Selected Plant Metabolites Involved in Oxidation-Reduction Processes during Bud Dormancy and Ontogenetic Development in Sweet Cherry Buds (Prunus avium L.)
Abstract
:1. Introduction
2. Results and Discussion
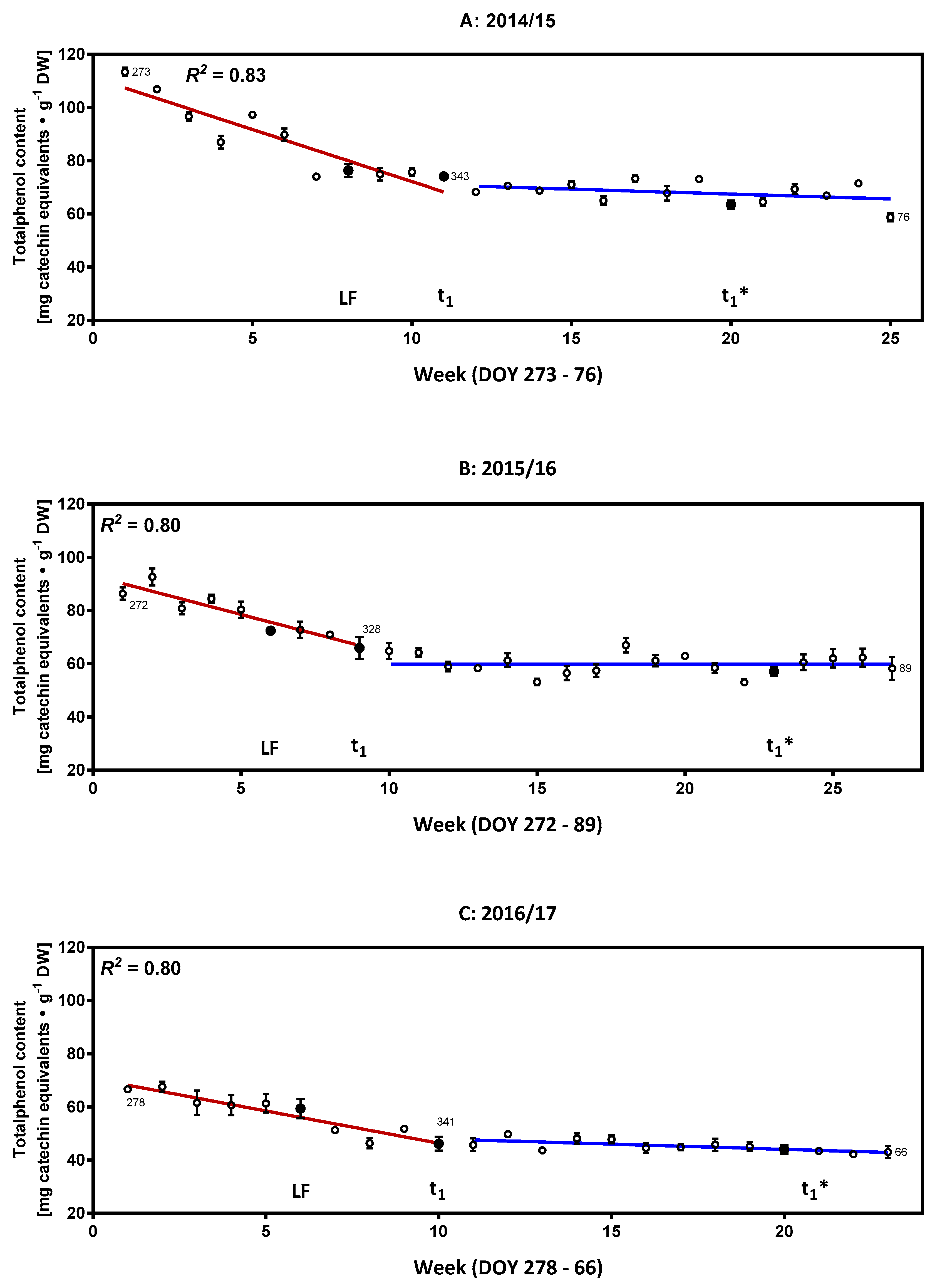
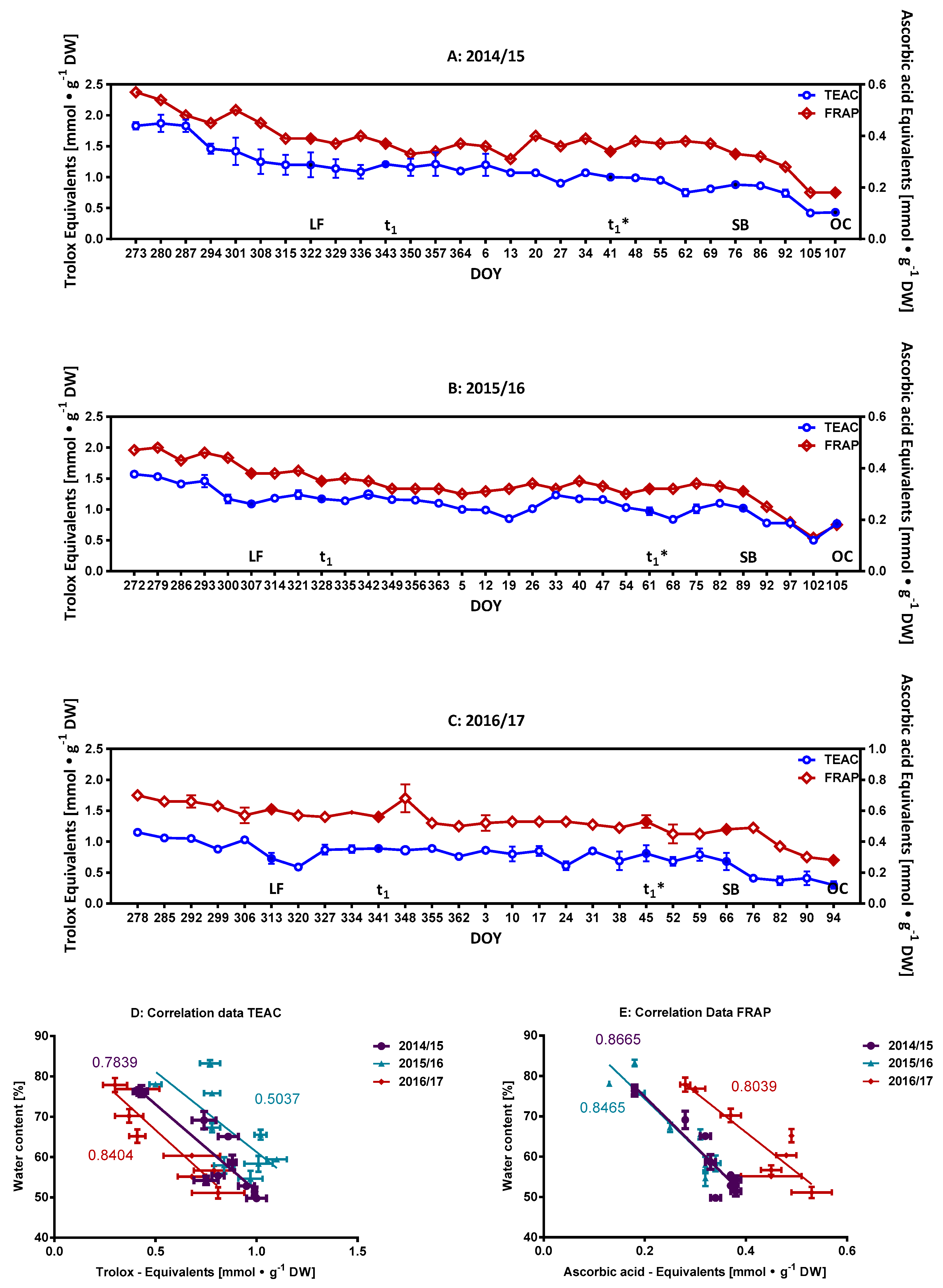
2.1. Characterization of the Phenolic Compounds and the Antioxidative Potential of the Flower Buds
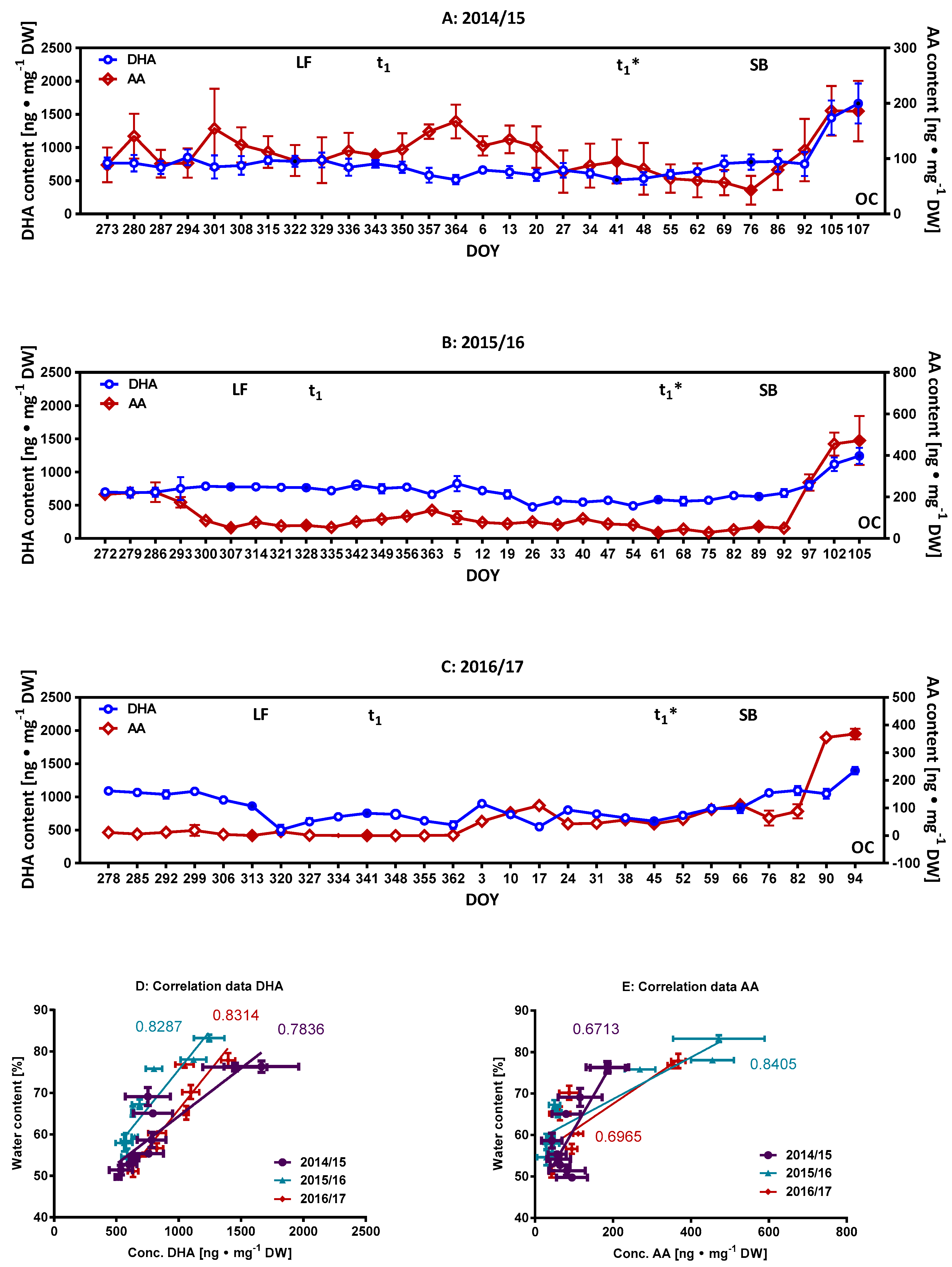
2.2. Monitoring of Ascorbate/Dehydroascorbate during Dormancy
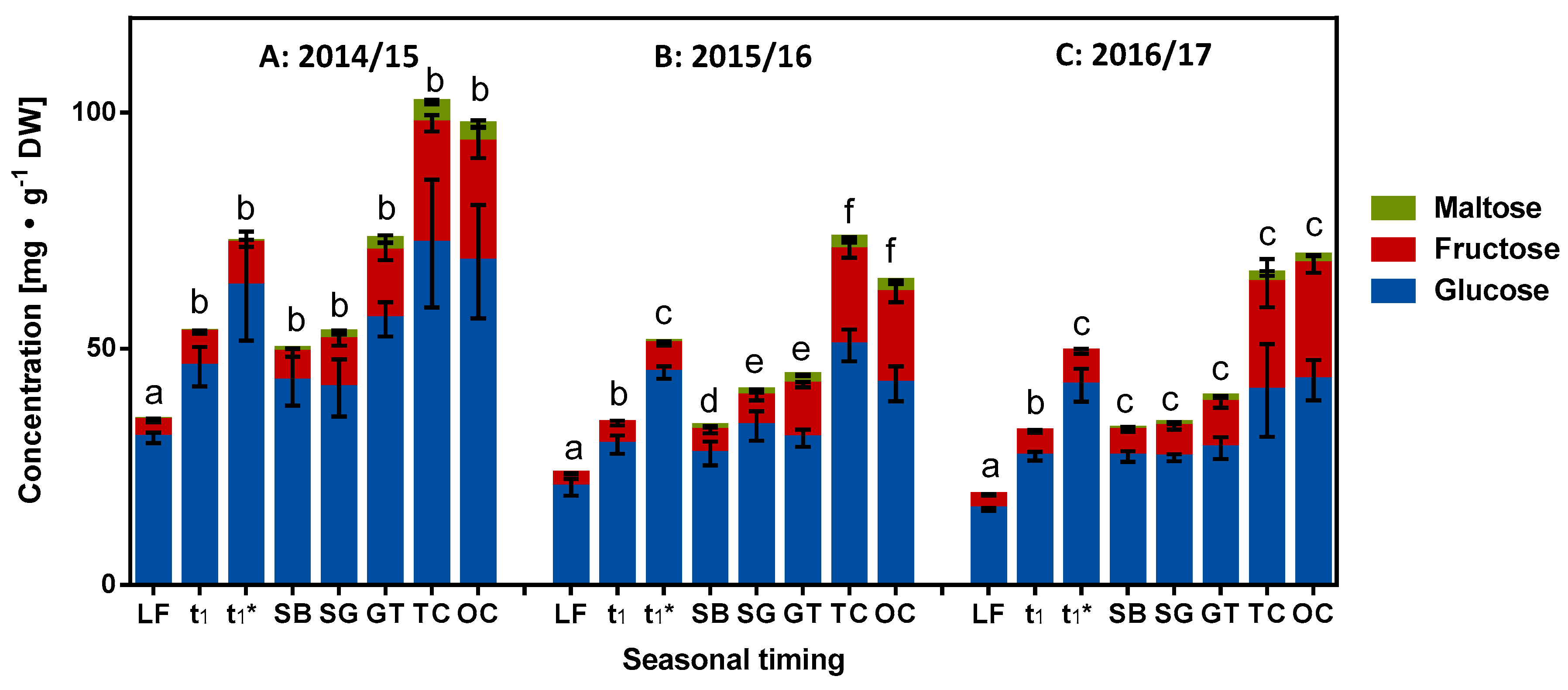
2.3. Role of Reducing Sugars in Dormancy
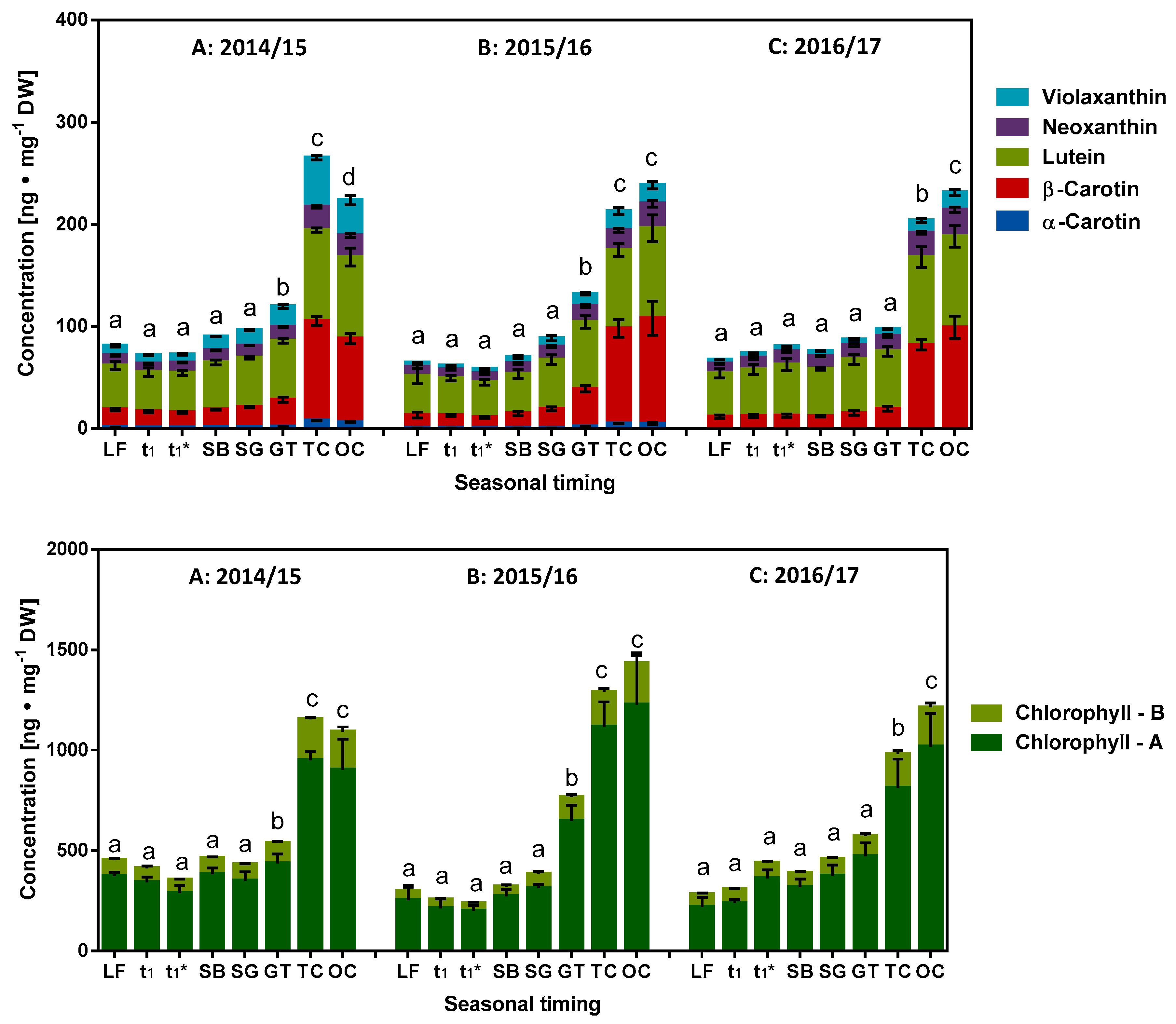
2.4. Role of Fat Soluble Redox Metabolites in Dormancy
2.5. Non-Targeted Analysis of the Metabolites
3. Materials and Methods
3.1. Chemicals
3.2. Study Design and Sampling
Bud and Twig Sampling
3.3. Phenolic Compounds
3.3.1. Extraction of Phenolic Compounds
3.3.2. Total Content of Phenolic Compounds
3.3.3. Determination of Antioxidative Capacity
3.3.4. Identification and Quantification of the Major Phenolic Compounds HPLC-DAD-ESI-MSn
3.4. Quantification of Ascorbic Acid (AA) and Dehydroascorbic Acid (DHA) by LC-MS/MS Analysis
3.5. Targeted Analysis of Reducing Sugars
3.6. Targeted Analysis of the Fat Soluble Redox Metabolites (Carotenoids and Chlorophylls) by UHPLC-DAD-TOF-MS
3.7. Non-Targeted Analysis of the Metabolites by UHPLC-Q-TOF-MS
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chmielewski, F.M.; Gotz, K.P.; Homann, T.; Huschek, G.; Rawel, H.M. Identification of endodormancy release for cherries (Prunus Avium L.) by abscisic acid and sugars. J. Hortic. 2017, 4, 210. [Google Scholar]
