In Vitro Anticandidal Activity and Mechanism of a Polyoxovanadate Functionalized by Zn-Fluconazole Complexes
Abstract
:1. Introduction
2. Results and Discussion
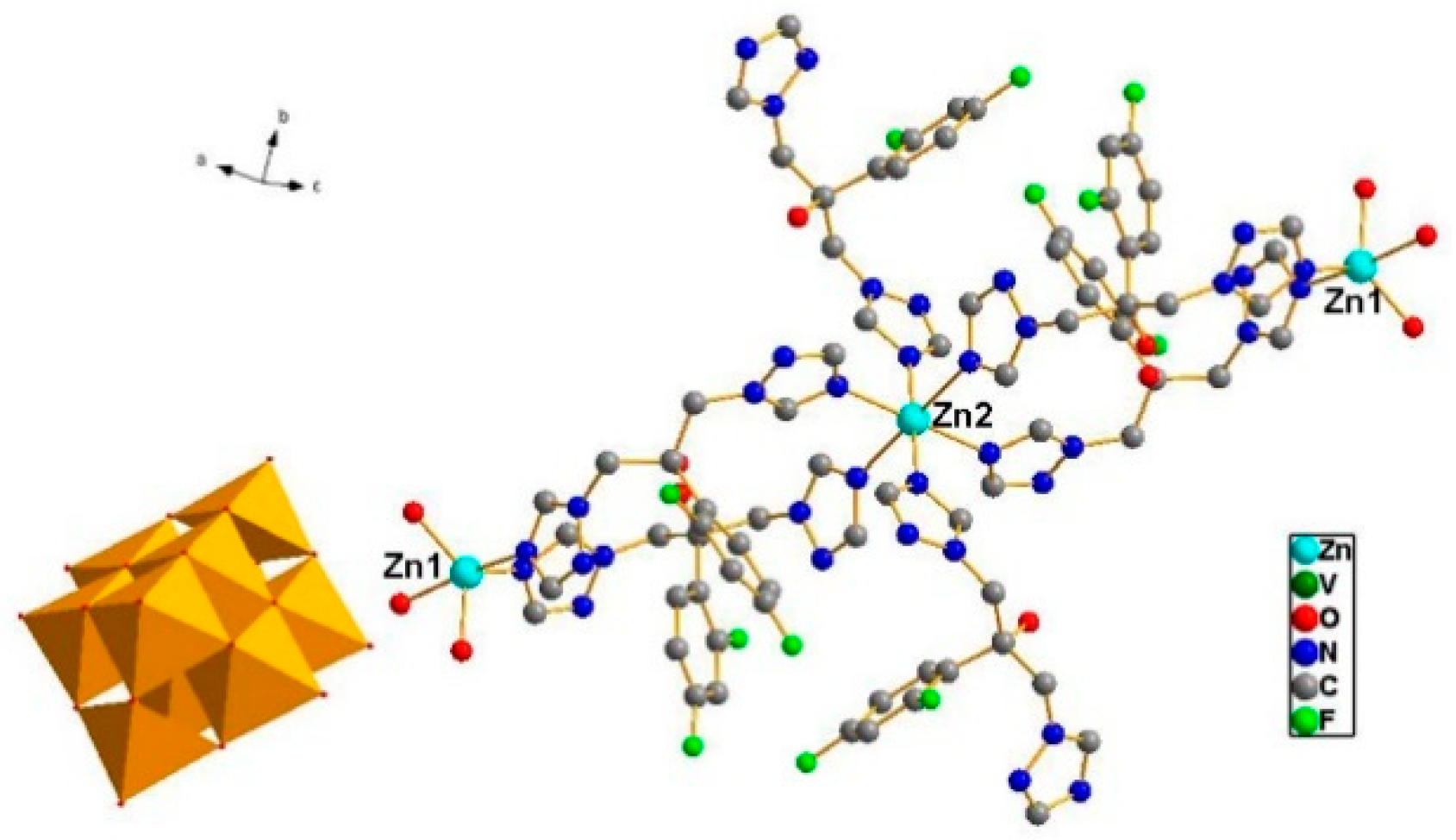
2.1. Structure Description of ZnFLC
2.2. FT-IR of ZnFLC
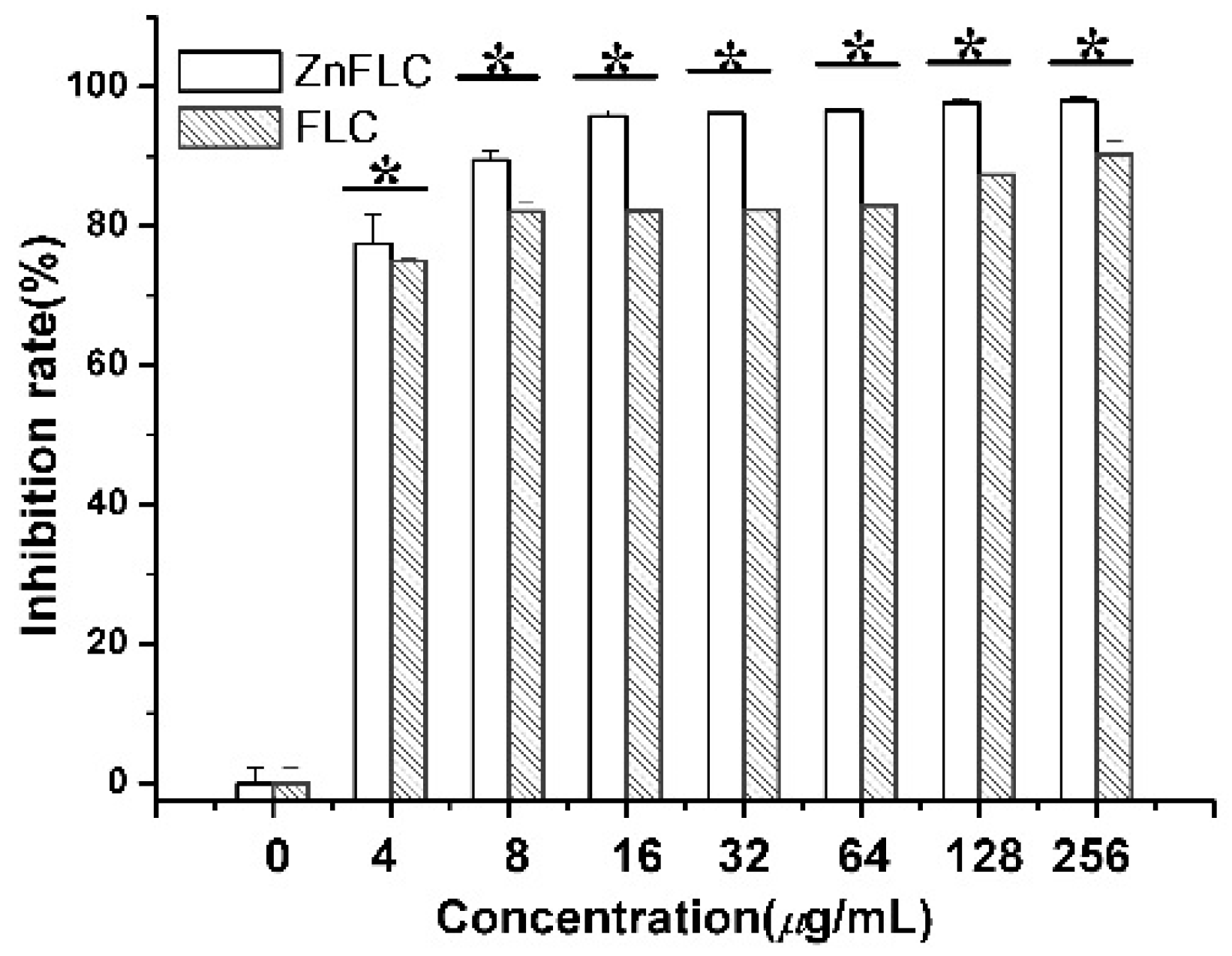
2.3. Antifungal Susceptibility Testing
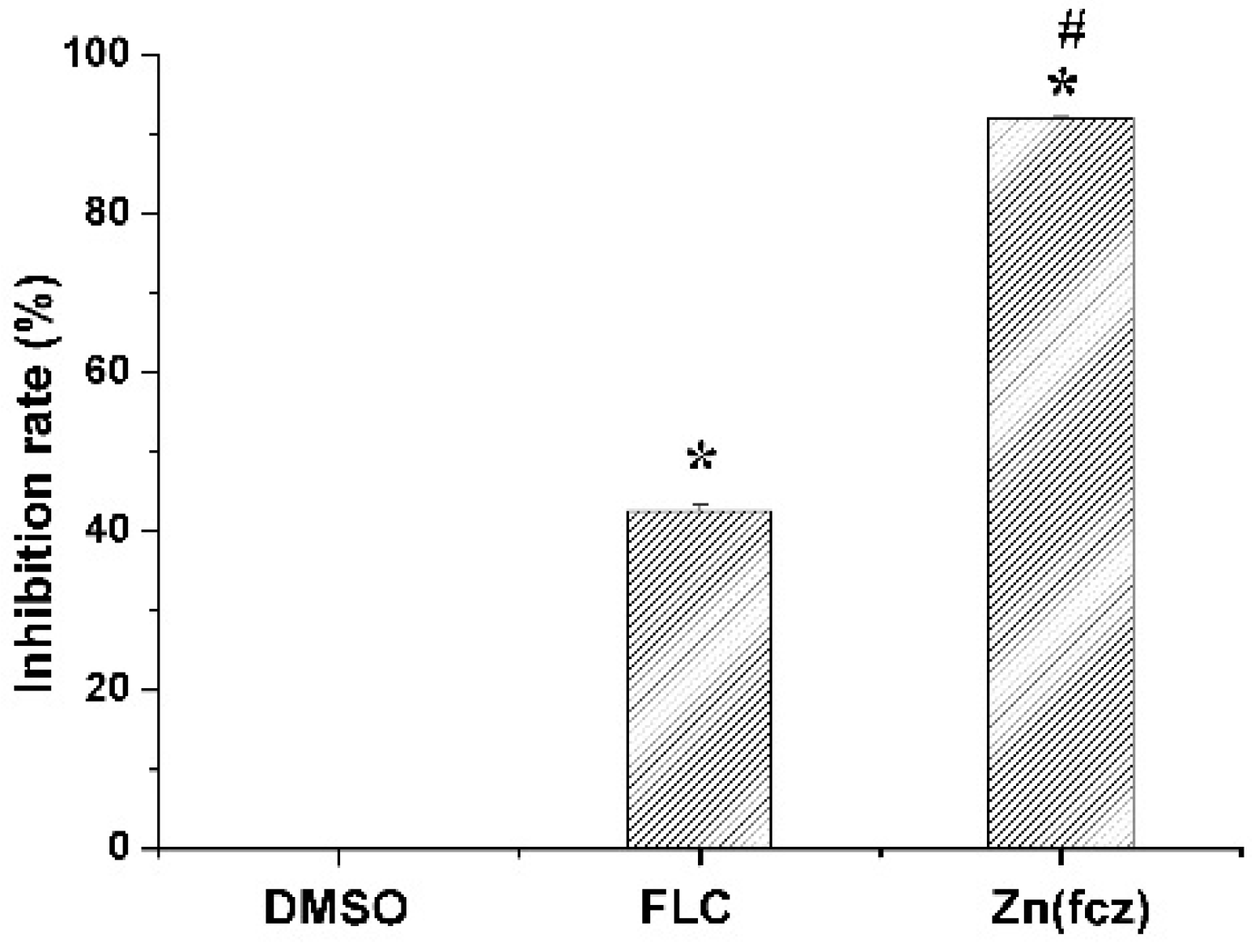
2.4. Inhibitory of ZnFLC on C. albicans HL973
2.5. Growth Inhibition Curves
2.6. Cell Living/Dead Analysis on HL973
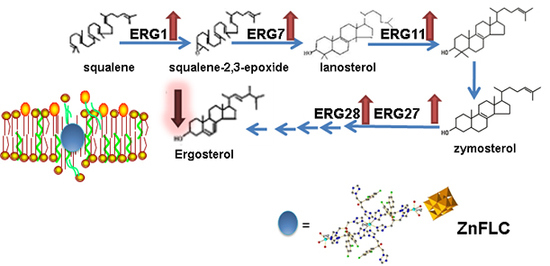
2.7. Assessment of Ergosterol Content
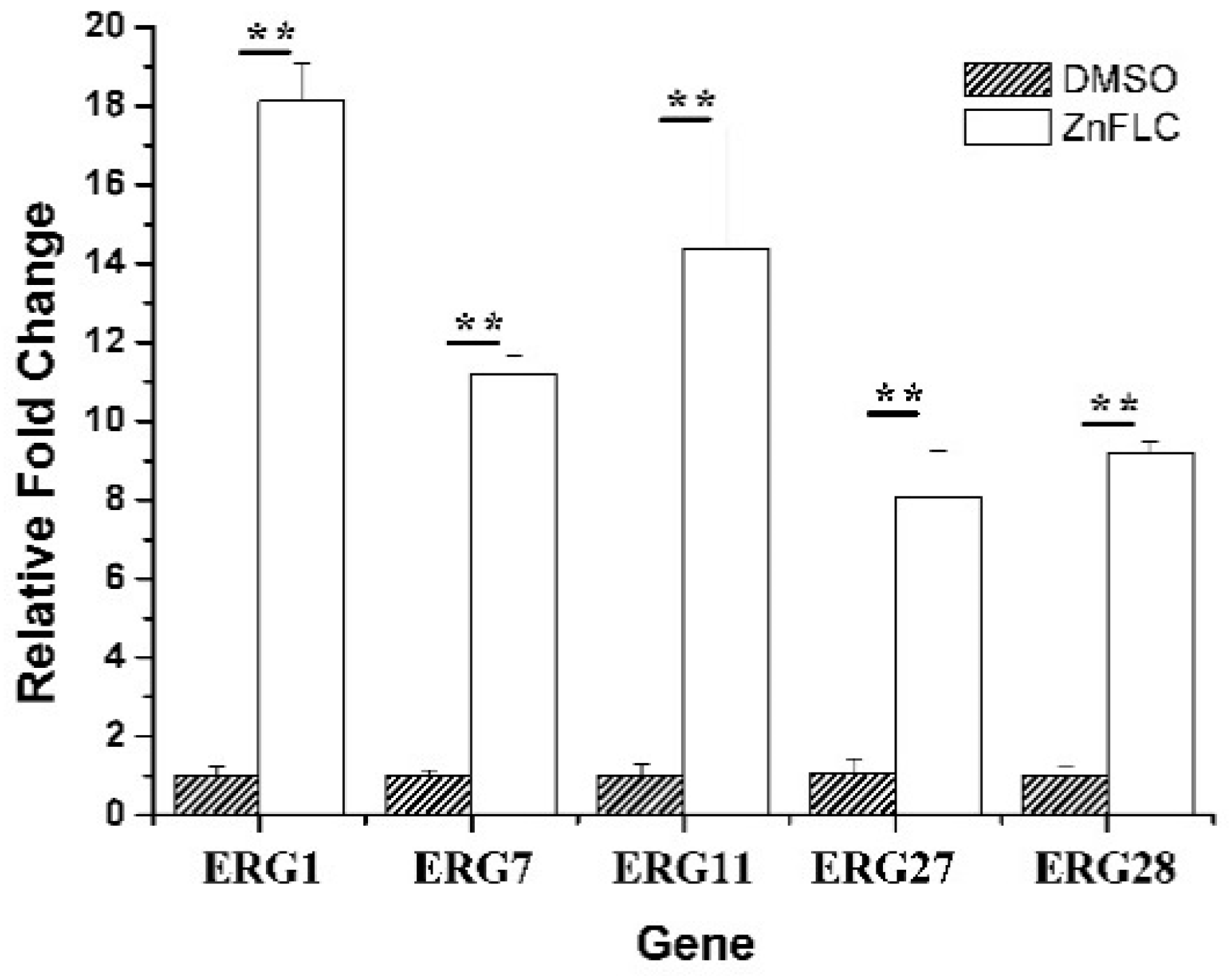
2.8. The Level of Ergosterol Biosynthesis Related Genes
3. Materials and Methods
3.1. Chemicals and Machines
3.2. Synthesis and Characterization of Zn3(FLZ)6V10O28·10H2O
3.3. X-ray Crystallography
3.4. Fungal Isolates and Culture Conditions
3.5. Determination of MIC of ZnFLC
3.6. MTS-Reduction Assay
3.7. Growth Inhibition Curves
3.8. AO/EB Double Staining
3.9. Assessment of Ergosterol Content
3.10. Real-Time PCR
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Morace, G.; Borghi, E. Fungal infections in ICU patients: Epidemiology and the role of diagnostics. Minerva Anestesiol. 2010, 76, 950–956. [Google Scholar] [PubMed]
