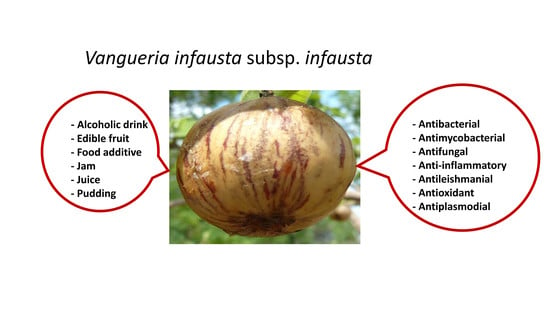
Nutraceutical and Ethnopharmacological Properties of Vangueria infausta subsp. infausta
Abstract
:1. Introduction
2. Materials and Methods
3. Medicinal Uses
4. Food Value
5. Phytochemical Constituents and Nutritional Composition of Vangueria infausta Subsp. infausta
6. Pharmacological Activities of Vangueria infausta subsp. infausta
6.1. Antibacterial Activity
6.2. Antimycobacterial Activity
6.3. Antifungal Activity
6.4. Anti-Inflammatory Activity
6.5. Antileishmanial Activity
6.6. Antioxidant Activity
6.7. Antiplasmodial Activity
6.8. Antifeedant Activity
6.9. Prostaglandin Synthesis Inhibitory Activity
6.10. Cytotoxicity Activity
6.11. Toxicity Activity
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bridson, D.M. Rubiaceae. In Flora Zambesiaca; Pope, G.V., Ed.; Royal Botanic Gardens: Kew, London, UK, 1998; Volume 5, pp. 249–250. [Google Scholar]
- Hyde, M.A.; Wursten, B.T.; Ballings, P.; Coates Palgrave, M. Flora of Zimbabwe: Species information: Vangueria infausta subsp. infausta. Available online: https://www.zimbabweflora.co.zw/speciesdata/species.php?species_id=155720 (accessed on 13 April 2018).
- Coates Palgrave, M. Keith Coates Palgrave Trees of Southern Africa; Struik Publishers: Cape Town, South Africa, 2002. [Google Scholar]
- Bremer, B. A review of molecular phylogenetic studies of Rubiaceae. Ann. MO Bot. Gard. 2009, 96, 4–26. [Google Scholar] [CrossRef]
- Lantz, H.; Bremer, B. Phylogeny of the complex Vanguerieae (Rubiaceae) genera Fadogia, Rytigynia, and Vangueria with close relatives and a new circumscription of Vangueria. Plant Syst. Evol. 2005, 253, 159–183. [Google Scholar] [CrossRef]
- Palmer, E.; Pitman, P. Trees of Southern Africa Covering all Known Indigenous Species in the Republic of South Africa, South West Africa, Botswana, Lesotho and Swaziland; A.A. Balkema: Cape Town, South Africa, 1972. [Google Scholar]
- Schmidt, E.; Lotter, M.; McCleland, W. Trees and Shrubs of Mpumalanga and Kruger National Park; Jacana Publishers: Johannesburg, South Africa, 2002. [Google Scholar]
- Behr, K. Vanguria infausta Burch subsp. infausta; National Botanical Garden: Pretoria, South Africa, 2004; Available online: http://pza.sanbi.org/vangueria-infausta. (accessed on 11 December 2017).
