Graminex Pollen: Phenolic Pattern, Colorimetric Analysis and Protective Effects in Immortalized Prostate Cells (PC3) and Rat Prostate Challenged with LPS
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals and Standards
2.2. HPLC-PDA Analyses
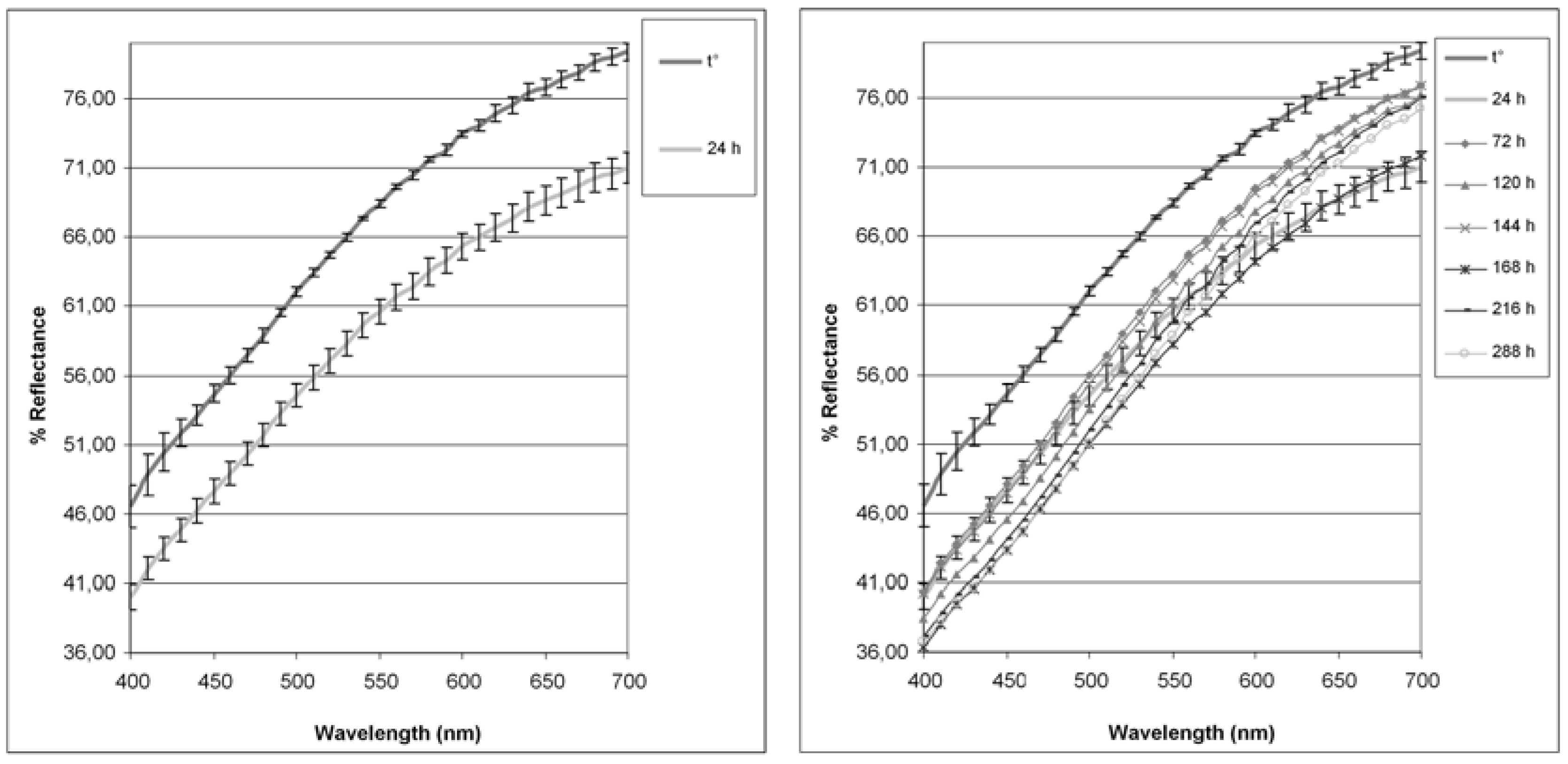
2.3. Colorimetric Analysis
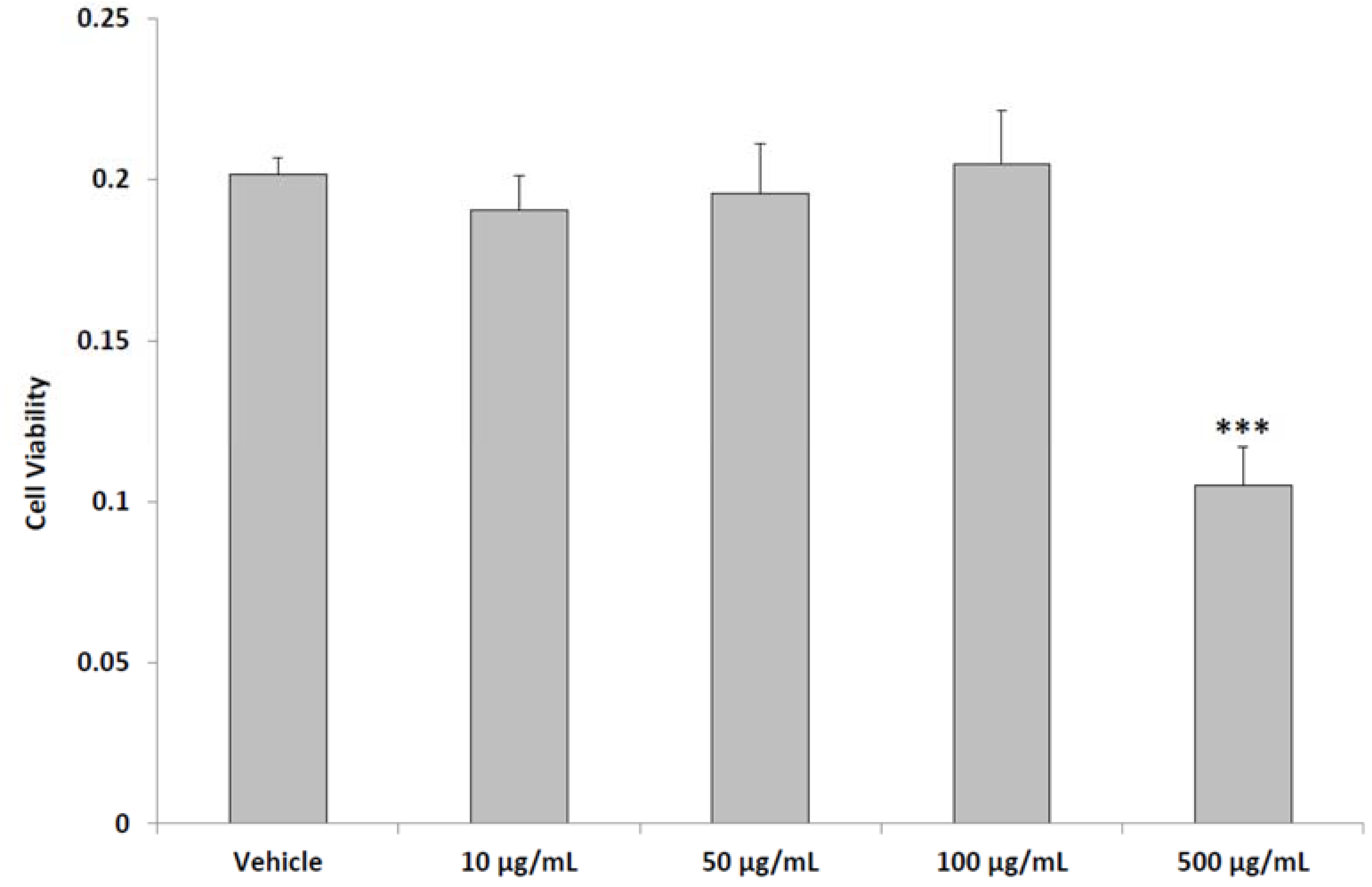
2.4. In Vitro Studies
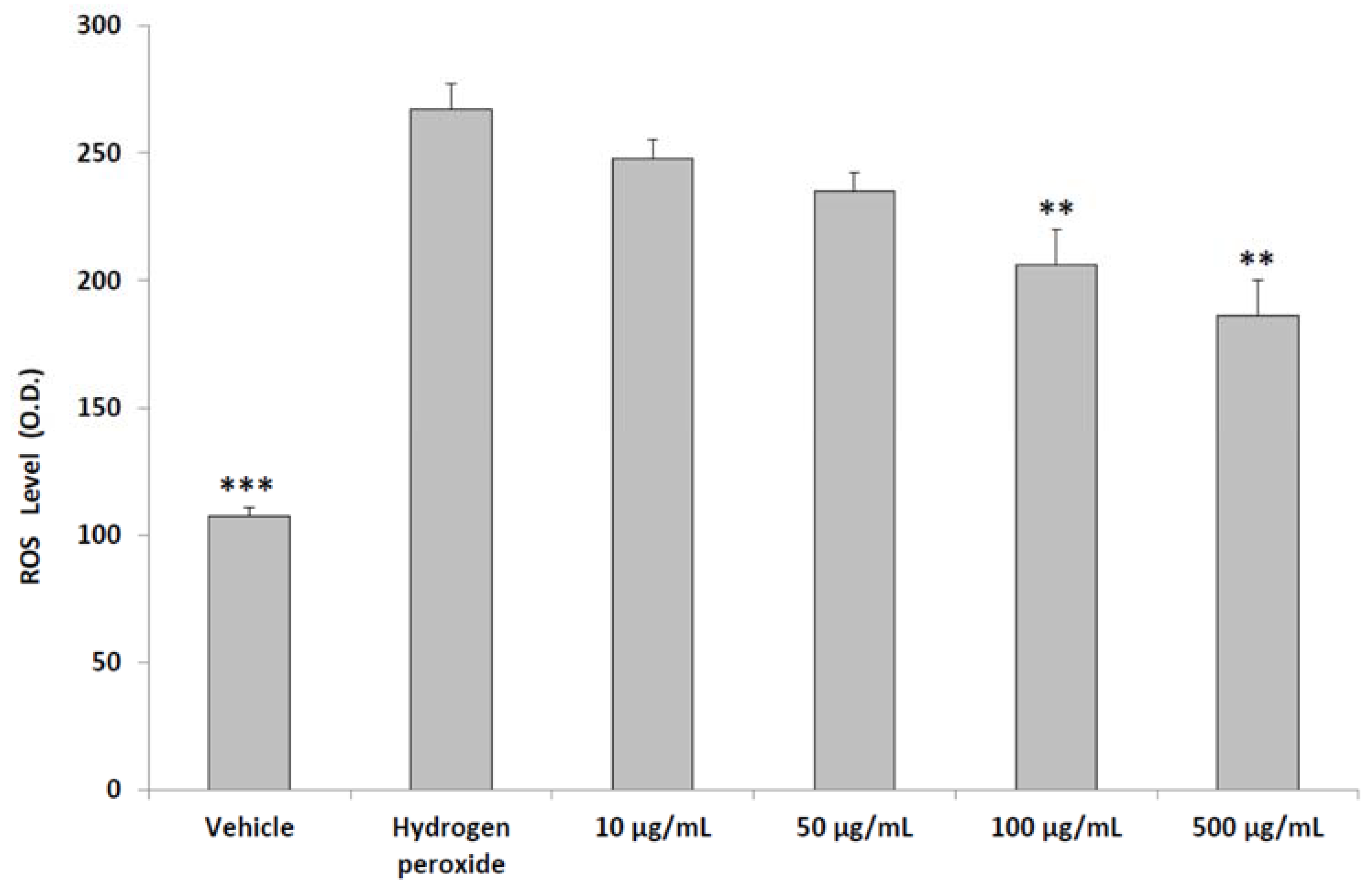
2.5. ROS Generation
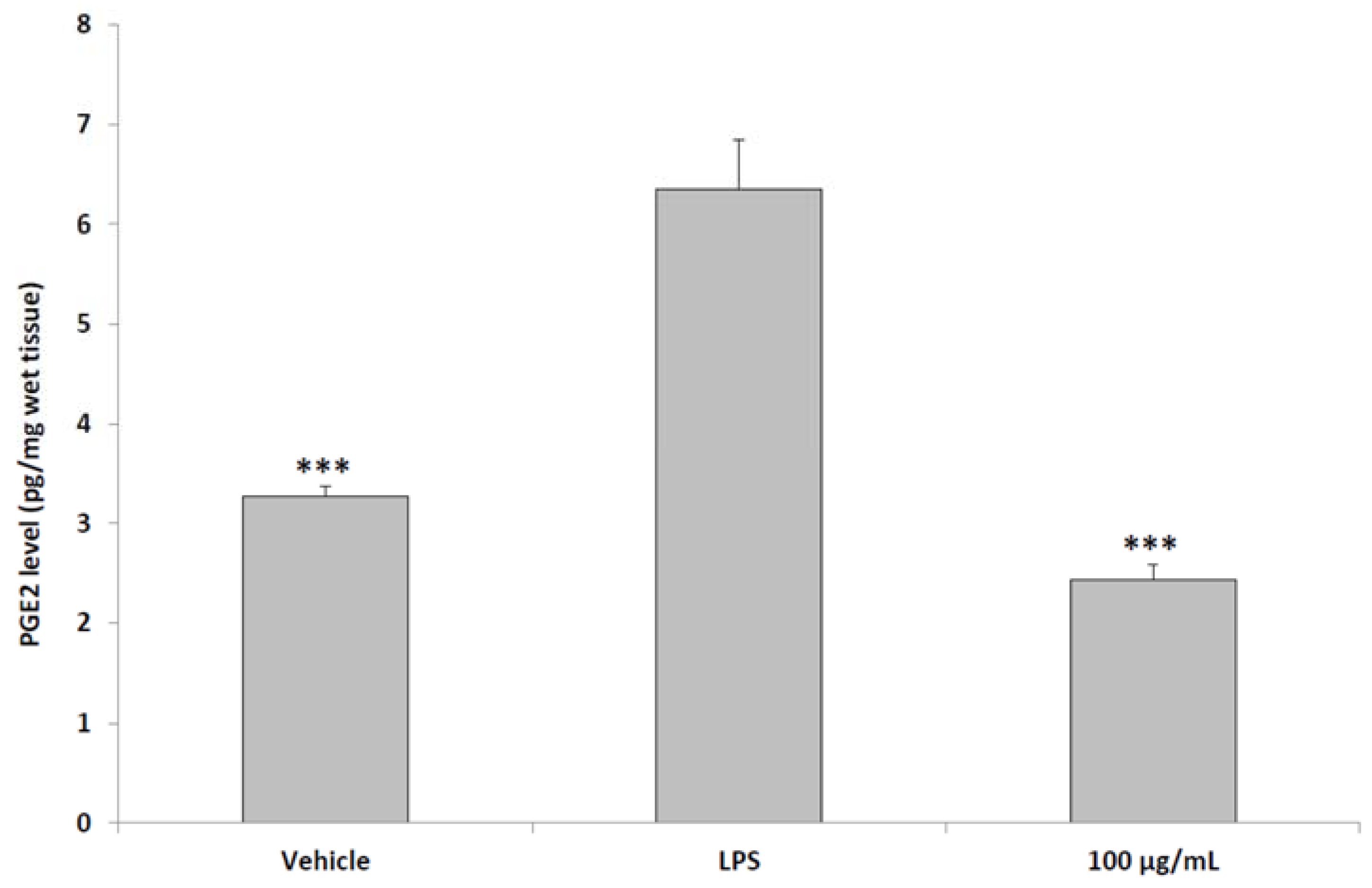
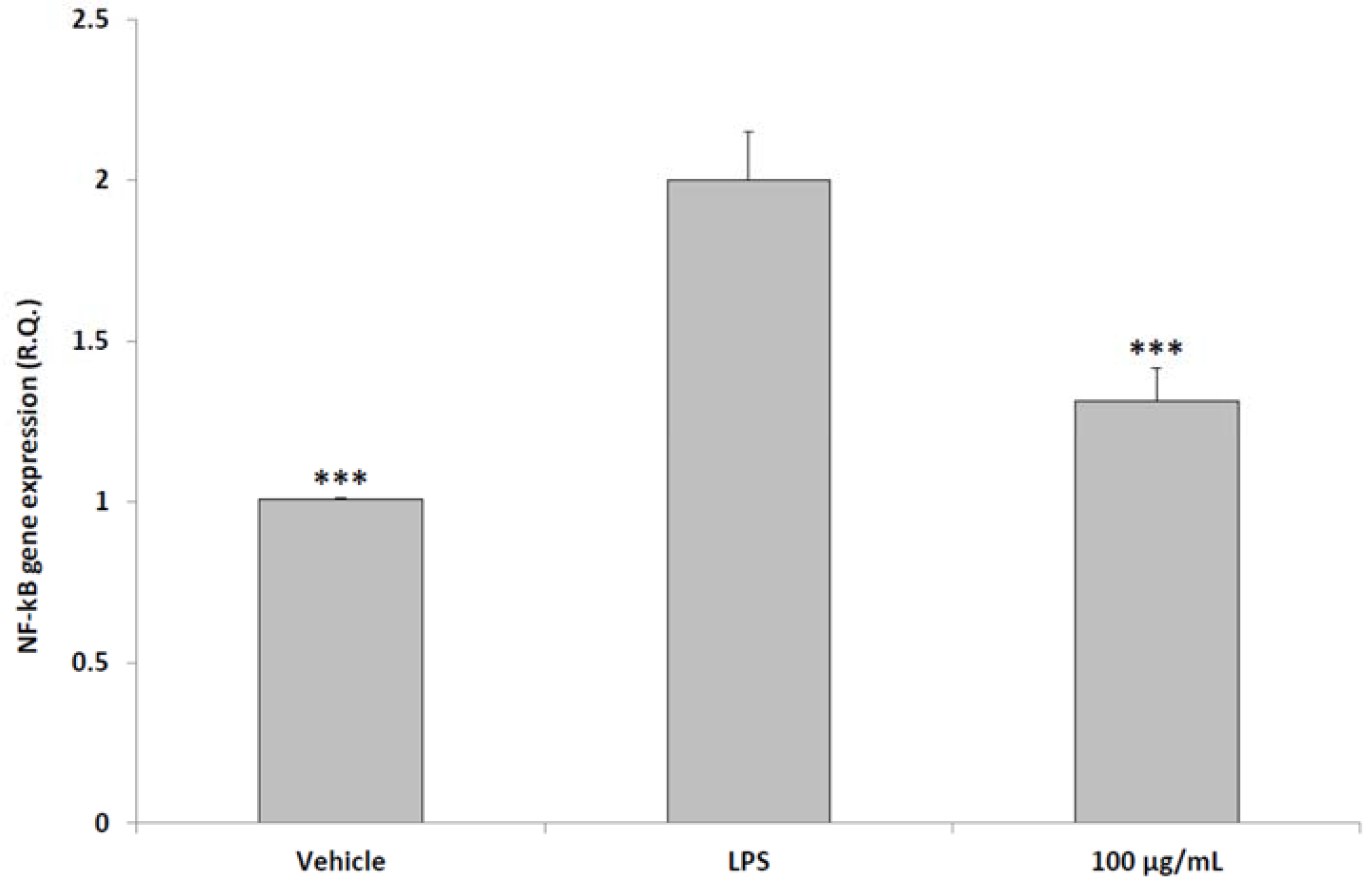
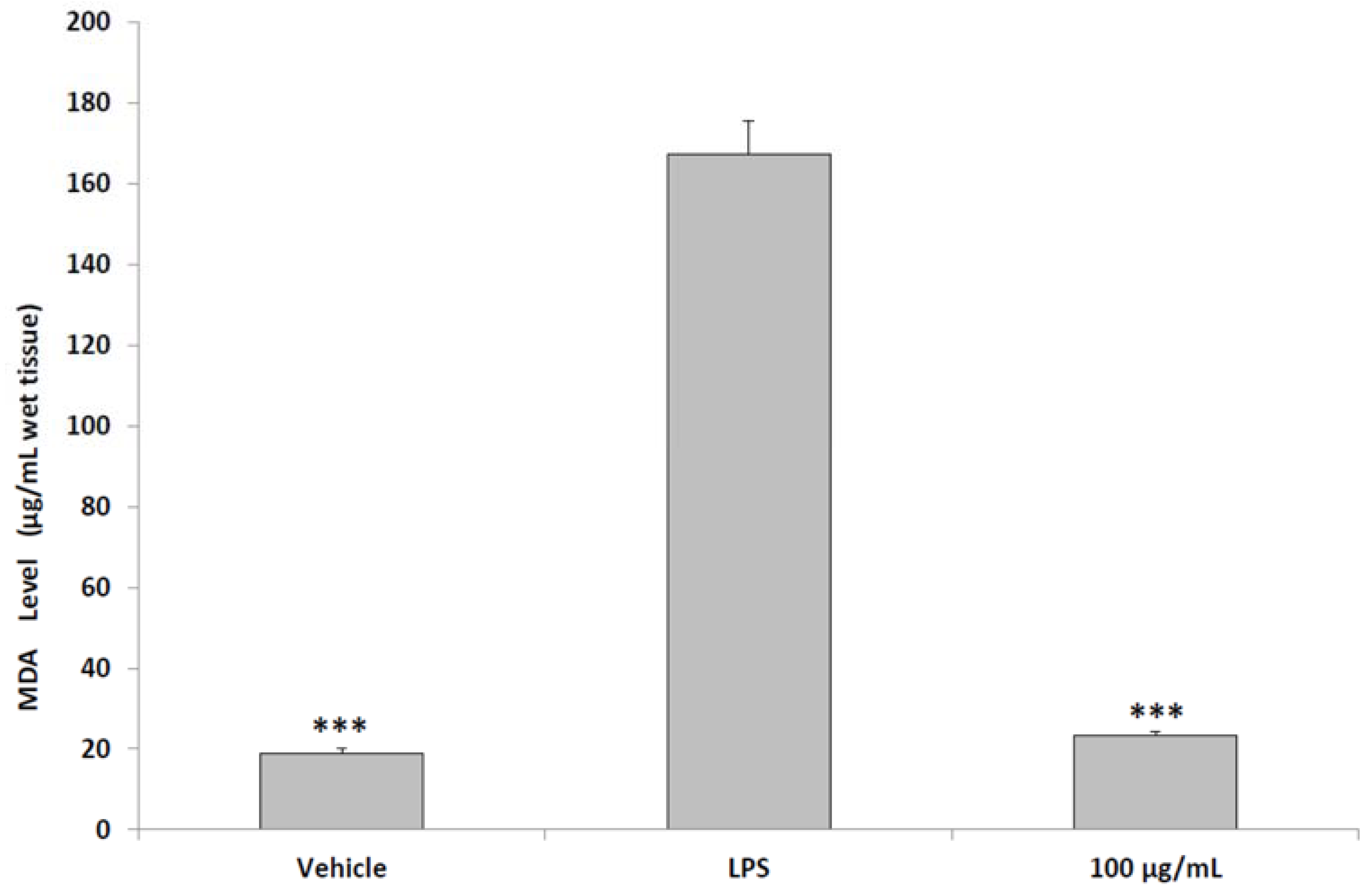
2.6. Ex Vivo Studies
2.7. Total Antioxidant Activity by Phosphomolybdenum Assay
2.8. Radical Scavenging Activity
2.9. Reducing Power (CUPRAC and FRAP Tests)
2.10. Metal Chelating Activity on Ferrous Ions
2.11. Enzyme Inhibition
2.11.1. Cholinesterase Inhibition
2.11.2. α-Amylase Inhibition
2.11.3. α-Glucosidase Inhibition
2.11.4. Tyrosinase Inhibition
2.12. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Steenkamp, V.; Gouws, M.C.; Gulumian, M.; Elgorashi, E.E.; van Staden, J. Studies on antibacterial, anti-inflammatory and anti-oxidant activity of herbal remedies used in the treatment of benign prostatic hyperplasia and prostatitis. J. Ethnopharmacol. 2006, 103, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Marzano, R.; Dinelli, N.; Ales, V.; Bertozzi, M.A. Effectiveness on urinary symptoms and erectile function of Prostamev Plus® vs. only extract Serenoa repens. Arch. Ital. Urol. Androl. 2015, 87, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Song, G.H.; Liu, G.T. Investigation of the effect of traditional Chinese medicine on pain and inflammation in chronic nonbacterial prostatitis in rats. Andrologia 2016, 48, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Verze, P.; La Rocca, R.; Anceschi, U.; De Nunzio, C.; Mirone, V. The role of flower pollen extract in managing patients affected by chronic prostatitis/chronic pelvic pain syndrome: A comprehensive analysis of all published clinical trials. BMC Urol. 2017, 17, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Verze, P.; La Rocca, R.; Palmieri, A.; Tiscione, D.; Luciani, L.G.; Mazzoli, S.; Mirone, V.; Malossini, G. The clinical efficacy of pollen extract and vitamins on chronic prostatitis/chronic pelvic pain syndrome is linked to a decrease in the pro-inflammatory cytokine interleukin-8. World J. Mens Health 2017, 35, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Negri, G.; Teixeira, E.W.; Alves, M.L.; Moreti, A.C.; Otsuk, I.P.; Borguini, R.G.; Salatino, A. Hydroxycinnamic acid amide derivatives, phenolic compounds and antioxidant activities of extracts of pollen samples from southeast Brazil. J. Agric. Food Chem. 2011, 59, 5516–5522. [Google Scholar] [CrossRef] [PubMed]