- Yapar, N.; Pullu kcu, H.; Avkan-Oguz, V.; Sayin-Kutlu, S.; Ertugrul, B.; Sacar, S.; Cetin, B.; Kaya, O. Evaluation of species distribution and risk factors of candidemia: A multicenter case-control study. Med. Mycol. 2011, 49, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Kett, D.H.; Azoulay, E.; Echeverria, P.M.; Vincent, J.L.; ICU EPI. Candida bloodstream infections in intensive care units: Analysis of the extended prevalence of infection in intensive care unit study. Crit. Care Med. 2011, 39, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Zirkel, J.; Klinker, H.; Kuhn, A.; Abele-Horn, M.; Tappe, D.; Turnwald, D.; Einsele, H.; Heinz, W.J. Epidemiology of Candida blood stream infections in patients with hematological malignancies or solid tumors. Med. Mycol. 2012, 50, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, G.; Milillo, L.; Marinosci, F.; Lo Muzio, L.; Serpico, R.; Antonaci, S.; Giannelli, G.; Milillo, L.; Marinosci, F.; Lo Muzio, L.; et al. Altered expression of integrins and basement membrane proteins in malignant and pre-malignant lesions of oral mucosa. J. Biol. Regul. Homeost. Agents 2001, 15, 375–380. [Google Scholar] [PubMed]
- Matthaiou, D.K.; Christodoulopoulou, T.; Dimopoulos, G. How to treat fungal infections in ICU patients. BMC Infect. Dis. 2015, 15, 205. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Biswas, D.; Kotwal, A.; Thakuria, B.; Kakati, B.; Chauhan, B.S.; Patras, A. Ibuprofen-mediated reversal of fluconazole resistance in clinical isolates of Candida. J. Clin. Diagn. Res. 2015, 9, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Crunkhorn, S. Fungal infection: Protecting from Candida albicans. Nat. Rev. Drug Discov. 2016, 15, 604. [Google Scholar] [CrossRef] [PubMed]