- Setshogo, M.P.; Venter, F. Trees of Botswana: Names and Distribution; Southern African Botanical Diversity Network Report No. 18; Southern African Botanical Diversity Network: Pretoria, South Africa, 2003. [Google Scholar]
- Motlhanka, D.M.T.; Motlhanka, P.; Selebatso, T. Edible indigenous wild fruit plants of eastern Botswana. Int. J. Poultry Sci. 2008, 7, 457–460. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Gilbreath, G.G.; Solio, J.; Lutura, M.; Lutuluo, R.; Kunguru, K.; Wood, N.; Mathenge, S.G. Plant use of the Maasai of Sekenani valley, Maasai Mara, Kenya. J. Ethnobiol. Ethnomed. 2006, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Gakuubi, M.M.; Wanzala, W. A survey of plants and plant products traditionally used in livestock health management in Buuri district, Meru County, Kenya. J. Ethnobiol. Ethnomed. 2012, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, P.; Morganti, M.; Mancini, M.; Signorini, M.A. Traditional healers and laypeople: A qualitative and quantitative approach to local knowledge on medicinal plants in Muda (Mozambique). J. Ethnopharmacol. 2011, 138, 543–563. [Google Scholar] [CrossRef] [PubMed]
- Ráice, R.T. Aroma Components in Vangueria infausta L.: Characterization of Components Using GC-MS and Aroma Loss During Drying. Ph.D. Thesis, Lund University, Lund, Sweden, 2014. [Google Scholar]
- Raice, R.T.; Sjoholm, I.; Francisco, J.C.; Bergenstahl, B. Identification of volatile components isolated from indigenous fruits of Mozambique, maphilwa (Vangueria infausta). Procedia Food Sci. 2011, 1, 404–407. [Google Scholar] [CrossRef]
- Magaia, T.; Uamusse, A.; Sjoholm, I.; Skoog, K. Proximate analysis of wild fruits of Mozambique. Sci. World J. 2013, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Raice, R.T.; Sjoholm, I.; Wang, H.-I.; Bergenståhl, B. Characterization of volatile components extracted from Vangueria infausta (African medlar) by using GC–MS. J. Essent. Oil Res. 2015, 27, 76–81. [Google Scholar] [CrossRef]
- Cheikhyoussef, A.; Shapi, M.; Matengu, K.; Ashekele, H.M. Ethnobotanical study of indigenous knowledge on medicinal plant use by traditional healers in Oshikoto region, Namibia. J. Ethnobiol. Ethnomed. 2011, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Cheikhyoussef, A.; Mapaure, I.; Shapi, M. The use of some indigenous plants for medicinal and other purposes by local communities in Namibia with emphasis on Oshikoto region: A review. Res. J. Med. Plant 2011, 5, 406–419. [Google Scholar]
- Maroyi, A.; Cheikhyoussef, A. A comparative study of medicinal plants used in rural areas of Namibia and Zimbabwe. Indian J. Trad. Knowl. 2015, 14, 401–406. [Google Scholar]
- Williams, V.L. Hawkers of Health: An Investigation of the Faraday Street Traditional Medicine Market in Johannesburg, Gauteng; Directorate for Nature Conservation, DACEL: Johannesburg, South Africa, 2003; Available online: http://wiredspace.wits.ac.za/bitstream/handle/10539/5313/Faraday%20Report.pdf (accessed on 16 April 2018).
- Van Wyk, B.-E. The potential of South African plants in the development of new food and beverage products. S. Afr. J. Bot. 2011, 77, 857–868. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; Gericke, N. People’s Plants: A Guide to Useful Plants of Southern Africa; Briza Publications: Pretoria, South Africa, 2000. [Google Scholar]
- Corrigan, B.M.; Van Wyk, B.-E.; Geldenhuys, C.J.; Jardine, J.M. Ethnobotanical plant uses in the KwaNibela Peninsula, St Lucia, South Africa. S. Afr. J. Bot. 2011, 77, 346–359. [Google Scholar] [CrossRef]
- Mthethwa, N.S.; Oyedeji, B.A.O.; Obi, L.C.; Aiyegoro, O.A. Anti-staphylococcal, anti-HIV and cytotoxicity studies of four South African medicinal plants and isolation of bioactive compounds from Cassine transvaalensis (Burtt. Davy) codd. BMC Complement. Altern. Med. 2014, 14, 512. [Google Scholar] [CrossRef] [PubMed]
- Chauke, M.A.; Shai, L.J.; Mogale, M.A.; Tshisikhawe, M.P.; Mokgotho, M.P. Medicinal plant use of villagers in the Mopani district, Limpopo province, South Africa. Afr. J. Trad. Complement. Altern. Med. 2015, 12, 9–26. [Google Scholar] [CrossRef]
- Masevhe, N.A.; McGaw, L.J.; Eloff, J.N. The traditional use of plants to manage candidiasis and related infections in Venda, South Africa. J. Ethnopharmacol. 2015, 168, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Chinsamy, M.; Koitsiwe, M. Traditional knowledge of medicinal and food plant uses for sustainable community livelihoods: A case of Batswana communities in South Africa. J. Soc. Sci. 2016, 46, 146–154. [Google Scholar] [CrossRef]
- Tshikalange, T.E.; Mophuting, B.C.; Mahore, J.; Winterboer, S.; Lall, N. An ethnobotanical study of medicinal plants used in villages under Jongilanga Tribal Council, Mpumalanga, South Africa. Afr. J. Trad. Complement. Altern. Med. 2016, 13, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Mokganya, M.G.; Tshisikhawe, M.P.; Swelankomo, N.; Tshivhandekano, T.R.; Ramovha, L.I.; Masevhe, N.A.; Ligavha-Mbelengwa, M.H.; Mocheki, T.A. An evaluation of additional uses of some wild edible fruit plants of the Vhembe District Municipality in the Limpopo province, South Africa. Indian J. Trad. Knowl. 2018, 17, 276–281. [Google Scholar]
- Long, C. Swaziland’s Flora: Siswati Names and Uses; Swaziland National Trust Commission: Mbambane, Swaziland, 2005; Available online: http://www.sntc.org.sz/index.asp (accessed on 11 December 2017).