- Chmielewski, F.M.; Gotz, K.P. Identification and timing of dormant and ontogenetic phase for sweet cherries in northeast Germany for modelling purposes. J. Hortic. 2017, 4, 205. [Google Scholar]
- Zheng, C.L.; Halaly, T.; Acheampong, A.K.; Takebayashi, Y.; Jikumaru, Y.; Kamiya, Y.; Or, E. Abscisic acid (ABA) regulates grape bud dormancy, and dormancy release stimuli may act through modification of ABA metabolism. J. Exp. Bot. 2015, 66, 1527–1542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Yu, D.; Tian, X.L.; Liu, C.Y.; Gai, S.P.; Zheng, G.S. Differential expression proteins associated with bud dormancy release during chilling treatment of tree peony (Paeonia suffruticosa). Plant Biol. 2015, 17, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Takemura, Y.; Kuroki, K.; Jiang, M.F.; Matsumoto, K.; Tamura, F. Identification of the expressed protein and the impact of change in ascorbate peroxidase activity related to endodormancy breaking in Pyrus pyrifolia. Plant Physiol. Biochem. 2015, 86, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, F.M.; Baldermann, S.; Gotz, K.P.; Homann, T.; Schumacher, F.; Huschek, G.; Rawel, H. Abscisic acid related metabolites in sweet cherry buds. J. Hort. 2018, 5, 221. [Google Scholar]
- Chmielewski, F.M.; Gotz, K.P. Performance of models for the beginning of sweet cherry blossom under current and changed climate conditions. Agric. For. Meteorol. 2016, 218, 85–91. [Google Scholar] [CrossRef]
