Cold Priming Induced Tolerance to Subsequent Low Temperature Stress is Enhanced by Melatonin Application during Recovery in Wheat
Abstract
:1. Introduction
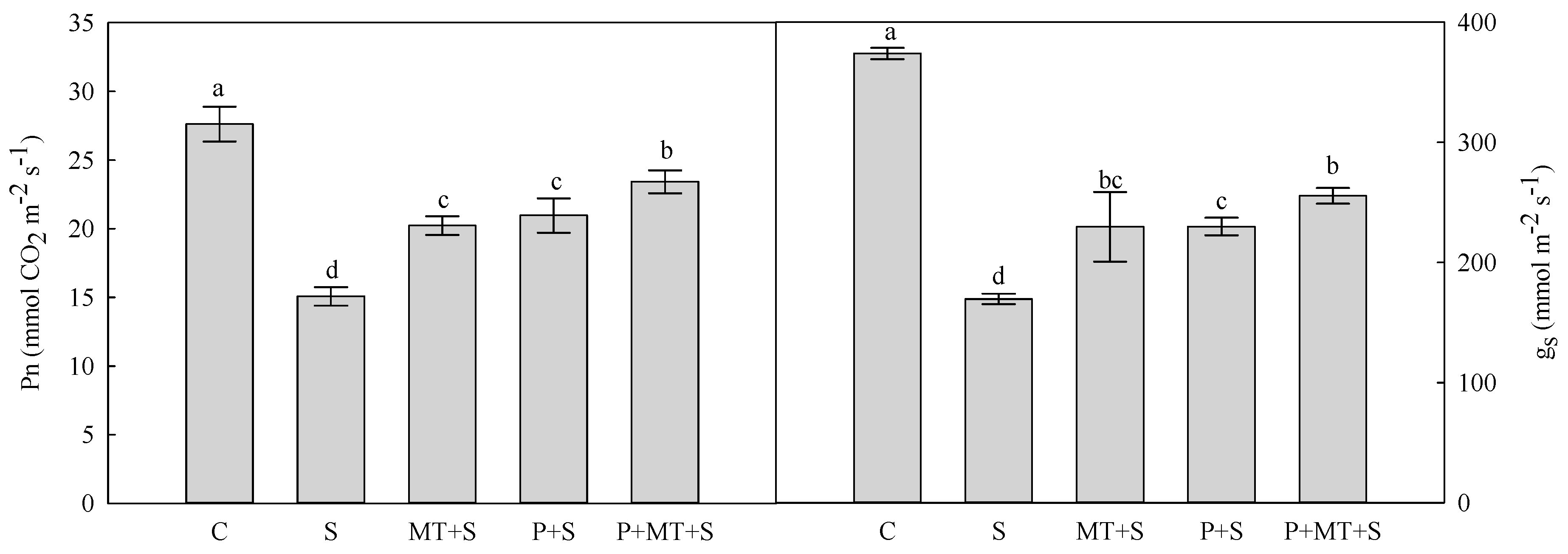
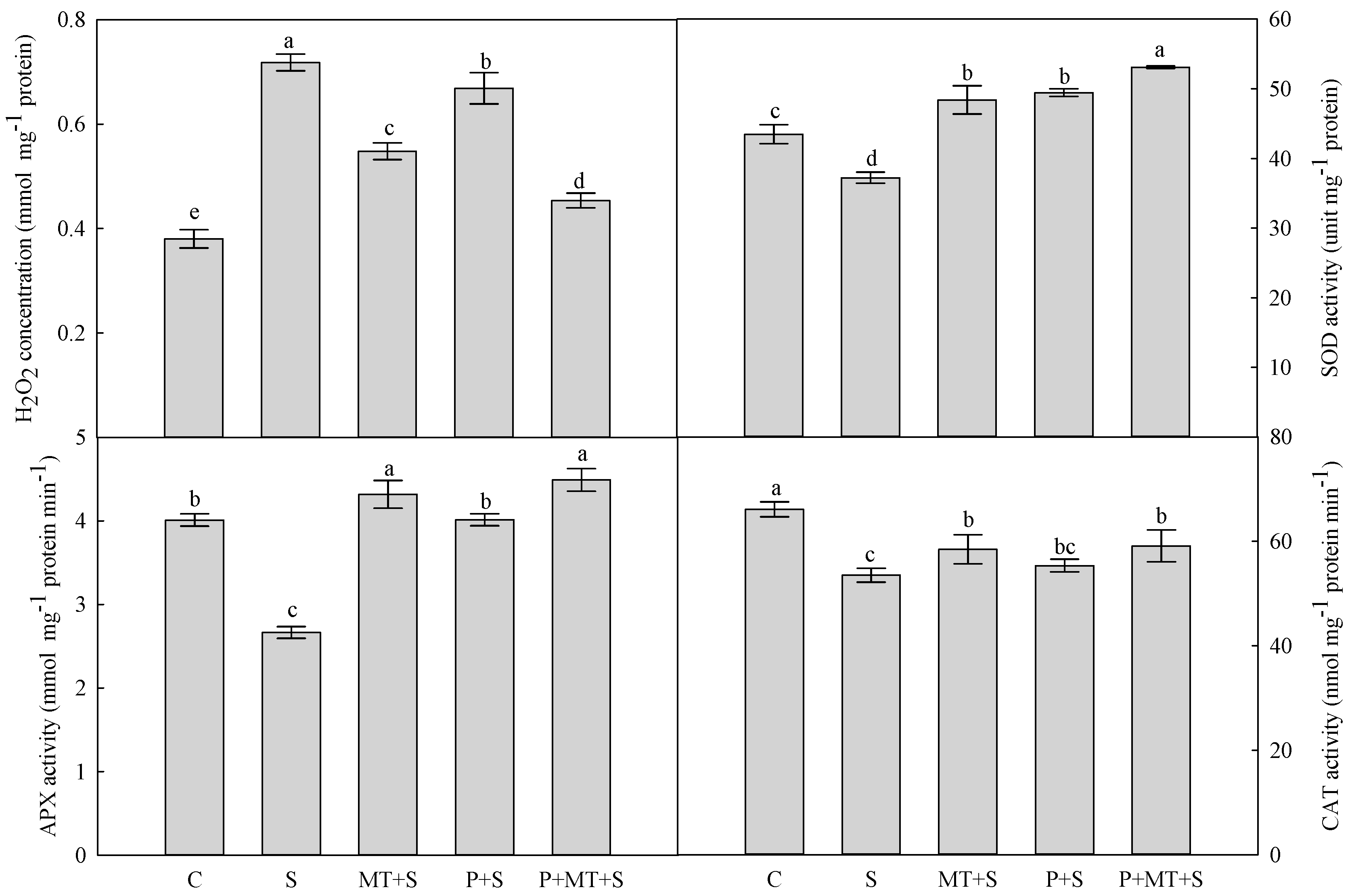
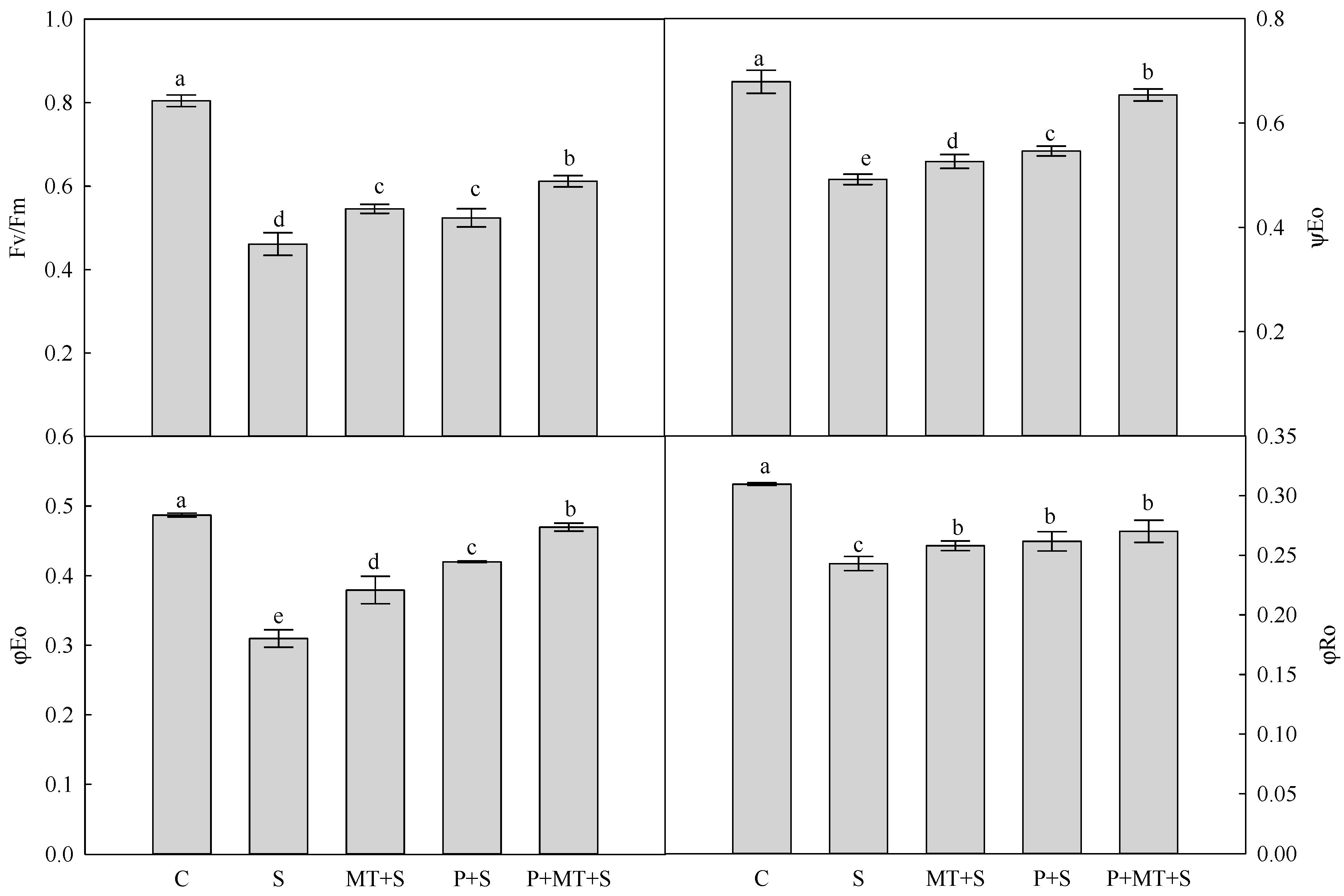
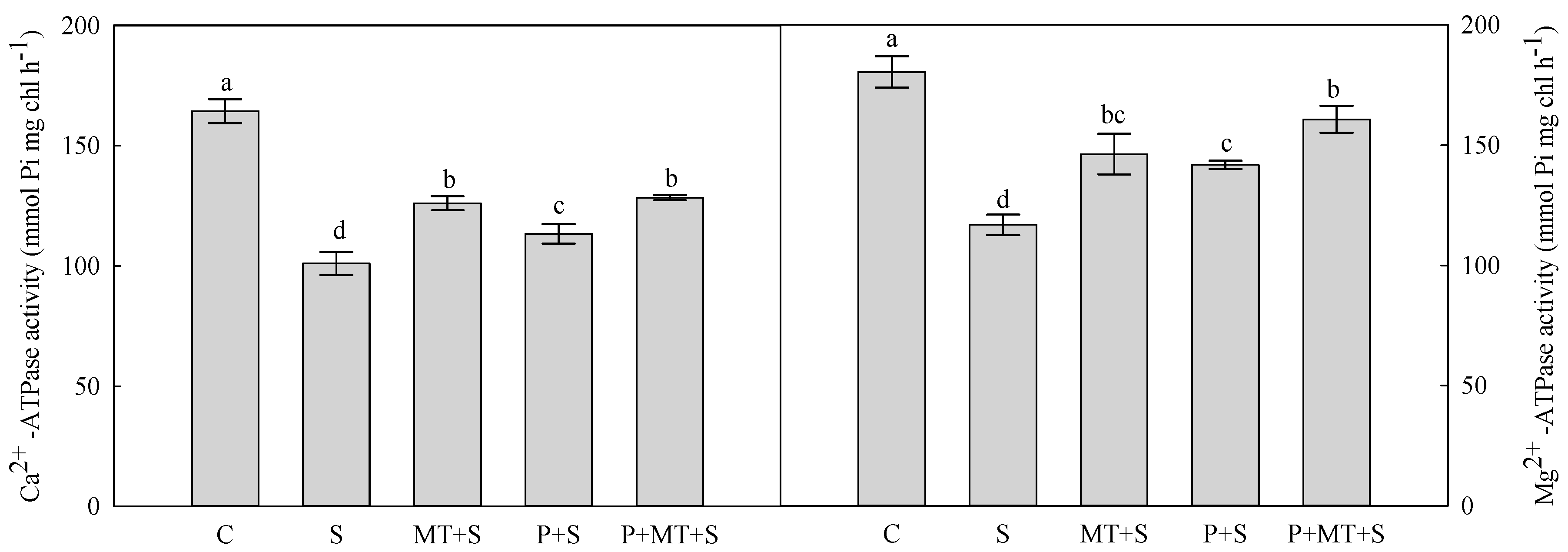
2. Results
3. Discussion
4. Materials and Methods
4.1. Experimental Setup
4.2. Gas Exchange and Chl a Fluorescence Transient
4.3. Membrane Stability Index
4.4. H2O2 Concentration and Antioxidant Enzyme Activity
4.5. RNA Extraction and qRT-PCR
4.6. Chloroplasts Isolation and ATPase Activity
4.7. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li, X.; Jiang, H.; Liu, F.; Cai, J.; Dai, T.; Cao, W.; Jiang, D. Induction of chilling tolerance in wheat during germination by pre-soaking seed with nitric oxide and gibberellin. Plant Growth Regul. 2013, 71, 31–40. [Google Scholar] [CrossRef]
- Shahryar, N.; Maali-Amiri, R. Metabolic acclimation of tetraploid and hexaploid wheats by cold stress-induced carbohydrate accumulation. J. Plant Physiol. 2016, 204, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hao, C.; Zhong, J.; Liu, F.; Cai, J.; Wang, X.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D. Mechano-stimulated modifications in the chloroplast antioxidant system and proteome changes are associated with cold response in wheat. BMC Plant Biol. 2015, 15, 219. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.-H.; Skinner, D.Z. Alteration of antioxidant enzyme gene expression during cold acclimation of near-isogenic wheat lines. Plant Sci. 2003, 165, 1221–1227. [Google Scholar] [CrossRef]