- Linnenberg, O.; Moors, M.; Sole-Daura, A.; Lopez, X.; Baumer, C.; Kentzinger, E.; Pyckhout-Hintzen, W.; Monakhov, K.Y. Molecular characteristics of a mixed-valence polyoxovanadate {VIV/V18O42} in solution and at the liquid–surface interface. J. Phys. Chem. C 2017, 121, 10419–10429. [Google Scholar] [CrossRef]
- Procissi, D.; Shastri, A.; Rousochatzakis, I.; Al Rifai, M.; Kogerler, P.; Luban, M.; Suh, B.J.; Borsa, F. Magnetic susceptibility and spin dynamics of a polyoxovanadate cluster: A proton NMR study of a model spin tetramer. Phys. Rev. B 2004, 69, 094436. [Google Scholar] [CrossRef]
- Palii, A.; Aldoshin, S.; Tsukerblat, B.; Borras-Almenar, J.J.; Clemente-Juan, J.M.; Cardona-Serra, S.; Coronado, E. Electric field generation and control of bipartite quantum entanglement between electronic spins in mixed valence polyoxovanadate [GeV14O40]8–. Inorg. Chem. 2017, 56, 9547–9554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Schmitt, W. From platonic templates to archimedean solids: Successive construction of nanoscopic {V16As8}, {V16As10}, {V20As8}, and {V24As8} polyoxovanadate cages. J. Am. Chem. Soc. 2011, 133, 11240–11248. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Tabussum, S.; Zheng, C. Mixed-metal oxide phases containing decavanadate clusters: Synthesis and crystal structures of {(H2O)2K-μ-(H2O)3-M(H2O)3}2[V10O28] (M=Co, Ni). J. Clust. Sci. 2001, 12, 583–594. [Google Scholar] [CrossRef]