- Bonvehí, J.S.; Torrentó, M.S.; Lorente, E.C. Evaluation of polyphenolic and flavonoid compounds in honeybee-collected pollen produced in Spain. J. Agric. Food Chem. 2001, 49, 1848–1853. [Google Scholar] [CrossRef]
- Iglesias-Gato, D.; Carsten, T.; Vesterlund, M.; Pousette, A.; Schoop, R.; Norstedt, G. Androgen-independent effects of Serenoa repens extract (Prostasan®) on prostatic epithelial cell proliferation and inflammation. Phytother. Res. 2012, 26, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. An hydroalcoholic chamomile extract modulates inflammatory and immune response in HT29 cells and isolated rat colon. Phytother. Res. 2016, 30, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Cesa, S.; Casadei, M.A.; Cerreto, F.; Paolicelli, P. Infant milk formulas: Effect of storage conditions on the stability of powdered products towards autoxidation. Foods 2015, 4, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Cesa, S. Malondialdehyde contents in infant milk formulas. J. Agric. Food Chem. 2004, 52, 2119–2122. [Google Scholar] [CrossRef] [PubMed]
- De Monte, C.; Carradori, S.; Chimenti, P.; Secci, D.; Mannina, L.; Alcaro, F.; Petzer, A.; N’Da, C.I.; Gidaro, M.C.; Costa, G.; et al. New insights into the biological properties of Crocus sativus L.: Chemical modifications, human monoamine oxidases inhibition and molecular modeling studies. Eur. J. Med. Chem. 2014, 82, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Ceylan, R.; Guler, G.O.; Carradori, S.; Uysal, S.; Aktumsek, A. Enzyme inhibitory effect and antioxidant properties of Astragalus lagurus extracts. Curr. Enzym. Inhib. 2016, 12, 177–182. [Google Scholar] [CrossRef]
- Clydesdale, F.M.; Ahmed, E.M. Colorimetry-methodology and applications. CRC Crit. Rev. Food Sci. Nutr. 1978, 10, 243–301. [Google Scholar] [CrossRef] [PubMed]
- Sintiprungrat, K.; Singhto, N.; Sinchaikul, S.; Chen, S.T.; Thongboonkerd, V. Alterations in cellular proteome and secretome upon differentiation from monocyte to macrophage by treatment with phorbol myristate acetate: Insights into biological processes. J. Proteom. 2010, 73, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.; Recinella, L.; Locatelli, M.; Guglielmi, P.; Secci, D.; Leporini, L.; Chiavaroli, A.; Leone, S.; Martinotti, S.; Brunetti, L.; et al. Protective Effects Induced by Microwave-Assisted Aqueous Harpagophytum Extract on Rat Cortex Synaptosomes Challenged with Amyloid β-Peptide. Phytother. Res. 2017, 31, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Di Nisio, C.; Recinella, L.; Orlando, G.; Ferrante, C.; Chiavaroli, A.; Leone, S.; Di Michele, P.; Shohreh, R.; Vacca, M. Obestatin inhibits dopamine release in rat hypothalamus. Eur. J. Pharmacol. 