- Gusta, L.V.; Wisniewski, M. Understanding plant cold hardiness: An opinion. Physiol. Plant. 2013, 147, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Truong, T.T.; Barrero, J.M.; Jacobsen, J.V.; Hocart, C.H.; Gubler, F. A role for jasmonates in the release of dormancy by cold stratification in wheat. J. Exp. Bot. 2016, 67, 3497–3508. [Google Scholar] [CrossRef] [PubMed]
- Dahal, K.; Kane, K.; Sarhan, F.; Grodzinski, B.; Huner, N.P.A. Cold acclimation inhibits CO2-dependent stimulation of photosynthesis in spring wheat and spring rye. Bot. Bot. 2012, 90, 433–444. [Google Scholar] [CrossRef]
- Ruelland, E.; Vaultier, M.-N.; Zachowski, A.; Hurry, V. Chapter 2 Cold Signalling and Cold Acclimation in Plants, in Advances in Botanical Research; Jean-Claude, K., Michel, D., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 35–150. [Google Scholar]
- Siddiqui, K.S.; Cavicchioli, R. Cold-adapted enzymes. Annu. Rev. Biochem. 2006, 75, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Zhu, J.; Zhu, J.-K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Winfield, M.O.; Lu, C.; Wilson, I.D.; Coghill, J.A.; Edward, K.J. Plant responses to cold: Transcriptome analysis of wheat. Plant Biotechnol. J. 2010, 8, 749–771. [Google Scholar] [CrossRef] [PubMed]
- Cevtkovic, J.; Muller, K.; Baier, M. The effect of cold primng on the fitness of Arabidopsis thaliana accessions under natural and controlld conditios. Sci. Rep. 2017, 7, 44055. [Google Scholar] [CrossRef] [PubMed]
- Pomeroy, M.; Andrews, C.; Fedak, G. Cold hardening and dehardening responses in winter wheat and winter barley. Can. J. Plant Sci. 1975, 55, 529–535. [Google Scholar] [CrossRef]