- Hayashi, Y. Hetero and lacunary polyoxovanadate chemistry: Synthesis, reactivity and structural aspects. Coord. Chem. Rev. 2011, 255, 2270–2280. [Google Scholar] [CrossRef]
- Aureliano, M.; Crans, D.C. Decavanadate (V10O286−) and oxovanadates: Oxometalates with many biological activities. J. Inorg. Biochem. 2009, 103, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Costa Pessoa, J. Thirty years through vanadium chemistry. J. Inorg. Biochem. 2015, 147, 4–24. [Google Scholar] [CrossRef] [PubMed]
- Li, J.K.; Hu, C.W. Progress in polyoxovanadate chemistry. Chin. J. Inorg. Chem. 2015, 31, 1705–1725. [Google Scholar]
- Willsky, G.R. Vanadium in Biological Systems: Physiology and Biochemistry; Chasteen, N.D., Ed.; Kluwar Academic Publishers: London, UK, 1990; pp. 1–24. [Google Scholar]
- Fang, Y.; Hu, L.; Zhou, X.; Jaiseng, W.; Zhang, B.; Takami, T.; Kuno, T. A Genomewide Screen in Schizosaccharomyces pombe for Genes Affecting the Sensitivity of Antifungal Drugs That Target Ergosterol Biosynthesis. Antimicrob. Agents Chem. 2012, 56, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Sant, D.G.; Tupe, S.G.; Ramana, C.V.; Deshpande, M.V. Fungal cell membrane—Promising drug target for antifungal therapy. J. Appl. Microbiol. 2016, 121, 1498–1510. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gong, H.W.; Qi, Y.F.; Li, J.; Ji, X.F.; Sun, J.H.; Tian, R.; Bao, H.; Song, X.F.; Chen, Q.; et al. In vitro and in vivo antifungal activities and mechanism of heteropolytungstates against Candida species. Sci. Rep. UK 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Yamase, T.; Tajima, Y. Inhibitory effect of polyoxotungstates on the production of penicillin-binding proteins and β-lactamase against methicillin resistant Staphylococcus aureua. Biol. Pharm. Bull. 1999, 22, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Shiota, S.; Shimizu, M.; Sugiyama, J.; Morita, Y.; Mizushima, T.; Tsuchiya, T. Mechanisms of action of corilagin and tellimagrandin I that remarkably potentiate the activity of β-lactams against methicillin-resistant Staphylococcus aureus. Microbiol. Immunol. 2004, 48, 67–73. [Google Scholar] [CrossRef] [PubMed]
- De Backer, M.D.; Ilyina, T.; Ma, X.J.; Vandoninck, S.; Luyten, W.H.M.L.; Vanden Bossche, H. Genomic profiling of the response of Candida albicans to itraconazole treatment using a DNA microarray. Antimicrob. Agents Chem. 2001, 45, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Hoot, S.J.; Smith, A.R.; Brown, R.P.; White, T.C. An A643V amino acid substitution in Upc2p contributes to azole resistance in well-characterized clinical Isolates of Candida albicans. Antimicrob. Agents Chem. 2011, 55, 940–942. [Google Scholar] [CrossRef] [PubMed]
- Meletiadis, J.; Meis, J.F.G.M.; Mouton, J.W.; Donnelly, J.P.; Verweij, P.E. Diphenyl-2H-tetrazolium Bromide (MTT) methods of in vitro susceptibility testing of filamentous fungi and development of a new simplified method. J. Clin. Microbiol. 2000, 38, 2949–2954. [Google Scholar] [PubMed]
