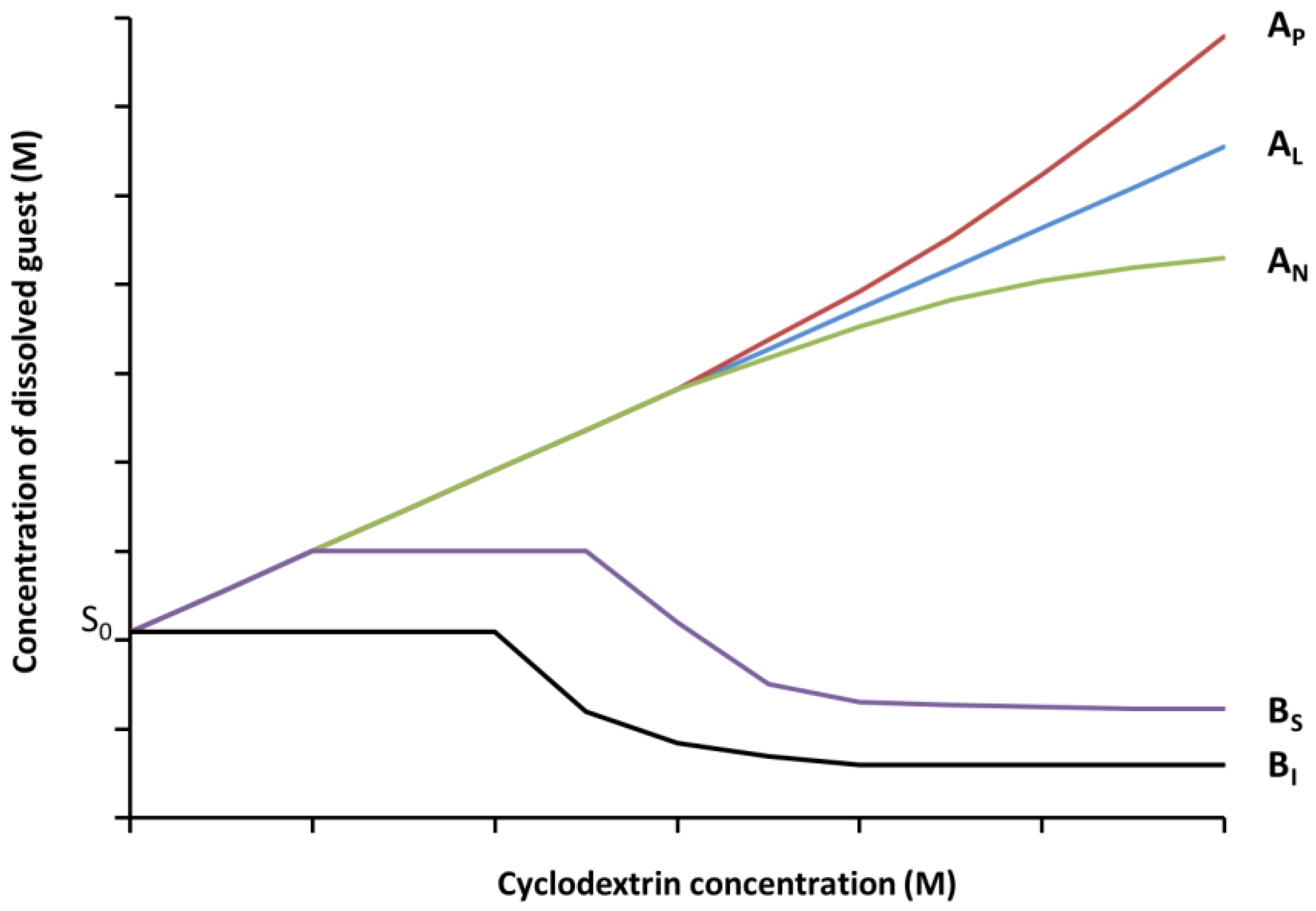
2.5. Phase Solubility Studies
2.6. Total Organic Carbon
2.7. Comparison of Formation Constants Obtained with Differents Methods
3. Conclusions
Acknowledgments
Conflicts of Interest
References
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, M.; Hadaruga, N.; Hadaruga, D.; Fourmentin, S. Cyclodextrins as encapsulation material for flavors and aroma. In Encapsulations; Nanotechnology in the AgriFood Industry; Elsevier: New York, NY, USA, 2016; pp. 127–192. ISBN 978-0-12-804307-3. [Google Scholar]
- Cabral Marques, H.M. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Rakmai, J.; Cheirsilp, B.; Mejuto, J.C.; Torrado-Agrasar, A.; Simal-Gándara, J. Physico-chemical characterization and evaluation of bio-efficacies of black pepper essential oil encapsulated in hydroxypropyl-beta-cyclodextrin. Food Hydrocoll. 2017, 65, 157–164. [Google Scholar] [CrossRef]
- Rakmai, J.; Cheirsil, B.; Torrado-Agrasar, A.; Simal-Gándara, J.; Mejuto, J.C. Encapsulation of yarrow essential oil in hydroxypropyl-beta-cyclodextrin: Physiochemical characterization and evaluation of bio-efficacies. CyTA J. Food 2017, 15, 409–417. [Google Scholar] [CrossRef]
- Rakmai, J.; Cheirsilp, B.; Mejuto, J.C.; Simal-Gándara, J.; Torrado-Agrasar, A. Antioxidant and antimicrobial properties of encapsulated guava leaf oil in hydroxypropyl-beta-cyclodextrin. Ind. Crops Prod. 2018, 111, 219–225. [Google Scholar] [CrossRef]
- Ciobanu, A.; Mallard, I.; Landy, D.; Brabie, G.; Nistor, D.; Fourmentin, S. Inclusion interactions of cyclodextrins and crosslinked cyclodextrin polymers with linalool and camphor in Lavandula angustifolia essential oil. Carbohydr. Polym. 2012, 87, 1963–1970. [Google Scholar] [CrossRef]
- Decock, G.; Landy, D.; Surpateanu, G.; Fourmentin, S. Study of the retention of aroma components by cyclodextrins by static headspace gas chromatography. J. Incl. Phenom. Macrocycl. Chem. 2008, 62, 297–302. [Google Scholar] [CrossRef]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Promising applications of cyclodextrins in food: Improvement of essential oils retention, controlled release and antiradical activity. Carbohydr. Polym. 2015, 131, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Reineccius, T.A.; Reineccius, G.A.; Peppard, T.L. Flavor release from cyclodextrin complexes: Comparison of alpha, beta, and gamma types. J. Food Sci. 2003, 68, 1234–1239. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in aqueous solution: A review. J. Pharm. Biomed. Anal. 2014, 101, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Decock, G.; Fourmentin, S.; Surpateanu, G.G.; Landy, D.; Decock, P.; Surpateanu, G. Experimental and theoretical study on the inclusion compounds of aroma components with β-cyclodextrins. Supramol. Chem. 2006, 18, 477–482. [Google Scholar] [CrossRef]
- Kfoury, M.; Landy, D.; Ruellan, S.; Auezova, L.; Greige-gerges, H.; Fourmentin, S. Nootkatone encapsulation by cyclodextrins: Effect on water solubility and photostability. Food Chem. 2017, 236, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ji, H.; Zhou, X.; Wang, L. Green synthesis of natural benzaldehyde from cinnamon oil catalyzed by hydroxypropyl-β-cyclodextrin. Tetrahedron 2010, 66, 9888–9893. [Google Scholar] [CrossRef]
- Jiang, S.; Li, J.N.; Jiang, Z.T. Inclusion reactions of β-cyclodextrin and its derivatives with cinnamaldehyde in Cinnamomum loureirii essential oil. Eur. Food Res. Technol. 2010, 230, 543–550. [Google Scholar] [CrossRef]
- Ciobanu, A.; Landy, D.; Fourmentin, S. Complexation efficiency of cyclodextrins for volatile flavor compounds. Food Res. Int. 2013, 53, 110–114. [Google Scholar] [CrossRef]
- Ciobanu, A.; Mallard, I.; Landy, D.; Brabie, G.; Nistor, D.; Fourmentin, S. Retention of aroma compounds from Mentha piperita essential oil by cyclodextrins and crosslinked cyclodextrin polymers. Food Chem. 2013, 138, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, M.; Auezova, L.; Fourmentin, S.; Greige-Gerges, H. Investigation of monoterpenes complexation with hydroxypropyl-beta-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2014, 80, 51–60. [Google Scholar] [CrossRef]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Ruellan, S.; Fourmentin, S. Cyclodextrin, an efficient tool for trans-anethole encapsulation: Chromatographic, spectroscopic, thermal and structural studies. Food Chem. 2014, 164, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Tanemura, I.; Saito, Y.; Ueda, H.; Sato, T. Solubility method using static head-space gas chromatography for determination of the stability constants of fragrance materials with 2-hydroxypropyl-β-cyclodextrin. Chem. Pharm. Bull. 1998, 46, 540–541. [Google Scholar] [CrossRef]