- Li, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Jiang, D. Cold priming drives the sub-cellular antioxidant systems to protect photosynthetic electron transport against subsequent low temperature stress in winter wheat. Plant Physiol. Biochem. 2014, 82, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Brestic, M.; Tan, D.-X.; Zivcak, M.; Zhu, X.; Liu, S.; Song, F.; Reiter, R.J.; Liu, F. Melatonin alleviates low PS I limited carbon assimilation under elevated CO2 and enhances the cold tolerance of offspring in chlorophyll b-deficient mutant wheat. J. Pineal Res. 2018, 64, e12453. [Google Scholar] [CrossRef] [PubMed]
- Kobylińska, A.; Borek, S.; Posmyk, M.M. Melatonin redirects carbohydrates metabolism during sugar starvation in plant cells. J. Pineal Res. 2018. [Google Scholar] [CrossRef]
- Wang, L.; Feng, C.; Zheng, X.; Guo, Y.; Zhou, F.; Shan, D.; Liu, X.; Kong, J. Plant mitochondria synthesize melatonin and enhance the tolerance of plants to drought stress. J. Pineal Res. 2017, 63, e12429. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, H.Y.; Back, K. Rice histone deacetylase 10 and Arabidopsis histone deacetylase 14 genes encode N-acetylserotonin deacetylase, which catalyzes conversion of N-acetylserotonin into serotonin, a reverse reaction for melatonin biosynthesis in plants. J. Pineal Res. 2017, 64, e12460. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Choi, G.H.; Back, K. Cadmium-induced melatonin synthesis in rice requires light, hydrogen peroxide, and nitric oxide: Key regulatory roles for tryptophan decarboxylase and caffeic acid O-methyltransferase. J. Pineal Res. 2017, 63, e12441. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Back, K. Overexpression of rice serotonin N acetyltransferase 1 in transgenic rice plants confers resistance to cadmium and senescence and increases grain yield. J. Pineal Res. 2017, 62, e12392. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, J.-P.; Scott, E.; Liu, J.