2010, 641, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.; Orlando, G.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; Vacca, M.; et al. Central inhibitory effects on feeding induced by the adipo-myokine irisin. Eur. J. Pharmacol. 2016, 791, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Menghini, L.; Leporini, L.; Vecchiotti, G.; Locatelli, M.; Carradori, S.; Ferrante, C.; Zengin, G.; Recinella, L.; Chiavaroli, A.; Leone, S.; et al. Crocus sativus L. stigmas and byproducts: Qualitative fingerprint, antioxidant potentials and enzyme inhibitory activities. Food Res. Int. 2018, 109, 91–98. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Brunetti, L.; Orlando, G.; Recinella, L.; Ferrante, C.; Leone, S.; Di Michele, P.; Di Nisio, C.; Vacca, M. Resveratrol inhibits isoprostane production in young and aged rat brain. J. Biol. Regul. Homeost. Agents 2010, 24, 441–446. [Google Scholar] [PubMed]
- Brunetti, L.; Ferrante, C.; Orlando, G.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; et al. Orexigenic effects of endomorphin-2 (EM-2) related to decreased CRH gene expression and increased dopamine and norepinephrine activity in the hypothalamus. Peptides 2013, 48, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Orlando, G.; Ferrante, C.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; et al. Peripheral chemerin administration modulates hypothalamic control of feeding. Peptides 2014, 51, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Uchiyama, M.; Fukuzawa, K. Thiobarbituric acid value on fresh homogenate of rat as a parameter of lipid peroxidation in aging, CCl4 intoxication, and vitamin E deficiency. Biochem. Med. 1980, 23, 302–311. [Google Scholar] [CrossRef]
- Zengin, G.; Locatelli, M.; Carradori, S.; Mocan, A.; Aktumsek, A. Total phenolics, flavonoids, condensed tannins content of eight Centaurea species and their broad inhibitory activities against cholinesterase, tyrosinase, α-amylase and α-glucosidase. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 195–200. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Recinella, L.; Ferrante, C.; Locatelli, M.; Carradori, S.; Leporini, L.; Leone, S.; Martinotti, S.; Brunetti, L.; Vacca, M.; et al. Crocus sativus, Serenoa repens and Pinus massoniana extracts modulate inflammatory response in isolated rat prostate challenged with LPS. J. Biol. Regul. Homeost. Agents 2017, 31, 531–541. [Google Scholar] [PubMed]