- Chuine, I.; Bonhomme, M.; Legave, J.M.; de Cortazar-Atauri, I.G.; Charrier, G.; Lacointe, A.; Ameglio, T. Can phenological models predict tree phenology accurately in the future? The unrevealed hurdle of endodormancy break. Glob. Chang. Biol. 2016, 22, 3444–3460. [Google Scholar] [CrossRef] [PubMed]
- Hanin, M.; Brini, F.; Ebel, C.; Toda, Y.; Takeda, S.; Masmoudi, K. Plant dehydrins and stress tolerance: Versatile proteins for complex mechanisms. Plant Signal Behav. 2011, 6, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Yamane, H.; Kashiwa, Y.; Kakehi, E.; Yonemori, K.; Mori, H.; Hayashi, K.; Iwamoto, K.; Tao, R.; Kataoka, I. Differential expression of dehydrin in flower buds of two Japanese apricot cultivars requiring different chilling requirements for bud break. Tree Physiol. 2006, 26, 1559–1563. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.D.; Wei, Z.G.; Shen, Z.; Lu, T.C.; Cheng, Y.X.; Wang, B.C.; Yang, C.P. Comparative temporal analyses of the Pinus sylvestris L. var. mongolica litv. apical bud proteome from dormancy to growth. Mol. Biol. Rep. 2011, 38, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Dhuli, P.; Rohloff, J.; Strimbeck, G.R. Metabolite changes in conifer buds and needles during forced bud break in Norway spruce (Picea abies) and European silver fir (Abies alba). Front. Plant Sci. 2014, 5, 706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safer, S.; Cicek, S.S.; Pieri, V.; Schwaiger, S.; Schneider, P.; Wissemann, V.; Stuppner, H. Metabolic