- Cong, L.; Liao, Y.; Yang, S.; Yang, R. In Vitro Activity of berberine alone and in combination with antifungal drugs against planktonic forms and biofilms of trichosporon asahii. Mycopathologia 2017, 182, 829–837. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound 1 are available from the authors. |


| Compound | ZnFLC |
|---|---|
| Formula | C78H72F12N36O44V10Zn3 |
| Formula weight | 3151.23 |
| T(K) | 300(2) |
| Crystal system, space group | Monoclinic, P21/c |
| Unit cell dimensions | a = 16.983(2) Å, α = 90° |
| b = 17.773(2) Å, β = 110.3 (4)° | |
| c = 20.033(3) Å, γ = 90° | |
| Volume (Å3) | 5670.3(1) |
| Z, ρcalcd (g cm−3) | 2, 1.846 |
| μ (mm−1) | 1.526 |
| F(000) | 3144 |
| Crystal size | 0.32 × 0.25 × 0.21 mm |
| Theta range for data collection | 2.29–25.08° |
| Limiting indices | −20 ≤ h ≤ 20, −21 ≤ k ≤ 21, −23 ≤ l ≤ 23 |
| Reflections collected/unique | 79,761/10,019 [Rint = 0.1083] |
| Completeness to θ = 25.08 | 99.6% |
| Max. and min. transmission | 0.726 and 0.639 |
| Data/restraints/parameters | 10,019/0/826 |
| Goodness-of-fit on F2 | 1.068 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0672, wR2 = 0.1777 |
| R indices (all data) | R1 = 0.1043, wR2 = 0.2253 |
| Largest diff. peak and hole | 1.357 and −0.767 e Å−3 |
| Strains | MIC80 | MIC50 | ||
|---|---|---|---|---|
| FCZ | ZnFLC | FCZ | ZnFLC | |
| C. albicans | ||||