- Locatelli, M.; Zengin, G.; Uysal, A.; Carradori, S.; De Luca, E.; Bellagamba, G.; Aktumsek, A.; Lazarova, I. Multicomponent pattern and biological activities of seven Asphodeline taxa: Potential sources of natural-functional ingredients for bioactive formulations. J. Enzyme Inhib. Med. Chem. 2017, 32, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.D.; Lou, Y.J. A steroid fraction of chloroform extract from bee pollen of Brassica campestris induces apoptosis in human prostate cancer PC-3 cells. Phytother. Res. 2007, 21, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.; Moita, E.; Valentão, P.; Fernandes, F.; Monteiro, P.; Andrade, P.B. Effects of colored and noncolored phenolics of Echium plantagineum L. bee pollen in Caco-2 cells under oxidative stress induced by tert-butyl hydroperoxide. J. Agric. Food Chem. 2015, 63, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Mahaldashtian, M.; Makoolati, Z.; Ghorbanian, M.T.; Naghdi, M.; Kouhpayeh, S.A. In vitro cytotoxicity effects of date palm (Phoenix dactylifera L.) pollen on neonate mouse spermatogonial stem cells. Nat. Prod. Res. 2015, 29, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Fatrcová-Šramková, K.; Nôžková, J.; Máriássyová, M.; Kačániová, M. Biologically active antimicrobial and antioxidant substances in the Helianthus annuus L. bee pollen. J. Environ. Sci. Health B 2016, 51, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Melucci, D.; Locatelli, M.; Locatelli, C.; Zappi, A.; De Laurentiis, F.; Carradori, S.; Campestre, C.; Leporini, L.; Zengin, G.; Picot, C.M.N.; et al. A comparative assessment of biological effects and chemical profile of Italian Asphodeline lutea extracts. Molecules 2018, 23, 461. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Ferrante, C.; Carradori, S.; Secci, D.; Leporini, L.; Chiavaroli, A.; Leone, S.; Recinella, L.; Orlando, G.; Martinotti, S.; et al. Optimization of aqueous extraction and biological activity of Harpagophytum procumbens root on ex vivo rat colon inflammatory model. Phytother. Res. 2017, 31, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Orlando, G.; Michelotto, B.; Recinella, L.; Di Nisio, C.; Ciabattoni, G.; Vacca, M. Identification of 8-iso-prostaglandin F(2alpha) in rat brain neuronal endings: A possible marker of membrane phospholipid peroxidation. Life Sci. 2002, 71, 2447–2455. [Google Scholar] [CrossRef]
- Brunetti, L.; Michelotto, B.; Orlando, G.; Recinella, L.; Di Nisio, C.; Ciabattoni, G.; Vacca, M. Aging increases amyloid beta-peptide-induced 8-iso-prostaglandin F2alpha release from rat brain. Neurobiol. Aging 2004, 25, 125–129. [Google Scholar] [CrossRef]