fingerprinting of Leontopodium species (Asteraceae) by means of 1H NMR and HPLC-ESI-MS. Phytochemistry 2011, 72, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Errard, A.; Ulrichs, C.; Kuhne, S.; Mewis, I.; Drungowski, M.; Schreiner, M.; Baldermann, S. Single-versus multiple-pest infestation affects differently the biochemistry of tomato (Solanum lycopersicum ‘Ailsa Craig’). J. Agric. Food Chem. 2015, 63, 10103–10111. [Google Scholar] [CrossRef] [PubMed]
- Dauwe, R.; Holliday, J.A.; Aitken, S.N.; Mansfield, S.D. Metabolic dynamics during autumn cold acclimation within and among populations of Sitka spruce (Picea sitchensis). New Phytol. 2012, 194, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Shiryaeva, L.; Antti, H.; Schroder, W.P.; Strimbeck, R.; Shiriaev, A.S. Pair-wise multicomparison and OPLS analyses of cold-acclimation phases in Siberian spruce. Metabolomics 2012, 8, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Angelcheva, L.; Mishra, Y.; Antti, H.; Kjellsen, T.D.; Funk, C.; Strimbeck, R.G.; Schroder, W.P. Metabolomic analysis of extreme freezing tolerance in Siberian spruce (Picea obovata). New Phytol. 2014, 204, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Hutzler, P.; Fischbach, R.; Heller, W.; Jungblut, T.P.; Reuber, S.; Schmitz, R.; Veit, M.; Weissenbock, G.; Schnitzler, J.P. Tissue localization of phenolic compounds in plants by confocal laser scanning microscopy. J. Exp. Bot. 1998, 49, 953–965. [Google Scholar] [CrossRef]