- Moeder, C.; O’Brien, T.; Thompson, R.; Bicker, G. Determination of stoichiometric coefficients and apparent formation constants for α- and β-CD complexes of terpenes using reversed-phase liquid chromatography. J. Chromatogr. A 1996, 736, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Wada, T.; Inoue, Y. Studies on molecular recognition in supramolecular systems. Part 31: Circular dichroism spectral studies of molecular and chiral recognition of aliphatic alcohols by 6-modified β-cyclodextrins. Tetrahedron 2001, 57, 7153–7161. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Zhang, H.Y.; Yang, Y.W.; Ding, F. Correlation between thermodynamic behavior and structure in the complexation of modified β-cyclodextrins and bile salts. Supramol. Chem. 2014, 16, 371–379. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Chen, Y. Spectrophotometric and calorimetric titration studies on molecular recognition of camphor and borneol by nucleobase- modified β-cyclodextrins. J. Phys. Chem. B 2007, 111, 12211–12218. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.J.; Kohli, K.; Ali, J.; Anwer, M.K.; Jamil, S.; Ahmed, M.M. Physicochemical characterizations and dissolution behavior of curcumin and α-cyclodextrin molecular inclusion complexes. Der Pharm. Lett. 2014, 6, 1–7. [Google Scholar]
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT Food Sci. Technol. 2013, 51, 86–93. [Google Scholar] [CrossRef]
- Karathanos, V.T.; Mourtzinos, I.; Yannakopoulou, K.; Andrikopoulos, N.K. Study of the solubility, antioxidant activity and structure of inclusion complex of vanillin with β-cyclodextrin. Food Chem. 2007, 101, 652–658. [Google Scholar] [CrossRef]
- Liang, H.; Yuan, Q.; Vriesekoop, F.; Lv, F. Effects of cyclodextrins on the antimicrobial activity of plant-derived essential oil compounds. Food Chem. 2012, 135, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Mazzobre, M.F.; dos Santos, C.I.; Buera, M. Solubility and stability of β-cyclodextrin-terpineol inclusion complex as affected by water. Food Biophys. 2011, 6, 274–280. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Kalogeropoulos, N.; Papadakis, S.E.; Konstantinou, K.; Karathanos, V.T. Encapsulation of nutraceutical monoterpenes in β-cyclodextrin and modified starch. J. Food Sci. 2008, 73, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.H.; Kamimura, J.A.; Hill, L.E.; Gomes, C.L. Characterization of carvacrol beta-cyclodextrin inclusion complexes as delivery systems for antibacterial and antioxidant applications. LWT Food Sci. Technol. 2015, 60, 583–592. [Google Scholar] [CrossRef]
- Tao, F.; Hill, L.E.; Peng, Y.; Gomes, C.L. Synthesis and characterization of β-cyclodextrin inclusion complexes of thymol and thyme oil for antimicrobial delivery applications. LWT Food Sci. Technol. 2014, 59, 247–255. [Google Scholar] [CrossRef]
- Waleczek, K.J.; Cabral Marques, H.M.; Hempel, B.; Schmidt, P.C. Phase solubility studies of pure (−)-α-bisabolol and camomile essential oil with β-cyclodextrin. Eur. J. Pharm. Biopharm. 2003, 55, 247–251. [Google Scholar] [CrossRef]
- Zeng, Z.; Fang, Y.; Ji, H. Side chain influencing the interaction between β-cyclodextrin and vanillin. Flavour Fragr. J. 2012, 378–385. [Google Scholar] [CrossRef]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Development of a Total Organic Carbon method for the quantitative determination of solubility enhancement by cyclodextrins: Application to essential oils. Anal. Chim. Acta 2016, 918, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Sanemasa, I.; Kobayashi, T.; Deguchi, T. Formation constants of cyclodextrin inclusion complexes with iodine in aqueous solutions. Bull. Chem. Soc. Jpn. 1985, 58, 1033–1036. [Google Scholar] [CrossRef]
- Sanemasa, I.; Fujiki, M.; Deguchi, T. A new method for determining cyclodextrin complex formation constants with electrolytes in aqueous medium. Bull. Chem. Soc. Jpn. 1988, 61, 1163–1167. [Google Scholar] [CrossRef]
- Osajima, T.; Deguchi, T.; Sanemasa, I. Association of cycloalkanes with cyclodextrins. Bull. Chem. Soc. Jpn. 1991, 64, 2705–2709. [Google Scholar] [CrossRef]
- Kolb, B. Headspace sampling with capillary columns. J. Chromatogr. A 1999, 842, 163–205. [Google Scholar] [CrossRef]
- Snow, N.H.; Slack, G.C. Head-Space analysis in modern gas chromatography. TrAC Trends Anal. Chem. 2002, 21, 608–617. [Google Scholar] [CrossRef]
- Kolb, B.; Ettre, L.S. Static Headspace-Gas Chromatography Theory and Practice, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Qu, Q.; Tucker, E.; Christian, S.D. Solubilization of synthetic perfumes by nonionic surfactants and by sulfoalkyl ether β-CDs. J. Incl. Phenom. Macrocycl. Chem. 2003, 45, 83–89. [Google Scholar] [CrossRef]
- Wu, J.S.; Zheng, J.Z.; Toda, K.; Sanemasa, I. Association of alcohol-cyclodextrin in aqueous medium determined by headspace gas chromatography. Anal. Sci. 1999, 15, 701–703. [Google Scholar] [CrossRef] [Green Version]
- Saito, Y. Determination of the stability constants of benzene and alkylbenzenes with α-cyclodextrin by static head-space gas chromatography. Chem. Pharm. Bull. 1997, 10, 1711–1713. [Google Scholar] [CrossRef]
- Zheng, J.Z.; Wu, J.S.; Toda, K.; Sanemasa, I. Association of n-alcohol with p-sulfonato calixarenes in an aqueous medium determined by headspace gas chromatography. Bull. Chem. Soc. Jpn. 2001, 74, 505–506. [Google Scholar] [CrossRef]
- Fourmentin, S.; Outirite, M.; Blach, P.; Landy, D.; Ponchel, A.; Monflier, E.; Surpateanu, G. Solubilisation of chlorinated solvents by cyclodextrin derivatives. A study by static headspace gas chromatography and molecular modelling. J. Hazard. Mater. 2007, 141, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Landy, D.; Fourmentin, S.; Salome, M.; Surpateanu, G. Analytical improvement in measuring formation constants of inclusion complexes between β-cyclodextrin and phenolic compounds. J. Incl. Phenom. 2000, 38, 187–198. [Google Scholar] [CrossRef]