- Moita, E.; Gil-Izquierdo, A.; Sousa, C.; Ferreres, F.; Silva, L.R.; Valentão, P.; Domínguez-Perles, R.; Baenas, N.; Andrade, P.B. Integrated analysis of COX-2 and iNOS derived inflammatory mediators in LPS-stimulated RAW macrophages pre-exposed to Echium plantagineum L. bee pollen extract. PLoS ONE 2013, 8, e59131. [Google Scholar] [CrossRef] [PubMed]
- Shirahama, T.; Sakakura, C. Overexpression of cyclooxygenase-2 in squamous cell carcinoma of the urinary bladder. Clin. Cancer Res. 2001, 7, 558–561. [Google Scholar] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Piva, R.; Belardo, G.; Santoro, M.G. NF-kappaB: A stress-regulated switch for cell survival. Antioxid. Redox Signal. 2006, 8, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Hartman, Z.C.; Yang, X.Y.; Glass, O.; Lei, G.; Osada, T.; Dave, S.S.; Morse, M.A.; Clay, T.M.; Lyerly, H.K. HER2 overexpression elicits a proinflammatory IL-6 autocrine signaling loop that is critical for tumorigenesis. Cancer Res. 2011, 71, 4380–4391. [Google Scholar] [CrossRef] [PubMed]
- Thurmond, P.; Yang, J.H.; Li, Y.; Lerner, L.B.; Azadzoi, K.M. Structural modifications of the prostate in hypoxia, oxidative stress, and chronic ischemia. Korean J. Urol. 2015, 56, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Rifaioglu, M.M.; Nacar, A.; Yuksel, R.; Yonden, Z.; Karcioglu, M.; Zorba, O.U.; Davarci, I.; Sefil, N.K. Antioxidative and anti-inflammatory effect of thymoquinone in an acute Pseudomonas prostatitis rat model. Urol. Int. 2013, 91, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Menghini, L.; Malatesta, L.; De Luca, E.; Bellagamba, G.; Uysal, S.; Aktumsek, A.; Locatelli, M. Comparative study of biological activities and multicomponent pattern of two wild Turkish species: Asphodeline anatolica and Potentilla speciosa. J. Enzyme Inhib. Med. Chem. 2016, 31, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Fu, J.; Zhao, Z.; Kong, X.; Huang, H.; Luo, L.; Yin, Z. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7 cells through suppressing NF-kappaB and JNK/AP-1 activation. Int. Immunopharmacol. 2009, 9, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.W.; Xie, Z.X.; Wang, B.F.; Zhong, Z.H.; Chen, X.Y.; Sun, Y.H.; Sun, Q.F.; Yang, G.Y.; Bian, L.G. Carvacrol protects neuroblastoma SH-SY5Y cells against Fe(2+)-induced apoptosis by suppressing activation of MAPK/JNK-NF-κB signaling pathway. Acta Pharmacol. Sin. 2015, 36, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Dong, Y.; Huo, R.; Wen, K.; Zhang, Y.; Liang, G. Rutin inhibits neuroinflammation and provides neuroprotection in an experimental rat model of subarachnoid hemorrhage, possibly through suppressing the RAGE-NF-κB inflammatory signaling pathway. Neurochem. Res. 2016, 41, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Peyrat-Maillard, M.N.; Bonnely, S.; Berset, C. Determination of the antioxidant activity of phenolic compounds by coulometric detection. Talanta 2000, 51, 709–716. [Google Scholar] [CrossRef]