- Vagiri, M.; Ekholm, A.; Andersson, S.C.; Johansson, E.; Rumpunen, K. An optimized method for analysis of phenolic compounds in buds, leaves, and fruits of black currant (Ribes nigrum L.). J. Agric. Food Chem. 2012, 60, 10501–10510. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, N.; Wang, Y.; Jiang, D.; Feng, X. Characterization of phenolic compounds from early and late ripening sweet cherries and their antioxidant and antifungal activities. J. Agric. Food Chem. 2017, 65, 5413–5420. [Google Scholar] [CrossRef] [PubMed]
- Commisso, M.; Bianconi, M.; Di Carlo, F.; Poletti, S.; Bulgarini, A.; Munari, F.; Negri, S.; Stocchero, M.; Ceoldo, S.; Avesani, L.; et al. Multi-approach metabolomics analysis and artificial simplified phytocomplexes reveal cultivar-dependent synergy between polyphenols and ascorbic acid in fruits of the sweet cherry (Prunus avium L.). PLoS ONE 2017, 12, e0180889. [Google Scholar] [CrossRef] [PubMed]
- Neugart, S.; Baldermann, S.; Hanschen, F.S.; Klopsch, R.; Wiesner-Reinhold, M.; Schreiner, M. The intrinsic quality of brassicaceous vegetables: How secondary plant metabolites are affected by genetic, environmental, and agronomic factors. Sci. Hortic. 2018, 233, 460–478. [Google Scholar] [CrossRef]
- Fiol, M.; Adermann, S.; Neugart, S.; Rohn, S.; Mugge, C.; Schreiner, M.; Krumbein, A.; Kroh, L.W. Highly glycosylated and acylated flavonols isolated from kale (Brassica oleracea var. sabellica)—Structure-antioxidant activity relationship. Food Res. Int. 2012, 47, 80–89. [Google Scholar] [CrossRef]