- Saito, Y.; Tanamura, I.; Ueda, H.; Sato, T. Simultaneous determination of the stability constants for fragrance materials with 2-hydroxypropyl-β-cyclodextrin by static headspace gas chromatography. Chem. Pharm. Bull. 1998, 46, 1777–1779. [Google Scholar] [CrossRef]
- Saito, Y.; Hashizaki, K.; Taguchi, H.; Tomono, K.; Goto, H.; Ogawa, N. Determination of the stability constants in alkanol/alpha-cyclodextrin mixed system. Drug Dev. Ind. Pharm. 2000, 26, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Fourmentin, S.; Ciobanu, A.; Landy, D.; Wenz, G. Space filling of β-cyclodextrin and β-cyclodextrin derivatives by volatile hydrophobic guests. Beilstein J. Org. Chem. 2013, 9, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, M.; Pipkin, J.D.; Antle, V.; Fourmentin, S. Captisol®: An efficient carrier and solubilizing agent for essential oils and their components. Flavour Fragr. J. 2017, 32, 340–346. [Google Scholar] [CrossRef]
- Szaniszló, N.; Fenyvesi, É.; Balla, J. Structure-stability study of cyclodextrin complexes with selected volatile hydrocarbon contaminants of soils. J. Incl. Phenom. Macrocycl. Chem. 2005, 53, 241–248. [Google Scholar] [CrossRef]
- Misawa, K.; Saito, Y.; Hashizaki, K.; Taguchi, H.; Ogawa, N.; Ueda, H. Stability constants for 1:1 complexes formed between 2-hydroxypropyl-β-cyclodextrin with an average substitution degree of 4.4 and benzene and alkylbenzenes as guests by modified static head-space gas chromatography method. J. Incl. Phenom. Macrocycl. Chem. 2005, 53, 237–240. [Google Scholar] [CrossRef]
- Saito, Y.; Tanemura, I.; Sato, T.; Ueda, H. Interaction of fragrance materials with 2-hydroxypropyl-beta-cyclodextrin by static and dynamic head-space methods. Int. J. Cosmet. Sci. 1999, 21, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.; Bloor, D.; Tawarah, K.; Wynjones, E. Kinetic and equilibrium studies associated with the formation of inclusion-compounds involving normal-nutanol and normal-pentanol in aqueous cyclodextrin solutions. J. Chem. Soc. Faraday Trans. 1986, 82, 2111–2121. [Google Scholar] [CrossRef]
- Saito, Y.; Misawa, K.; Hashizaki, K.; Taguchi, H.; Ogawa, N.; Ueda, H. A modified method using static head-space gas chromatography for determining the stability constants of 1-alkanol/alpha-cyclodextrin complexation. Chem. Pharm. Bull. 2004, 52, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Hashizaki, K.; Taguchi, H.; Ogawa, N. Complexation of butylbenzenes with 2-hydroxypropyl-cyclodextrins in aqueous solution. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2003, 38, 771–777. [Google Scholar] [CrossRef]
- Becker, L.F.; Schwarz, D.H.; Wenz, G. Synthesis of uniform cyclodextrin thioethers to transport hydrophobic drugs. Beilstein J. Org. Chem. 2014, 10, 2920–2927. [Google Scholar] [CrossRef] [PubMed]
- Blach, P.; Fourmentin, S.; Landy, D.; Cazier, F.; Surpateanu, G. Cyclodextrins: A new efficient absorbent to treat waste gas streams. Chemosphere 2008, 70, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Asztemborska, M.; Bielejewska, A.; Duszczyk, K.; Sybilska, D. Comparative study on camphor enantiomers behavior under the conditions of gas-liquid chromatography and reversed-phase high-performance liquid chromatography systems modified with α- and β-cyclodextrins. J. Chromatogr. A 2000, 874, 73–80. [Google Scholar] [CrossRef]
- Ceborska, M.; Szwed, K.; Asztemborska, M.; Wszelaka-Rylik, M.; Kicińska, E.; Suwińska, K. Study of β-cyclodextrin inclusion complexes with volatile molecules geraniol and α-terpineol enantiomers in solid state and in solution. Chem. Phys. Lett. 2015, 641, 44–50. [Google Scholar] [CrossRef]
- Hildebrand, J.H.; Benesi, H.A. Interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Scatchard, G. The attractions of proteins for small molecules and ions. Ann. N. Y. Acad. Sci. 1949, 51, 660–672. [Google Scholar] [CrossRef]
- Scott, R.L. Some comments on the Benesi-Hildebrand equation. Recl. Trav. Chim. Pays-Bas 1956, 75, 787–789. [Google Scholar] [CrossRef]
- Wang, R.; Yu, Z. Validity and reliability of Benesi-Hildebrand method. Acta Phys.-Chim. Sin. 2007, 23, 1353–1359. [Google Scholar] [CrossRef]
- Astray, G.; Mejuto, J.C.; Morales, J.; Rial-Otero, R.; Simal-Gandara, J. Factors controlling flavors binding constants to cyclodextrins and their applications in foods. Food Res. Int. 2010, 43, 1212–1218. [Google Scholar] [CrossRef]
- Hernández-Sánchez, P.; López-Miranda, S.; Lucas-Abellán, C.; Núñez-Delicado, E. Complexation of eugenol (EG), as main component of clove oil and as pure compound, with β- and HP-β–CDs. Food Nutr. Sci. 2012, 3, 716–723. [Google Scholar] [CrossRef]
- Yang, Y.; Song, L.X. Study on the inclusion compounds of eugenol with α-, β-, γ- and heptakis (2,6-di-O-methyl)-β-cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2005, 53, 27–33. [Google Scholar] [CrossRef]
- Pendergast, D.D.; Connors, K.A. Improved competitive indicator methods for the study of α-cyclodextrin complexes. J. Pharm. Sci. 1984, 73, 1779–1783. [Google Scholar] [CrossRef] [PubMed]