-W.; Guo, S.; Li, Y.; Zhang, L.; Han, W.-Y. Exogenous melatonin alleviates cold stress by promoting antioxidant defense and redox homeostasis in Camellia sinensis L. Molecules 2018, 23, 165. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.-H.; Huang, B.; Ding, C.-B.; Zhang, Z.-W.; Chen, Y.-E.; Hu, C.; Zhou, L.-J.; Huang, Y.; Liao, J.-Q.; Yuan, S.; et al. Effects of melatonin on anti-oxidative systems and photosystem II in cold-stressed rice seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Jarvis, A.J.; Mansfield, T.A.; Davies, W.J. Stomatal behaviour, photosynthesis and transpiration under rising CO2. Plant Cell Environ. 1999, 22, 639–648. [Google Scholar] [CrossRef]
- Sangwan, V.; Foulds, I.; Singh, J.; Dhindsa, R.S. Cold-activation of Brassica napus BN115 promoter is mediated by structural changes in membranes and cytoskeleton, and requires Ca2+ influx. Plant J. 2001, 1, 1–12. [Google Scholar] [CrossRef]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Liang, C.; Wang, G.-P.; Luo, Y.; Wang, W. The protection of wheat plasma membrane under cold stress by glycine betaine overproduction. Biol. Plant. 2010, 54, 83–88. [Google Scholar] [CrossRef]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.; Cuypers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. The Water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tan, D.X.; Jiang, D.; Liu, F. Melatonin enhances cold tolerance in drought-primed wild-type and abscisic acid-deficient mutant barley. J. Pineal Res. 2016, 61, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Brestic, M.; Zivcak, M.; Hauptvogel, P.; Misheva, S.; Kocheva, K.; Yang, X.; Li, X.; Allakhverdiev, S.I. Wheat plant selection for high yields entailed improvement of leaf anatomical and biochemical