- Ali, M.; Homann, T.; Kreisel, J.; Khalil, M.; Puhlmann, R.; Kruse, H.P.; Rawel, H. Characterization and modeling of the interactions between coffee storage proteins and phenolic compounds. J. Agric. Food Chem. 2012, 60, 11601–11608. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, H.; Sugiura, T.; Ito, D. Changes in hydrogen peroxide content in flower buds of Japanese pear (Pyrus pyrifolia Nakai) in relation to breaking of endodormancy. J. Jpn. Soc. Hortic. Sci. 2002, 71, 610–616. [Google Scholar] [CrossRef]
- Ning, D.L.; Liu, C.C.; Liu, J.W.; Shen, Z.; Chen, S.; Liu, F.; Wang, B.C.; Yang, C.P. Label-free quantitative proteomics analysis of dormant terminal buds of poplar. Mol. Biol. Rep. 2013, 40, 4529–4542. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.J.; Qi, T.T.; Xu, L.; Lu, L.L.; Xiao, M. Recent progress in the enzymatic glycosylation of phenolic compounds. J. Carbohydr. Chem. 2016, 35, 1–23. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry antioxidants: From farm to table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef] [PubMed]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef] [PubMed]
- Hoque, E. Effects of para-hydroxyacetophenone and its glucoside on the enzymic oxidation of indole-3-acetic-acid. Agric. Biol. Chem. Tokyo 1989, 53, 239–240. [Google Scholar]
- Gotz, K.P.; Chmielewski, F.M.; Godeke, K.; Wolf, K.; Jander, E.; Sievers, S.; Homann, T.; Huschek, G.; Rawel, H.M. Assessment of amino acids during winter rest and ontogenetic development in sweet cherry buds (Prunus avium. L.). Sci. Hortic. 2017, 222, 102–110. [Google Scholar] [CrossRef]