- Sadrerafi, K.; Moore, E.E.; Lee, M.W. Association constant of β-cyclodextrin with carboranes, adamantane, and their derivatives using displacement binding technique. J. Incl. Phenom. Macrocycl. Chem. 2015, 83, 159–166. [Google Scholar] [CrossRef]
- Selvidge, L.A.; Eftink, M.R. Spectral displacement techniques for studying the binding of spectroscopically transparent ligands to cyclodextrins. Anal. Biochem. 1986, 154, 400–408. [Google Scholar] [CrossRef]
- Guo, Q.X.; Luo, S.H.; Liu, Y.C. Substituent effects on the driving force for inclusion complexation of alpha- and beta-cyclodextrin with monosubstituted benzene derivatives. J. Incl. Phenom. Mol. Recognit. Chem. 1998, 30, 173–182. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Jiang, Z.T.; Li, R. Complexation of allyl isothiocyanate with β-cyclodextrin and its derivatives and molecular microcapsule of allyl isothiocyanate in β-cyclodextrin. Eur. Food Res. Technol. 2007, 225, 407–413. [Google Scholar] [CrossRef]
- Kfoury, M.; Landy, D.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Effect of cyclodextrin complexation on phenylpropanoids’ solubility and antioxidant activity. Beilstein J. Org. Chem. 2014, 10, 2322–2331. [Google Scholar] [CrossRef] [PubMed]
- Belyakova, L.A.; Lyashenko, D.Y. Complex formation between benzene carboxylic acids and beta-cyclodextrin. Appl. Spectrosc. 2008, 75, 314–318. [Google Scholar] [CrossRef]
- Kfoury, M.; Landy, D.; Ruellan, S.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Determination of formation constants and structural characterization of cyclodextrin inclusion complexes with two phenolic isomers: Carvacrol and thymol. Beilstein J. Org. Chem. 2016, 12, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Jenita, M.J.; Mohandass, T.; Rajendiran, N. Spectral and molecular modeling studies on hydroxybenzaldehydes with native and modified cyclodextrins. J. Fluoresc. 2014, 24, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Zhou, D.; Fang, Y.X.; Ji, H.B. Shape-selective separation of geraniol and nerol via noncovalent interactionswith β-cyclodextrin. Sep. Sci. Technol. 2016, 51, 168–180. [Google Scholar] [CrossRef]
- Azzi, J.; Danjou, P.E.; Landy, D.; Ruellan, S.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. The effect of cyclodextrin complexation on the solubility and photostability of nerolidol as pure compound and as main constituent of cabreuva essential oil. Beilstein J. Org. Chem. 2017, 13, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, M.; Balan, R.; Landy, D.; Nistor, D.; Fourmentin, S. Investigation of the complexation of essential oil components with cyclodextrins. Supramol. Chem. 2015, 27, 1–10. [Google Scholar] [CrossRef]
- Dsouza, R.N.; Pischel, U.; Nau, W.M. Fluorescent dyes and their supramolecular host/guest complexes with macrocycles in aqueous solution. Chem. Rev. 2011, 111, 7941–7980. [Google Scholar] [CrossRef] [PubMed]
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Imamura, M.; Ikehara, K.; Hama, Y. Fluorescence enhancement of benzene derivatives by forming inclusion complexes with β-cyclodextrin in aqueous solutions. J. Phys. Chem. 1981, 85, 1820–1823. [Google Scholar] [CrossRef]
- Zhan, H.; Jiang, Z.T.; Wang, Y.; Li, R.; Dong, T.S. Molecular microcapsules and inclusion interactions of eugenol with β-cyclodextrin and its derivatives. Eur. Food Res. Technol. 2008, 227, 1507–1513. [Google Scholar] [CrossRef]
- Ochocka, R.J. Fluorescence enhancement of two terpenes commonly present in essential oils. J. Pharm. Biomed. Anal. 1993, 10, 809–812. [Google Scholar] [CrossRef]
- Uzaşçi, S.; Erim, F.B. Enhancement of native fluorescence intensity of berberine by (2-hydroxypropyl)-β-cyclodextrin in capillary electrophoresis coupled by laser-induced fluorescence detection: Application to quality control of medicinal plants. J. Chromatogr. A 2014, 1338, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Favrelle, A.; Gouhier, G.; Guillen, F.; Martin, C.; Mofaddel, N.; Petit, S.; Mundy, K.M.; Pitre, S.P.; Wagner, B.D. Structure-binding effects: Comparative binding of 2-anilino-6-naphthalenesulfonate by a series of alkyl- and hydroxyalkyl-substituted β-cyclodextrins. J. Phys. Chem. B 2015, 119, 12921–12930. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.D.; Fitzpatrick, S.J.A. Comparision of the host-guest inclusion complexes of 1,8-ANS and 2,6-ANS in parent and modified cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2000, 38, 467–478. [Google Scholar] [CrossRef]
- Divakar, S.; Maheswaran, M.M. Structural studies on inclusion compounds of β-cyclodextrin with some substituted phenols. J. Incl. Phenom. Mol. Recognit. Chem. 1997, 27, 113–126. [Google Scholar] [CrossRef]
- Yañez, C.; Günther, G. Is the determination of the association constant of cyclodextrin inclusion complexes dependent on the technique. J. Chil. Chem. Soc. 2014, 59, 2523–2525. [Google Scholar] [CrossRef]
- Schneider, H.-J.; Hacket, F.; Rüdiger, V.; Ikeda, H. NMR Studies of cyclodextrins and cyclodextrin complexes. Chem. Rev. 1998, 98, 1755–1786. [Google Scholar] [CrossRef] [PubMed]