| HL973 | 64 | 4 | 16 | 2 |
| HL963 | 64 | 32 | 4 | 1 |
| HL996 | 2 | 4 | 1 | 0.5 |
| HL27 | 2 | 1 | 1 | 0.5 |
| HL3929 | >256 | 128 | >256 | 64 |
| HL3973 | 16 | 8–16 | 8 | 4 |
| HL3863 | 16 | 8 | 4 | 0.5 |
| HL3084 | 16 | 32 | 4 | 8–16 |
| HL3961 | 4 | 2 | 1 | 0.5 |
| HL17034 | 8 | 16 | 4 | 4 |
| HL3916 | 64 | 64 | 8 | 16 |
| HL3974 | 16 | 4 | 0.5 | 0.5 |
| HL3970 | 16 | 32 | 0.5 | 2 |
| HL3968 | 32 | 8 | 4 | 1 |
| ATCC 90028 | 1 | 1 | 0.25 | 0.5 |
| C. glabrat | ||||
| HL981 | >256 | 64–128 | 128–256 | 32 |
| C. krusei | ||||
| HL946 | >256 | 64–128 | >256 | 32 |
| C. parapsilosis | ||||
| ATCC 22019 | 2 | 1 | 1 | 0.5 |
| C. tropicalis | ||||
| ATCC 750 | 4 | 8 | 4 | <4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Yang, W.; Zhao, M.; Tian, R.; Zhang, B.; Qi, Y. In Vitro Anticandidal Activity and Mechanism of a Polyoxovanadate Functionalized by Zn-Fluconazole Complexes. Molecules 2018, 23, 1122. https://doi.org/10.3390/molecules23051122
Guo S, Yang W, Zhao M, Tian R, Zhang B, Qi Y. In Vitro Anticandidal Activity and Mechanism of a Polyoxovanadate Functionalized by Zn-Fluconazole Complexes. Molecules. 2018; 23(5):1122. https://doi.org/10.3390/molecules23051122
Chicago/Turabian StyleGuo, Shuanli, Wei Yang, Mingming Zhao, Rui Tian, Boyu Zhang, and Yanfei Qi. 2018. "In Vitro Anticandidal Activity and Mechanism of a Polyoxovanadate Functionalized by Zn-Fluconazole Complexes" Molecules 23, no. 5: 1122. https://doi.org/10.3390/molecules23051122