traits including tolerance to non-optimal temperature conditions. Photosynth. Res. 2018, 136, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Zivcak, M.; Bruckova, K.; Sytar, O.; Brestic, M.; Olsovska, K.; Allakhverdiev, S.I. Lettuce flavonoids screening and phenotyping by chlorophyll fluorescence excitation ratio. Planta 2017, 245, 1215–1229. [Google Scholar] [CrossRef] [PubMed]
- Brestic, M.; Zivcak, M.; Kunderlikova, K.; Allakhverdiev, S.I. High temperature specifically affects the photoprotective responses of chlorophyll b-deficient wheat mutant lines. Photosynth. Res. 2016, 130, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Brestic, M.; Zivcak, M.; Kunderlikova, K.; Sytar, O.; Shao, H.; Kalaji, H.M.; Allakhverdiev, S.I. Low PSI content limits the photoprotection of PSI and PSII in early growth stages of chlorophyll b-deficient wheat mutant lines. Photosynth. Res. 2015, 125, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Zivcak, M.; Kalaji, H.M.; Shao, H.-B.; Olsovska, K.; Brestic, M. Photosynthetic proton and electron transport in wheat leaves under prolonged moderate drought stress. J. Photochem. Photobiol. B Biol. 2014, 137, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Zivcak, M.; Brestic, M.; Balatova, Z.; Drevenakova, P.; Olsovska, K.; Kalaji, H.M.; Yang, X.; Allakhverdiev, S.I. Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. Photosynth. Res. 2013, 117, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Spoustová, P.; Synková, H.; Valcke, R.; Čeřovská, N. Chlorophyll a fluorescence as a tool for a study of the Potato virus Y effects on photosynthesis of nontransgenic and transgenic Pssu-ipt tobacco. Photosynthetica 2013, 51, 191–201. [Google Scholar] [CrossRef]