- Tsao, J.; Tsai, H.; Wu, C.; Lin, P.; Su, S.; Chen, L.; Tsai, F.; Tsai, Y. Release of paeonol-β-CD complex from thermo-sensitive poly(N-isopropylacrylamide) hydrogels. Int. J. Pharm. 2010, 402, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Terekhova, I.; Koźbiał, M.; Kumeev, R.; Gierycz, P. Complex formation of native and hydroxypropylated cyclodextrins with benzoic acid in aqueous solution: Volumetric and 1H NMR study. Chem. Phys. Lett. 2011, 514, 341–346. [Google Scholar] [CrossRef]
- Nowakowski, M.; Ejchart, A. Complex formation of fenchone with α-cyclodextrin: NMR titrations. J. Incl. Phenom. Macrocycl. Chem. 2014, 79, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrazza, R.; Rossi, B.; Guella, G. DOSY-NMR and raman investigations on the self-aggregation and cyclodextrin complexation of vanillin. J. Phys. Chem. B 2014, 118, 7147–7155. [Google Scholar] [CrossRef] [PubMed]
- Lantz, A.W.; Rodriguez, M.A.; Wetterer, S.M.; Armstrong, D.W. Estimation of association constants between oral malodor components and various native and derivatized cyclodextrins. Anal. Chim. Acta 2006, 557, 184–190. [Google Scholar] [CrossRef]
- Hansen, L.D.; Fellingham, G.W.; Russell, D.J. Simultaneous determination of equilibrium constants and enthalpy changes by titration calorimetry: Methods, instruments, and uncertainties. Anal. Biochem. 2011, 409, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Chaires, J.B. Possible origin of differences between Van’t Hoff and calorimetric enthalpy estimates. Biophys. Chem. 1997, 64, 15–23. [Google Scholar] [CrossRef]
- Bertaut, E.; Landy, D. Improving ITC studies of cyclodextrin inclusion compounds by global analysis of conventional and non-conventional experiments. Beilstein J. Org. Chem. 2014, 10, 2630–2641. [Google Scholar] [CrossRef]
- Hallén, D.; Schön, A.; Shehatta, I.; Wadsö, I. Microcalorimetric titration of α-cyclodextrin with some straight-chain alkan-1-ols at 288.15, 298.15 and 308.15 K. J. Chem. Soc. Faraday Trans. 1992, 88, 2859–2863. [Google Scholar] [CrossRef]
- Schmidtchen, F.P. The anatomy of the energetics of molecular recognition by calorimetry: Chiral discrimination of camphor by α-cyclodextrin. Chem. Eur. J. 2002, 8, 3522–3529. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Mayhew, M.P.; Goldberg, R.N.; Ross, P.D.; Yamashoji, Y.; Inoue, Y. Thermodynamic and nuclear magnetic resonance study of the reactions of α- and β-cyclodextrin with acids, aliphatic amines, and cyclic alcohols. J. Phys. Chem. B 1997, 101, 87–100. [Google Scholar] [CrossRef]
- Rekharsky, M.; Inoue, Y. 1:1 and 1:2 Complexation thermodynamics of γ-cyclodextrin with N-carbobenzyloxy aromatic amino acids and ω-phenylalkanoic acids. J. Am. Chem. Soc. 2000, 122, 10949–10955. [Google Scholar] [CrossRef]
- Gómez-Orellana, I.; Hallén, D. The thermodynamics of the binding of benzene to β-cyclodextrin in aqueous solution. Thermochim. Acta 1993, 221, 183–193. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, E.C.; Yang, Y.W.; Zhang, H.Y.; Fan, Z.; Ding, F.; Cao, R. Thermodynamics of the molecular and chiral recognition of cycloalkanols and camphor by modified β-cyclodextrins possessing simple aromatic Tethers. J. Org. Chem. 2004, 69, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, G.L.; Faulkner, J.R., Jr.; Han, S.M.; Armstrong, D.W. Substituent effects on the binding of phenols to cyclodextrins in aqueous solution. J. Phys. Chem. 1989, 93, 6863–6867. [Google Scholar] [CrossRef]
- Castronuovo, G.; Elia, V.; Iannone, A.; Niccoli, M.; Velleca, F. Factors determining the formation of complexes between α-cyclodextrin and alkylated substances in aqueous solutions: A calorimetric study at 25 °C. Carbohydr. Res. 2000, 325, 278–286. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Solvent and guest isotope effects on complexation thermodynamics of α-, β-, and 6-amino-6-deoxy-β-cyclodextrins. J. Am. Chem. Soc. 2002, 124, 12361–12371. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, M.; Kimura, T. Enthalpy and entropy changes on molecular inclusion of 1-heptanol into α- and β-cyclodextrin cavities in aqueous solutions. Thermochim. Acta 2004, 416, 51–54. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Schwarz, F.P.; Tewari, Y.B.; Goldberg, R.N.; Tanaka, M.; Yamashoji, Y. Thermodynamic and NMR study of the interactions of cyclodextrins with cyclohexane derivatives. J. Phys. Chem. 1994, 98, 4098–4103. [Google Scholar] [CrossRef]
- Moreira, R.; Bastos, M. The influence of glycerol on ligand binding equilibria between monoalcohols and α-cyclodextrin. J. Chem. Thermodyn. 2000, 32, 1539–1550. [Google Scholar] [CrossRef]
- Rüdiger, V.; Eliseev, A.; Simova, S.; Schneider, H.J.; Blandamer, M.J.; Cullis, P.M.; Meyer, A.J. Conformational, calorimetric and NMR spectroscopic studies on inclusion complexes of cyclodextrins with substituted phenyl and adamantane derivatives. J. Chem. Soc. Perkin Trans. 2 1996, 2119–2123. [Google Scholar] [CrossRef]
- Fini, P.; Castagnolo, M.; Catucci, L.; Cosma, P.; Agostiano, A. The effects of increasing NaCl concentration on the stability of inclusion complexes in aqueous solution. J. Therm. Anal. Calorim. 2003, 73, 653–659. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase solubility techniques. In Advances in Analytical Chemistry Instrument; Wiley-Interscience: New York, NY, USA, 1965; Volume 4, pp. 56–63. [Google Scholar]