- Amusan, O.O.G.; Sukati, N.A.; Dlamini, P.S.; Sibandze, F.G. Some Swazi phytomedicines and their constituents. Afr. J. Biotechnol. 2007, 6, 267–272. [Google Scholar]
- Chhabra, S.C.; Mahunnah, R.L.A.; Mshiu, E.N. Plants used in traditional medicine in Eastern Tanzania. V. Angiosperms (Passifloraceae to Sapindaceae). J. Ethnopharmacol. 1991, 33, 143–157. [Google Scholar] [CrossRef]
- De Boer, H.J.; Kool, A.; Broberg, A.; Mziray, W.R.; Hedberg, I.; Levenfors, J.J. Antifungal and anti-bacterial activity of some herbal remedies from Tanzania. J. Ethnopharmacol. 2005, 96, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Amri, E.; Kisangau, D.P. Ethnomedicinal study of plants used in villages around Kimboza forest reserve in Morogoro, Tanzania. J. Ethnobiol. Ethnomed. 2012, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Nondo, R.S.O.; Zofou, D.; Moshi, M.J.; Erasto, P.; Wanji, S.; Ngemenya, M.N.; Titanji, V.P.K.; Kidukuli, A.W.; Masimba, P.J. Ethnobotanical survey and in vitro antiplasmodial activity of medicinal plants used to treat malaria in Kagera and Lindi regions, Tanzania. J. Med. Plants Res. 2015, 9, 179–192. [Google Scholar]
- Gelfand, M.; Drummond, R.B.; Mavi, S.; Ndemera, B. The Traditional Medical Practitioner in Zimbabwe: His Principles of Practice and Pharmacopoeia; Mambo Press: Gweru, Zimbabwe, 1985. [Google Scholar]
- Maroyi, A. Ethnobotanical study of medicinal plants used by people in Nhema communal area, Zimbabwe. J. Ethnopharmacol. 2011, 136, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Munodawafa, T. Screening of some Traditional Medicinal Plants from Zimbabwe for Biological and Anti-microbial Activity. Master’s Thesis, University of Zimbabwe, Harare, Zimbabwe, 2012. [Google Scholar]
- Pooley, E. Trees of Natal, Zululand and the Transkei; Natal Flora Publications Trust: Durban, South Africa, 1993. [Google Scholar]
- Ruffo, C.K.; Birnie, A.; Tengäs, B. Edible Wild Plants of Tanzania; RELMA Technical Handbook Series 27; Regional Land Management Unit (RELMA): Nairobi, Kenya, 2002. [Google Scholar]
- Siangulube, F.S. Local Vegetation Use and Traditional Conservation Practices in the Zambian Rural Community: Implications on Forest Stability. Master’s Thesis, Uppsala University, Uppsala, Sweden, 2007. [Google Scholar]
- Williams, V.L.; Balkwill, K.; Witkowski, E.T.F. A lexicon of plants traded in the Witwatersrand umuthi shops, South Africa. Bothalia 2001, 31, 71–98. [Google Scholar] [CrossRef]
- Okole, B.N.; Odhav, B. Commercialisation of plants in Africa. S. Afr. J. Bot. 2004, 70, 109–115. [Google Scholar] [CrossRef]