- Glässer, G.; Graefe, E.U.; Struck, F.; Veit, M.; Gebhardt, R. Comparison of antioxidative capacities and inhibitory effects on cholesterol biosynthesis of quercetin and potential metabolites. Phytomedicine 2002, 9, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Bagdas, D.; Gul, N.Y.; Topal, A.; Tas, S.; Ozyigit, M.O.; Cinkilic, N.; Gul, Z.; Etoz, B.C.; Ziyanok, S.; Inan, S.; et al. Pharmacologic overview of systemic chlorogenic acid therapy on experimental wound healing. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 1101–1116. [Google Scholar] [CrossRef] [PubMed]
- Arigesavan, K.; Sudhandiran, G. Carvacrol exhibits anti-oxidant and anti-inflammatory effects against 1,2-dimethylhydrazine plus dextran sodium sulfate induced inflammation associated carcinogenicity in the colon of Fischer 344 rats. Biochem. Biophys. Res. Commun. 2015, 461, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, R.; Kawanishi, H.; Tsujino, T.; Tsujita, M.; Nishisaka, N.; Horii, A.; Kishimoto, T. Clinical evaluation of long-term treatment using Cernitin pollen extract in patients with benign prostatic hyperplasia. Clin. Ther. 1995, 17, 82–87. [Google Scholar] [CrossRef]
- Togo, Y.; Ichioka, D.; Miyazaki, J.; Maeda, Y.; Kameyama, K.; Yasuda, M.; Hiyama, Y.; Takahashi, S.; Nagae, H.; Hirota, S.; et al. Oral administration of cernitin pollen extract (Cernilton®) for 30 days might be useful to avoid unnecessary biopsy in prostate biopsy candidates: A preliminary study. Int. J. Urol. 2018, 25, 479–485. [Google Scholar] [CrossRef] [PubMed]
- De Nunzio, C.; Kramer, G.; Marberger, M.; Montironi, R.; Nelson, W.; Schröder, F.; Sciarra, A.; Tubaro, A. The controversial relationship between benign prostatic hyperplasia and prostate cancer: The role of inflammation. Eur. Urol. 2011, 60, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.O.; Yumnam, S.; Jeong, K.W.; Kim, S.Y. Finasteride inhibits melanogenesis through regulation of the adenylate cyclase in melanocytes and melanoma cells. Arch. Pharm. Res. 2018, 41, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Shoskes, D.A.; Vij, S.C.; Shoskes, A.; Nyame, Y.; Gao, T. Development of a clinically relevant men’s health phenotype and correlation of systemic and urologic conditions. Urology 2018, 114, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Yamazaki, H.; Iwama, K.; Oota, Y.; Aibe, N.; Nakamura, S.; Yoshida, K.; Okabe, H.; Yamada, K. Potential risk of alpha-glucosidase inhibitor administration in prostate cancer external radiotherapy by exceptional rectal gas production: A case report. J. Med. Case Rep. 2014, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Cesa, S.; Carradori, S.; Bellagamba, G.; Locatelli, M.; Casadei, M.A.; Masci, A.; Paolicelli, P. Evaluation of processing effects on anthocyanin content and colour modifications of blueberry (Vaccinium spp.) extracts: Comparison between HPLC-DAD and CIELAB analyses. Food Chem. 2017, 232, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Tuberoso, C.I.G.; Jerković, I.; Sarais, G.; Congiu, F.; Marijanović, Z.; Kuś, P.M. Color evaluation of seventeen European unifloral honey types by means of spectrophotometrically determined CIE chromaticity coordinates. Food Chem. 2014, 145, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Jerković, I.; Tuberoso, C.I.G.; Baranović, G.; Marijanović, Z.; Kranjac, M.; Svečnjak, L.; Kuś, P.M. Characterization of summer savory (Satureja hortensis L.) honey by physico-chemical parameters and chromatographic/spectroscopic techniques (GC-FID/MS, HPLC-DAD, UV/VIS and FTIR-ATR). Croat. Chem. Acta 2015, 88, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Cesa, S.; Paolicelli, P.; Cerreto, F.; Casadei, M.A. Comparison between third derivative spectrophotometric method and HPLC-DAD method in detection of malondialdehyde in infant formulae, human and cow milks. J. Chem. Pharm. Res. 2012, 4, 221–230. [Google Scholar]
Sample Availability: Sample of the compounds are available from the authors. |
| Assays | Results * |
|---|---|
| Phosphomolybdenum (mg TEs/g) | 32.07 ± 2.39 |
| DPPH (mg TEs/g) | ni |
| ABTS (mg TEs/g) | 8.55 ± 0.33 |
| CUPRAC (mg TEs/g) | 9.11 ± 0.76 |
| FRAP (mg TEs/g) | 6.95 ± 0.25 |
| Metal Chelating (mg EDTAEs/g) | 1.85 ± 0.03 |
| Compound | µg/g of Pollen | Retention Time (min) | Wavelength (nm) |
|---|---|---|---|
| Gallic acid | 89.06 ± 8.25 | 4.99 | 271 |
| Catechin | nd | 13.36 | 278 |
| Chlorogenic acid | 101.77 ± 10.09 | 14.29 | 324 |
| p-OH benzoic acid | nd | 14.71 | 256 |
| Vanillic acid | nd | 17.31 | 260 |
| Epicatechin | nd | 18.30 | 278 |
| Syringic acid | nd | 18.50 | 274 |
| 3-OH benzoic acid | nd | 19.41 | 275 |
| 3-OH-4-MeO benzaldehyde | nd | 22.08 | 278 |
| p-coumaric acid | nd | 22.65 | 310 |
| Rutin | 122.29 ± 11.23 | 25.38 | 256 |
| Sinapinic acid | nd | 26.18 | 324 |
| t-ferulic acid | nd | 27.75 | 315 |
| Naringin | nd | 29.78 | 285 |