- Balogh, K.; Szaniszló, N.; H-Otta, K.; Fenyvesi, É. Can cyclodextrins really improve the selectivity of extraction of BTEX compounds? J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 457–462. [Google Scholar] [CrossRef]
- Matsuda, H.; Ito, K.; Fujiwara, Y.; Tanaka, M.; Taki, A.; Uejima, O.; Sumiyoshi, H. Complexation of various fragrance materials with 2-hydroxypropyl-β-cyclodextrin. Chem. Pharm. Bull. 1991, 39, 827–830. [Google Scholar] [CrossRef]
- Numanoglu, U.; Şen, T.; Tarimci, N.; Kartal, M.; Koo, O.M.Y.; Önyüksel, H. Use of cyclodextrins as a cosmetic delivery system for fragrance materials: Linalool and benzyl acetate. AAPS PharmSciTech 2007, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Fenyvesi, É.; Szejtli, J. Entrapment of iodine with cyclodextrins: Potential application of cyclodextrins in nuclear waste management. Environ. Sci. Technol. 1999, 33, 4495–4498. [Google Scholar] [CrossRef]
- Trapani, G.; Lopedota, A.; Franco, M.; Latrofa, A.; Liso, G. Effect of 2-hydroxypropyl-β-cyclodextrin on the aqueous solubility of the anaesthetic agent propofol (2,6-diisopropylphenol). Int. J. Pharm. 1996, 139, 215–218. [Google Scholar] [CrossRef]
- Bourgeois, W.; Burgess, J.E.; Stuetz, R.M. On-line monitoring of wastewater quality: A review. J. Chem. Technol. Biotechnol. 2001, 76, 337–348. [Google Scholar] [CrossRef]
| Guest | α-CD | β-CD | γ-CD |
|---|---|---|---|
| Benzene | 17 | 120 | 12 |
| Toluene | 33 | 140 | 20 |
| Ethylbenzene | 110 | 330 | 36 |
| o-Xylene | 22 | 300 | 34 |
| m-Xylene | 40 | 160 | 27 |
| p-Xylene | 72 | 240 | 8 |
| Guest | α-CD | β-CD | γ-CD | CRYSMEB | RAMEB | HP-β-CD | SBE-β-CD |
|---|---|---|---|---|---|---|---|
| trans-Anethole | 710 a 1163 a | 497 a 630 a 1040 a | 96 a | 877 a 740 a | 1110 a 1553 a | 981 a 1042 a | - |
| Benzene | 19 b 20 c | 128 d 111 c | 9 c | - | 110 c | 139 e 94 d 99 c | - |
| Benzyl acetate | - | - | - | - | - | 230 f | 124 g |
| Benzyl alcohol | 52 h | 64 h | 56 h | 53 h | 63 h | 12 g | |
| 1-Butanol | 73 i 74 j 81 k | 14 k | 2 k | - | - | - | - |
| iso-Butylbenzene | - | - | - | - | - | 7665 l | - |
| n-Butylbenzene | - | - | - | - | - | 3410 l | - |
| tert-Butylbenzene | - | 9503 d | - | - | - | 8224 l 1863 d | - |
| tert-Butylcyclohexane | - | 4092 d | - | - | - | 2036 d | - |
| Camphene | 598 a | 4825 a | 360 a | 6625 a | 6057 a | 3033 a | - |
| Camphor | 184 a | 2058 a | 1048 a | 1901 a | 1194 a | 1280 a | |
| Carbon tetrachloride | 40 m | 164 m | - | 215 m | 238 m | 218 m | - |
| β-Caryophyllene | - | 28,674 a | 4004 a | 11,488 a | 14,274 a | 4960 a | - |
| Chloroform | 34 m | 60 m | - | 55 m | 93 m | 61 m | - |
| Cinnamaldehyde | 236 h | 450 h | - | 595 h | 1696 h | 969 h | - |
| Citronellol | 223 h | 3141 h | - | 3290 h | 4048 h | 3290 f 2578 h | - |
| Cyclohexane | 164 c | 468 c 341 d | <10 c | - | 474 c | 363 c 227 d | - |
| p-Cymene | 140 a | 2505 a | 88 a | 2549 a | 3543 a | 2213 a | - |
| Dichloromethane | 21 m | 9 m | - | 9 m | 12 m | 10 m | - |
| Ethylbenzene | 131 c | 392 d 289 c | 125 c | - | 320 c | 303 d 248 c | - |
| Ethylcyclohexane | 2017 c | 646 c | 8 c | - | 738 c | 630 c | - |
| Eucalyptol | 13 a | 615 a | 742 a | 688 a | 673 a | 334 a | - |
| Eugenol | 94 h | 264 h | - | 454 h | 568 h | 270 f 462 h | - |
| Estragole | 478 a | 939 a | - | 1661 a | 1761 a | 1581 a | - |
| Geraniol | 90 h | 528 h | - | 977 h | 1100 h | 1340 f 712 h | - |
| 1-Heptanol | 1493 j 1586 n 2460 k | 985 k | 37 k | - | - | - | - |
| 1-Hexanol | 935 j 509 n 860 k | 260 k | 13 k | - | - | - | - |
| Isoeugenol | 85 a | 225 a | - | 263 a | 514 a | 441 a | - |
| cis-Jasmone | - | - | - | - | - | 1020 f | - |
| Lilial | 4387 a | 56,567 a | - | 147,617 a | 166,338 a | 112,205 a | - |
| Limonene | 1289 o | 3162 o | 116 o 70 p | 3668 o | 4386 o | 4630 f 5630 q 2787 o | 4125 p |
| Linalool | 32 o | 366 o | 138 o | 816 o | 833 o | 940 f 596 o | - |
| Linalyl acetate | - | - | - | - | - | 1330 f | - |
| Menthol | 82 a | 1731 a | 105 a | 2396 a | 1928 a | 1079 a | - |
| Menthone | 35 a | 656 a | 83 a | 989 a | 748 a | 664 a | - |
| Methylcyclohexane | 141 c | 332 c 295 d | 9 c | - | 374 c | 253 c 202 d | - |
| Methyl heptine carbonate | 2905 a | 226 a | - | 539 a | 485 a | 325 a | - |
| α-iso-Methylionone | 71 h | 9869 h | - | 15,632 h | 13,176 h | 5750 f 9789 h | - |
| Myrcene | 212 o | 1431 o | 138 o 126 p | 959 o | 1286 o | 1290 f 1360 q 575 o | 1116 p |
| 1-Nonanol | 13,400 k | 4900 k | 141 k | - | - | - | - |
| cis-Ocimene | 42 a | 432 a | 20 a | 622 a | 593 a | 538 a | - |
| trans-Ocimene | 46 a | 538 a | 26 a | 789 a | 640 a | 627 a | - |
| 1-Octanol | 2532 j 4820 k | 1910 k | 67 k | - | - | - | - |
| 1-Pentanol | 302 i 286 k 188 n 291 k | 61 k | 3 k | - | - | - | - |
| 2-Pentanol | 115 k | 25 k | 3 k | - | - | - | - |
| α-Pinene | 1778 o | 2588 o | 214 o 217 p | 2999 o | 2395 o | 5400 f 4750 q 1637 o | 1892 p |
| β-Pinene | 1018 o | 4587 o | 633 o 404 p | 5141 o | 4450 o | 6650 f 7070 q 3151 o | 4904 p |
| Pulegone | 30 a | 332 a | 82 a | 1025 a | 796 a | 676 a | - |
| Sabinene hydrate | 108 a | 2108 a | 708 a | 1308 a | 1882 a | 772 a | - |