- Mosina, G.K.E.; Maroyi, A.; Potgieter, M.J. Comparative analysis of plant use in peri-urban domestic gardens of the Limpopo Province, South Africa. J. Ethnobiol. Ethnomed. 2014, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Tairo, V.E. Utilization of Woody Plants During Times of Food Scarcity from Selected Drylands of Iringa District, Tanzania. Ph.D. Thesis, University of Nairobi, Nairobi, Kenya, 2011. [Google Scholar]
- Pakia, M. Plant Ecology and Ethnobotany of Two Sacred Forests (Kayas) at the Kenya Coast. Master’s Thesis, University of Natal, Durban, South Africa, 2000. [Google Scholar]
- Hedberg, I.; Staugård, F. Traditional Medicinal Plants: Traditional Medicine in Botswana; Ipeleng Publishers: Gaborone, Botswana, 1989. [Google Scholar]
- Morobe, I.C.; Mthethwa, N.S.; Bisi-Johnson, M.A.; Vasaikar, S.D.; Obi, C.L.; Oyedeji, A.O.; Kambizi, L.; Eloff, J.N.; Hattori, T. Cytotoxic effects and safety profiles of extracts of active medicinal plants from South Africa. J. Microbiol. Res. 2012, 2, 176–182. [Google Scholar]
- Arnold, H.-J.; Gulumian, M. Pharmacopoeia of traditional medicine in Venda. J. Ethnopharmacol. 1984, 12, 35–74. [Google Scholar] [CrossRef]
- Augustino, S.; Gillah, P.R. Medicinal plants in urban districts of Tanzania: Plants, gender roles and sustainable use. Int. For. Rev. 2005, 7, 44–58. [Google Scholar] [CrossRef]
- Hamisy, W.C.; Mwaseba, D.; Zilihona, I.E.; Mwihomeke, S.T. Status and Domestication Potential of Medicinal Plants in the Uluguru Mountain Area, Tanzania; Wildlife Conservation Society of Tanzania (WCST): Morogoro, Tanzania, 2000. [Google Scholar]
- Von Koenen, E. Medicinal, Poisonous and Edible Plants in Namibia; Klaus Hess Publishers: Windhoek, Namibia, 2001. [Google Scholar]
- Nafuka, S.N.; Mumbengegwi, D.R. Phytochemical analysis and in vitro anti-plasmodial activity of selected ethnomedicinal plants used to treat malaria associated symptoms in northern Namibia. Int. Sci. Technol. J. Namib. 2013, 2, 78–93. [Google Scholar]
- Morris, B. Chewa Medical Botany: A Study of Herbalism in Southern Malawi; International African Institute, Lit Verlag: Hamburg, Germany, 1996. [Google Scholar]
- Venter, A.; Venter, J.A. Making the Most of Indigenous Trees; Briza Publishers: Pretoria, South Africa, 1996. [Google Scholar]
- Fowler, D.G. Traditional Fever Remedies: A List of Zambian Plants; Kew Publishing: Royal Botanic Gardens, Kew, UK, 2011; Available online: http://www. giftshealth.org/ritam/news/Traditional_Fever_remedies 1.pdf (accessed on 11 January 2018).