- Kahkonen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- Scattino, C.; Castagna, A.; Neugart, S.; Chan, H.M.; Schreiner, M.; Crisosto, C.H.; Tonutti, P.; Ranieri, A. Post-harvest UV-B irradiation induces changes of phenol contents and corresponding biosynthetic gene expression in peaches and nectarines. Food Chem. 2014, 163, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.E.; Sabir, G. Comparison of two orthogonal liquid chromatographic methods for quantitation of sugars in food. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 902–919. [Google Scholar] [CrossRef]
- Cooke, J.E.; Eriksson, M.E.; Junttila, O. The dynamic nature of bud dormancy in trees: Environmental control and molecular mechanisms. Plant Cell Environ. 2012, 35, 1707–1728. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.B.; Shi, T.; Gao, Z.H.; Zhang, Z.; Zhang, J.Y. Differential expression of proteins associated with seasonal bud dormancy at four critical stages in Japanese apricot. Plant Biol. 2013, 15, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Gao, Z.; Wang, L.; Zhong, W.; Ni, Z.; Zhang, Z. Comparative proteomic and transcriptomic approaches to address the active role of GA4 in Japanese apricot flower bud dormancy release. J. Exp. Bot. 2013, 64, 4953–4966. [Google Scholar] [CrossRef] [PubMed]