| Sevoflurane | 18 r | 150 r | 9 r | - | - | 163 r | - |
| γ-Terpinene | 37 a | 1309 a | 40 a | 1950 a | 2066 a | 1488 a | - |
| α-Terpineol | 126 a | 1143 a | 89 a | 1223 a | 1287 a | 761 a | - |
| Thymol | - | - | - | - | - | 806 a | - |
| Toluene | 36 b 38 s 29 c | 142 s 158 d 172 c | 33 c | - | 171 s 144 c | 182 e 163 s 131 d 170 c | - |
| o-Xylene | 18 b 10 c | 184 c | 57 c | - | 225 c | 263 e 187 c | - |
| m-Xylene | 60 b 36 c | 100 c | 18 c | - | 216 c | 222 e 167 c | - |
| p-Xylene | 124 b 132 c | 218 c | 32 c | - | 300 c | 323 e 236 c | - |
| Method | Equation |
|---|---|
| Benesi-Hildebrand a | |
| Scott b | |
| Scatchard c |
| Guest | Method | α-CD | β-CD | γ-CD | CRYSMEB | RAMEB | HP-β-CD |
|---|---|---|---|---|---|---|---|
| Acetophenone | BH | 140 a | 188 a | - | - | - | - |
| Allyl isothiocyanate | SD | - | 36 b | - | - | - | - |
| trans-Anethole | SD | 927 c | 542 d | - | 1039 c | 1815 c | 845 d |
| Anisole | BH | - | 209 a | - | - | - | - |
| Anisyl alcohol | SD | - | 107 e | - | 130 e | 125 e | 156 e |
| Benzaldehyde | BH | 102 a | 150 a | - | - | - | - |
| Benzene | BH | 29 a | 194 a | - | - | - | - |
| Benzoic acid | BH | - | 120 f | - | - | - | - |
| Benzonitrile | BH | 78 a | 170 a | - | - | - | - |
| Benzyl alcohol | BH | 96 a | 143 a | - | - | - | - |
| SD | 63 e | - | 57 e | 55 e | 54 e | ||
| Benzyl chloride | BH | 204 a | 280 a | - | - | - | - |
| Bromobenzene | BH | 540 a | 322 a | - | - | - | - |
| Camphor | - | 3 g | 19 g | - | - | - | - |
| Carvacrol | SD | 454 h | 2620 h | 999 h | 2421 h | 3564 h | 2154 h |
| Chlorobenzene | BH | 112 a | 196 a | - | - | - | - |
| Cineole | - | 6 g | 29 g | - | - | - | - |
| Citral | - | 8 g | 31 g | - | - | - | - |
| 3,4-Dimethoxy benzaldehyde | BH | 98 i | 157 i | - | - | - | - |
| N,N-Dimethylaniline | BH | 172 a | 252 a | - | - | - | - |
| Estragole | SD | 335 j | 987 j | 52 j | 1584 j | 1916 j | 1508 j |
| Ethyl benzoate | BH | 361 a | 539 a | - | - | - | - |
| Ethyl phenyl ether | BH | 171 a | 308 a | - | - | - | - |
| N-Ethyl aniline | BH | 128 a | 217 a | - | - | - | - |
| Ethylbenzene | BH | 104 a | 389 a | - | - | - | - |
| Eugenol | BH | 4.95 × 104 k 10,633 l | 3.96 × 105 k | 1.47 × 105 k | - | - | 4555 l |
| - | 5 g | 23 g | - | - | - | - | |
| SD | 350 c | 322 e | - | 401 e | 521 e | 445 e | |
| Fluorobenzene | BH | 39 a | 91 a | - | - | - | - |
| Furaneol | - | 1.1 g | 7 g | - | - | - | - |
| Geraniol | BH | 51 m | - | - | - | - | |
| - | 9 g | 44 g | - | - | - | - | |
| 4-Hydroxy-3,5-dimethoxy benzaldehyde | BH | 269 i | 372 i | - | - | - | - |
| p-Hydroxybenzaldehyde | BH | 72 n | - | - | - | - | |
| Iodobenzene | BH | 1200 a | 846 a | - | - | - | - |
| Isoeugenol | SD | 178 c | 304 e | - | 240 e | 547 e | 452 e |
| Limonene | - | 14 g | 55 g | - | - | - | - |
| Menthol | - | 10 g | 35 g | - | - | - | - |
| N-Methylaniline | BH | 83 a | 131 a | - | - | - | - |
| Methyl benzoate | BH | 213 a | 317 a | - | - | - | - |
| Methyl cinnamate | - | 4 g | 20 g | - | - | - | - |
| Nerol | BH | - | 26 m | - | - | - | |
| Nerolidol | SD | - | - | - | - | 8168 o | |
| Nitrobenzene | BH | 89 a | 279 a | - | - | - | - |
| Nootkatone | - | 7 g | 32 g | - | - | - | - |
| N-Phenylacetamide | BH | 103 a | 157 a | - | - | - | - |
| Phenol | BH | 40 a | 95 a | - | - | - | - |
| Phenylacetylene | BH | 86 a | 230 a | - | - | - | - |
| Phenylamine | BH | 15 a | 86 a | - | - | - | - |
| Sabinene hydrate | SD | 108 p | 2108 p | 708 p | 1308 p | 1882 p | 772 p |
| α-Terpineol | SD | 126 p | 1143 p | 89 p | 1223 p | 1287 p | 761 p |
| Thymol | SD | 107 h | 1467 h | 233 h | 2386 h | 3337 h | 488 h |
| Toluene | BH | 36 a | 214 a | - | - | - | - |
| o-Vanillin | BH | 105 i | 250 i | - | - | - | - |
| Vanillin | BH | 163 i | 100 n 296 i | - | - | - | - |
| - | 1.6 g | 17 g | - | - | - | - | |
| p-Vinyl guaiacol | - | 2 g | 17 g | - | - | - | - |
| Guest | Method | α-CD | β-CD | HP-β-CD |
|---|---|---|---|---|
| Aniline | SC | - | 50 a | - |
| Benzene | SC | - | 196 a | - |
| Cinnamaldehyde | BH | - | - | 928 b |
| 3,4-Dimethoxybenzaldehyde | BH | 124 c | 343 c | - |
| N,N-Diethylaniline | SC | - | 862 a | - |
| N,N-Dimethylaniline | SC | - | 217 a | - |
| Ethoxybenzene | SC | - | 286 a | - |
| Eucalyptol | BH | - | - | 1200 d |
| SC | - | - | 1112 d | |
| Eugenol | BH | - | 357 e | 420 e |
| Geraniol | BH | - | - | 1320 d |
| SC | - | - | 1064 d | |
| 4-Hydroxy-3,5-dimethoxy benzaldehyde | BH | 373 c | 493 c | - |
| Limonene | BH | - | - | 1667 d |
| SC | - | - | 1700 d | |
| Linalool | BH | - | - | 1500 d |
| SC | - | - | 1260 d | |
| N-Methylaniline | SC | - | 53 a | - |
| Phenol | SC | - | 40 a | - |
| α-Pinene | BH | - | - | 2000 d |
| SC | - | - | 1842 d | |
| β-Pinene | BH | - | - | 1667 d |
| SC | - | - | 1671 d | |
| Pulegone | BH | - | - | 867 d |
| SC | - | - | 798 d | |
| Thymol | BH | - | - | 1400 d |
| SC | - | - | 1313 d | |
| Vanillin | BH | 295 c | 384 c | - |
| o-Vanillin | BH | 183 c | 320 c | - |
| Guest | Observed Signal | α-CD | β-CD | γ-CD | HP-α-CD | HP-β-CD | HP-γ-CD |
|---|---|---|---|---|---|---|---|
| Benzoic acid | δ | 842 a | 306 a | - | 731 a | 447 a | 551 a |
| Carvacrol | δ + D | - | 1736 b | - | - | - | - |