| 2,3-diMeO benzoic acid | nd | 30.36 | 299 |
| Benzoic acid | nd | 31.20 | 275 |
| o-coumaric acid | nd | 34.81 | 276 |
| Crocin | nd | 35.52 | 440 |
| Quercetin | 124.42 ± 12.01 | 40.57 | 367 |
| Harpagoside | nd | 45.49 | 280 |
| t-cinnamic acid | nd | 45.87 | 276 |
| Naringenin | nd | 46.74 | 290 |
| Safranal | nd | 47.00 | 330 |
| Carvacrol | 251.88 ± 25.03 | 49.95 | 275 |
| TOTAL | 689.41 ± 52.89 |
| Enzyme Inhibition | Result * |
|---|---|
| AChE Inhibition (mg GALAE/g) | 0.26 ± 0.01 |
| BChE Inhibition (mg GALAE/g) | 0.35 ± 0.01 |
| Tyrosinase inhibition (mg KAE/g) | 1.53 ± 0.11 |
| α-Amylase inhibition (mmol ACAE/g) | 0.05 ± 0.01 |
| α-Glucosidase inhibition (mmol ACAE/g) | 0.79 ± 0.10 |
| t° | 24 h | 72 h | 120 h | 144 h | 168 h | 216 h | 288 h | |
|---|---|---|---|---|---|---|---|---|
| L | 86.05 ± 0.44 | 81.14 ± 0.39 | 83.39 ± 0.47 | 82.25 ± 0.31 | 83.14 ± 0.40 | 80.61 ± 0.20 | 81.63 ± 0.46 | 81.11 ± 0.70 |
| a* | 1.71 ± 0.11 | 1.75 ± 0.03 | 2.66 ± 0.11 | 3.24 ± 0.04 | 2.86 ± 0.14 | 2.92 ± 0.12 | 3.52 ± 0.08 | 3.69 ± 0.32 |
| b* | 11.96 ± 0.41 | 12.05 ± 0.15 | 14.30 ± 0.24 | 15.33 ± 0.34 | 14.48 ± 0.52 | 14.71 ± 0.41 | 15.60 ± 0.37 | 16.29 ± 1.14 |
| C*ab | 12.09 ± 0.39 | 12.18 ± 0.14 | 14.54 ± 0.25 | 15.67 ± 0.34 | 14.75 ± 0.54 | 15.00 ± 0.42 | 15.99 ± 0.37 | 16.05 ± 0.72 |
| hab | 81.88 ± 0.79 | 81.78 ± 0.22 | 79.49 ± 0.31 | 78.05 ± 0.12 | 78.86 ± 0.13 | 78.80 ± 0.35 | 77.30 ±0.09 | 76.71 ± 0.56 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Locatelli, M.; Macchione, N.; Ferrante, C.; Chiavaroli, A.; Recinella, L.; Carradori, S.; Zengin, G.; Cesa, S.; Leporini, L.; Leone, S.; et al. Graminex Pollen: Phenolic Pattern, Colorimetric Analysis and Protective Effects in Immortalized Prostate Cells (PC3) and Rat Prostate Challenged with LPS. Molecules 2018, 23, 1145. https://doi.org/10.3390/molecules23051145
Locatelli M, Macchione N, Ferrante C, Chiavaroli A, Recinella L, Carradori S, Zengin G, Cesa S, Leporini L, Leone S, et al. Graminex Pollen: Phenolic Pattern, Colorimetric Analysis and Protective Effects in Immortalized Prostate Cells (PC3) and Rat Prostate Challenged with LPS. Molecules. 2018; 23(5):1145. https://doi.org/10.3390/molecules23051145
Chicago/Turabian StyleLocatelli, Marcello, Nicola Macchione, Claudio Ferrante, Annalisa Chiavaroli, Lucia Recinella, Simone Carradori, Gokhan Zengin, Stefania Cesa, Lidia Leporini, Sheila Leone, and et al. 2018. "Graminex Pollen: Phenolic Pattern, Colorimetric Analysis and Protective Effects in Immortalized Prostate Cells (PC3) and Rat Prostate Challenged with LPS" Molecules 23, no. 5: 1145. https://doi.org/10.3390/molecules23051145