- Bapela, M.J.; Meyer, J.J.M.; Kaiser, M. In vitro antiplasmodial screening of ethnopharmacologically selected South African plant species used for the treatment of malaria. J. Ethnopharmacol. 2014, 156, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Chinsembu, K.C.; Hedimbi, M. An ethnobotanical survey of plants used to manage HIV/AIDS opportunistic infections in Katima Mulilo, Caprivi region, Namibia. J. Ethnobiol. Ethnomed. 2010, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Amusan, O.O.G. Herbal medicine in Swaziland: An overview. In African Natural Plant Products: New Discoveries and Challenges in Chemistry and Quality; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2010; Volume 1021, pp. 31–49. [Google Scholar]
- McGaw, L.J.; Lall, N.; Meyer, J.J.M.; Eloff, J.N. The potential of South African plants against Mycobacterium infections. J. Ethnopharmacol. 2008, 119, 482–500. [Google Scholar] [CrossRef] [PubMed]
- De Wet, H.; Nkwanyana, M.N.; van Vuuren, S.F. Medicinal plants used for the treatment of diarrhoea in northern Maputaland, KwaZulu-Natal Province, South Africa. J. Ethnopharmacol. 2010, 130, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Nkwanyana, M.N. An Ethnobotanical and Antidiarrhoeal Investigation of Plants Used Traditionally in the Maputaland Area Homesteads. Master’s Thesis, University of Zululand, KwaDlangezwa, South Africa, 2013. [Google Scholar]
- Van Vuuren, S.F.; Nkwanyana, M.N.; de Wet, H. Antimicrobial evaluation of plants used for the treatment of diarrhoea in a rural community in northern Maputaland, KwaZulu-Natal, South Africa. BMC Complement. Altern. Med. 2015, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Steel, B.; Behr, K. A small tree for rocky gardens: Vangueria infausta Wild Medlar. Veld Flora 1986, 72, 88–89. [Google Scholar]
- Bryant, A.T. Zulu Medicine and Medicine-men; C Struik Publishers: Cape Town, South Africa, 1966. [Google Scholar]
- Mabogo, E.E.N. The Ethnobotany of the Vhavenda. Master’s Thesis, University of Pretoria, Pretoria, South Africa, 1990. [Google Scholar]
- Pakia, M.; Cooke, J.A. The ethnobotany of the Midzichenda tribes of the coastal forest areas in Kenya: 2. Medicinal plant uses. S. Afr. J. Bot. 2003, 69, 382–395. [Google Scholar] [CrossRef]
- Watt, J.M.; Breyer-Brandwijk, M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa; E. and S. Livingstone: London, UK, 1962. [Google Scholar]
- Adeniji, K.O.; Amusan, O.O.G.; Dlamini, P.S.; Enow-Orock, E.G.; Gamedze, S.T.; Gbile, Z.O.; Langa, A.D.; Makhubu, L.P.; Mahunnah, R.L.A.; Mshana, R.N.; et al. Traditional Medicine and Pharmacopoeia: Contributions to Ethnobotanical and Floristic Studies in Swaziland; Organisation of African Unity/Scientific, Technical and Research Commission: Mbambane, Swaziland, 1998. [Google Scholar]
- Chilimampunga, F.H. Utilization of Indigenous Fruits by Rural Communities in Mwanza District, Malawi. Master’s Thesis, University of Stellenbosch, Cape Town, South Africa, 2002. [Google Scholar]
- Nundkumar, N.; Ojewole, J.A.O. Studies on the antiplasmodial properties of some South African medicinal plants used as antimalarial remedies in Zulu folk medicine. Methods Find. Exp. Clin. Pharmacol. 2002, 24, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Abosi, A.O.; Mbukwa, E.; Majinda, R.R.T.; Raseroka, B.H. Vangueria infausta root bark: In vivo and in vitro antiplasmodial activity. Br. J. Biomed. Sci. 2006, 63, 129–133. [Google Scholar] [CrossRef]
- Munodawafa, T.; Chagonda, L.S.; Moyo, S.R. Antimicrobial and phytochemical screening of some Zimbabwean medicinal plants. J. Biol. Act. Prod. Nat. 2013, 3, 323–330. [Google Scholar] [CrossRef]
- Chaves, S.K.M.; Feitosa, C.M.; Araújo, L.S. Alkaloids pharmacological activities: Prospects for the development of phytopharmaceuticals for neurodegenerative diseases. Curr. Pharm. Biotechnol. 2016, 17, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Malik, E.M.; Muller, C.E. Anthraquinones as pharmacological tools and drugs. Med. Res. Rev. 2016, 36, 705–748. [Google Scholar] [CrossRef] [PubMed]