| m-Cresol | δ | 48 c | 125 c | 97 c | - | 130 c | - |
| (+)-Fenchone | δ | - | 550 d | - | - | - | - |
| (−)-Fenchone | δ | - | 523 d | - | - | - | - |
| Nootkatone | δ + D | - | 5750 e | - | - | - | - |
| Phenol | δ | 19 c | 60 c | 3 c | - | - | - |
| Thymol | δ + D | - | 1344 b | - | - | - | - |
| Vanillin | D | - | 9.8 f | - | - | - | - |
| Guest | α-CD | β-CD | ||||
|---|---|---|---|---|---|---|
| Kf | ΔH | −TΔS | Kf | ΔH | −TΔS | |
| Benzene | - | - | - | 107 a | −3.5 | −8.1 |
| (−)-Borneol | - | - | - | 19,750 b | −23.2 | −1.3 |
| (+)-Borneol | - | - | - | 18,640 b | −20.9 | −3.5 |
| 4-Bromophenol | 708 c | −25.6 | 9.2 | 851 c | −12.2 | −4.5 |
| 1-Butanol | 83 d | −10.7 | −0.2 | - | - | - |
| 100 e | −9.9 | −1.5 | - | - | - | |
| 80 f | −10.9 | 0.1 | - | - | - | |
| 9153 g | −7.9 | −14.7 | 1430 g | 3.0 | −21.0 | |
| 4-Chlorophenol | 295 c | −20.1 | 6 | 407 c | −11.9 | −3.0 |
| Cyclobutanol | 63 e | −5.5 | −4.8 | - | - | - |
| 30 i | −11.5 | 3 | 14 i | 3.7 | −10.2 | |
| Cycloheptanol | - | - | - | 2344 b | −11.6 | −7.6 |
| 25 e | −32 | 24 | - | - | - | |
| - | - | - | 2200 f | −12.4 | −6.7 | |
| 68 i | −12.5 | 2 | 2197 i | −12.4 | −6.7 | |
| Cyclohexanol | - | - | - | 707 b | −6.1 | −10.2 |
| - | - | - | 701 f | −6.3 | −9.9 | |
| 62 j | −12.8 | 2.7 | 776 j | −6.6 | −9.8 | |
| Cyclooctanol | - | - | - | 4425 b | −15.7 | −5.1 |
| 28 e | −40 | 32 | - | - | - | |
| 235 i | −3.9 | −9.5 | 4405 i | −16.4 | −4.4 | |
| Cyclopentanol | - | - | - | 168 b | −3.9 | −8.8 |
| - | - | - | 175 f | −4.6 | −8.2 | |
| 83 e | −8.8 | −2.1 | - | - | - | |
| 36 i | −11.5 | 2.6 | 172 i | −4.6 | −8.2 | |
| Ethanol | 7 e | −2.5 | −2.2 | - | - | - |
| 7184 g | −0.9 | −21.0 | 1319 g | 1.1 | −18.9 | |
| 1-Heptanol | 1168 d | −22.8 | 5.3 | - | - | - |
| 377 e | −20.6 | 5.9 | - | - | - | |
| 24,113 g | −34.6 | 9.6 | 17,459 g | −7.9 | −16.3 | |
| 1503 k | −28.6 | 10.4 | - | - | - | |
| 1-Hexanol | 705 d | −18.2 | 1.9 | - | - | - |
| 523 e | −13.9 | −1.6 | - | - | - | |
| 840 f | −17.5 | 0.8 | - | - | - | |
| 7788 g | −29.1 | 6.9 | 5871 g | 0.6 | −22.1 | |
| 906 k | −21.3 | 4.5 | - | - | - | |
| 4-Iodophenol | 881 l | −26.3 | 9.5 | 955 l | −16.1 | −0.9 |
| Methanol | 0 g | - | - | 0 g | - | - |
| 4-Methylphenol | 37 c | −17.7 | 8.6 | 251 c | −12.5 | −1.2 |
| 4-Nitrophenol | 104 l | −23 | 11.5 | 296 l | −10.2 | −3.9 |
| 219 c | −25.8 | 12.5 | 347 c | −12.0 | −2.4 | |
| 1-Nonanol | 480 e | −36.2 | 20.9 | - | - | - |
| Nootkatone | - | - | - | 5801 m | −14.4 | −7.1 |
| 1-Octanol | 220 e | −22.0 | 9.0 | - | - | - |
| 1-Pentanol | 246 d | −14.9 | 1.3 | - | - | - |
| 287 f | −14.7 | 0.7 | - | - | - | |
| 275 e | −11.8 | −2.1 | - | - | - | |
| 18,927 g | −13.9 | −10.4 | 9153 g | 2.2 | −24.8 | |
| 428 k | −13.8 | −1.2 | - | - | - | |
| Phenol | 37 c | −10.2 | 0.6 | 93 c | −12.2 | 1.2 |
| 1-Propanol | 23 d | −6.8 | −1.0 | - | - | - |
| 27 e | −6.1 | −2.1 | - | - | - | |
| 1319 g | −6.6 | −11.2 | 1168 g | 1.9 | −19.4 | |
| Guest | HPLC | SH-GC | UV-Visible | Sodium Thiosulfate Titration |
|---|---|---|---|---|
| trans-Anethole | - | - | 1510 a | - |
| Benzene | - | - | 121 b | - |
| Benzyl acetate | 306 c 275 d | - | - | - |
| Carvacrol | - | - | 2123 e | - |
| Citral | 1560 c | - | - | - |
| Citronellol | 3740 c | - | - | - |
| Estragole | - | - | 1412 a | - |
| Ethylbenzene | - | - | 435 b | - |
| Eugenol | - | - | 445 a | - |
| Iodine | - | - | - | 38 f |
| Isoeugenol | - | - | 449 a | - |
| (+)-Limonene | 3350 c | 4730 g | - | - |
| Linalool | 1610 c 720 d | 940 g | - | - |
| Linalyl acetate | 137 c | - | - | - |
| α-iso-Methylionone | 29,500 c | - | - | - |
| Myrcene | - | 1240 g | - | - |
| Nootkatone | - | - | 3700 h | - |
| (−)α-Pinene | - | 5780 g | - | - |
| (−)-β-Pinene | - | 7360 g | - | - |
| Propofol | - | - | 3972 i | - |
| Thymol | - | - | 1282 e | - |
| Toluene | - | - | 287 b | - |
| o-Xylene | - | - | 305 b | - |
| m-Xylene | - | - | 210 b | - |
| p-Xylene | - | - | 353 b | - |
| Guest | SH-GC | UV-Visible | Fluorescence | HPLC | ITC | NMR | Phase Solubility |
|---|---|---|---|---|---|---|---|
| trans-Anethole | 630 a 1040 a 497 a | 542 b | - | - | - | - | 537 c |
| Benzene | 128 d 111 e | 194 f | 196 g | - | 107 h | - | - |
| Camphor | 2058 a | 19 i | - | 350 j | - | - | - |
| Eugenol | 264 k | 3.96 × 105 l 23 i 322 m | 357 n | - | - | - | 513 c |
| Limonene | 3162 a 2230 a | 55 i | - | - | - | - | - |
| Phenol | - | 95 f | 40 g | - | 93 o | 120 p | - |
| Thymol | - | 1467 q | 1400 r | - | - | 1344 q | 1150 q |
| Toluene | 142 s 158 d 172 e | 214 f | - | - | - | - | - |
| Vanillin | - | 100 t 296 u 17 i | 384 u | - | - | 9.8 v | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kfoury, M.; Landy, D.; Fourmentin, S. Characterization of Cyclodextrin/Volatile Inclusion Complexes: A Review. Molecules 2018, 23, 1204. https://doi.org/10.3390/molecules23051204
Kfoury M, Landy D, Fourmentin S. Characterization of Cyclodextrin/Volatile Inclusion Complexes: A Review. Molecules. 2018; 23(5):1204. https://doi.org/10.3390/molecules23051204
Chicago/Turabian StyleKfoury, Miriana, David Landy, and Sophie Fourmentin. 2018. "Characterization of Cyclodextrin/Volatile Inclusion Complexes: A Review" Molecules 23, no. 5: 1204. https://doi.org/10.3390/molecules23051204