- Prassinos, C.; Rigas, S.; Kizis, D.; Vlahou, A.; Hatzopoulos, P. Subtle proteome differences identified between post-dormant vegetative and floral peach buds. J. Proteom. 2011, 74, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Considine, M.J.; Foyer, C.H. Redox regulation of plant development. Antioxid. Redox Signal. 2014, 21, 1305–1326. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compoundsare not or only partly available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldermann, S.; Homann, T.; Neugart, S.; Chmielewski, F.-M.; Götz, K.-P.; Gödeke, K.; Huschek, G.; Morlock, G.E.; Rawel, H.M. Selected Plant Metabolites Involved in Oxidation-Reduction Processes during Bud Dormancy and Ontogenetic Development in Sweet Cherry Buds (Prunus avium L.). Molecules 2018, 23, 1197. https://doi.org/10.3390/molecules23051197
Baldermann S, Homann T, Neugart S, Chmielewski F-M, Götz K-P, Gödeke K, Huschek G, Morlock GE, Rawel HM. Selected Plant Metabolites Involved in Oxidation-Reduction Processes during Bud Dormancy and Ontogenetic Development in Sweet Cherry Buds (Prunus avium L.). Molecules. 2018; 23(5):1197. https://doi.org/10.3390/molecules23051197
Chicago/Turabian StyleBaldermann, Susanne, Thomas Homann, Susanne Neugart, Frank-M. Chmielewski, Klaus-Peter Götz, Kristin Gödeke, Gerd Huschek, Getrud E. Morlock, and Harshadrai M. Rawel. 2018. "Selected Plant Metabolites Involved in Oxidation-Reduction Processes during Bud Dormancy and Ontogenetic Development in Sweet Cherry Buds (Prunus avium L.)" Molecules 23, no. 5: 1197. https://doi.org/10.3390/molecules23051197