- Chen, S.; Yang, J.; Zhang, M.; Strasser, R.J.; Qiang, S. Classification and characteristics of heat tolerance in Ageratina adenophora populations using fast chlorophyll a fluorescence rise O-J-I-P. Environ. Exp. Bot. 2016, 122, 126–140. [Google Scholar] [CrossRef]
- Zuo, Z.; Sun, L.; Wang, T.; Miao, P.; Zhu, X.; Liu, S.; Song, F.; Mao, H.; Li, X. Melatonin improves the photosynthetic carbon assimilation and antioxidant capacity in wheat exposed to nano-ZnO stress. Molecules 2017, 22, 1727. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Jiang, D. Exogenous abscisic acid application during grain filling in winter wheat improves cold tolerance of offspring’s seedlings. J. Agron. Crop Sci. 2014, 200, 467–478. [Google Scholar] [CrossRef]
Sample Availability: Samples of the melatonins are available from the authors. |



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Li, X.; Wang, Z.; Sun, Z.; Zhu, X.; Liu, S.; Song, F.; Liu, F.; Wang, Y. Cold Priming Induced Tolerance to Subsequent Low Temperature Stress is Enhanced by Melatonin Application during Recovery in Wheat. Molecules 2018, 23, 1091. https://doi.org/10.3390/molecules23051091
Sun L, Li X, Wang Z, Sun Z, Zhu X, Liu S, Song F, Liu F, Wang Y. Cold Priming Induced Tolerance to Subsequent Low Temperature Stress is Enhanced by Melatonin Application during Recovery in Wheat. Molecules. 2018; 23(5):1091. https://doi.org/10.3390/molecules23051091
Chicago/Turabian StyleSun, Luying, Xiangnan Li, Zongshuai Wang, Zhongwei Sun, Xiancan Zhu, Shengqun Liu, Fengbin Song, Fulai Liu, and Yongjun Wang. 2018. "Cold Priming Induced Tolerance to Subsequent Low Temperature Stress is Enhanced by Melatonin Application during Recovery in Wheat" Molecules 23, no. 5: 1091. https://doi.org/10.3390/molecules23051091