- Srikrishna, D.; Godugu, C.; Dubey, P.K. A review on pharmacological properties of coumarins. Mini-Rev. Med. Chem. 2016, 18, 113–141. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Melzig, M.F.; Fuchs, H.; Weng, A. Chemistry and pharmacology of saponins: Special focus on cytotoxic properties. Botanics Target Ther. 2011, 1, 19–29. [Google Scholar]
- Xu, X.-H.; Li, T.; Fong, C.M.V.; Chen, X.; Chen, X.-J.; Wang, Y.-T.; Huang, M.-Q.; Lu, J.-J. Saponins from Chinese medicines as anticancer agents. Molecules 2016, 21, 1326. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Oishi, K.; Abe, H.; Masuoka, C.; Okawa, M.; Ikeda, T.; Nohara, T. New iridoid glucosides from the aerial parts of Verbena brasiliensis. Chem. Pharm. Bull. 2006, 54, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Gunther, S.; Patterson, R.E.; Kristal, A.R.; Stratton, K.L.; White, E. Demographic and health-related correlates of herbal and specialty supplement use. J. Am. Diet. Assoc. 2004, 104, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Saika, D.; Deka, S.C. Cereals: From staple food to nutraceuticals. Int. Food Res. J. 2011, 18, 21–30. [Google Scholar]
- Kaur, G.; Mukundan, S.; Wani, V.; Kumar, M.S. Nutraceuticals in the management and prevention of metabolic syndrome. Austin J. Pharmacol. Ther. 2015, 3, 1–6. [Google Scholar]
- Chivandi, E.; Mukonowenzou, N.; Nyakudya, T.; Erlwanger, K.H. Potential of indigenous fruit-bearing trees to curb malnutrition, improve household food security, income and community health in sub-Saharan Africa: A review. Food Res. Int. 2015, 76, 980–985. [Google Scholar] [CrossRef]
- Aganga, A.A.; Adogla-Bessa, T.; Omphile, U.J.; Tshireletso, K. Significance of browses in the nutrition of Tswana goats. Arch. Zootec. 2000, 49, 469–480. [Google Scholar]
- Amarteifio, J.O.; Mosase, M.O. The chemical composition of selected indigenous fruits of Botswana. J. Appl. Sci. Environ. Manag. 2006, 10, 43–47. [Google Scholar] [CrossRef]
- Saka, J.D.K.; Msonthi, J.D. Nutritional value of edible fruits of indigenous wild trees in Malawi. For. Ecol. Manag. 1994, 64, 245–248. [Google Scholar] [CrossRef]
- Tairo, V.E.; Njoka, J.T.; Lukhoba, W.C.; Lyaruu, H.V.M. Nutritive and anti-nutritive qualities of mostly preferred edible woody plants in selected drylands of Iringa district, Tanzania. Pak. J. Nutr. 2011, 10, 786–791. [Google Scholar]
- Mothapo, M.J.; Mafeo, T.P.; Mamphiswana, N.D. Physico-chemical properties and selected nutritional components of wild medlar (Vangueria infausta) fruit harvested at two harvesting times. World J. Dairy Food Sci. 2014, 9, 79–85. [Google Scholar]
- Würger, G.; McGaw, L.J.; Eloff, J.N. Tannin content of leaf extracts of 53 trees used traditionally to treat diarrhoea is an important criterion in selecting species for further work. S. Afr. J. Bot. 2014, 90, 114–117. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Cheung, H.T.; Daniels, P.J.L.; Lewis, K.G.; McGhie, J.F. Triterpenoids. Part XXVI. The triterpenoids of Vangueria tomentosa. J. Chem. Soc. 1962, 5163–5175. [Google Scholar] [CrossRef]
- Mbukwa, E.; Chacha, M.; Majinda, R.R.T. Phytochemical constituents of Vangueria infausta: Their radical scavenging and antimicrobial activities. Arkivoc 2007, 9, 104–112. [Google Scholar]
- Abeer, T. Flavonoidal content of Vangueria infausta extract grown in Egypt: Investigation of its antioxidant activity. Int. Res. J. Pharm. 2011, 2, 157–161. [Google Scholar]
- Bapela, M.J. NMR-based Metabolomic Study of Medicinal Plants Used Against Malaria and the Isolation of Bioactive Alkaloids. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2016. [Google Scholar]
- Masoko, P. Ethnobotanical study of some selected medicinal plants used by traditional healers in Limpopo province (South Africa). Am. J. Res. Commun. 2013, 1, 8–23. [Google Scholar]
- Shai, L.J.; Chauke, M.A.; Magano, S.R.; Mogale, A.M.; Eloff, J.N. Antibacterial activity of sixteen plant species from Phalaborwa, Limpopo province, South Africa. J. Med. Plants Res. 2013, 7, 1899–1906. [Google Scholar]
- Mmushi, T.J.; Masoko, P.; Mdee, L.K.; Mokgotho, M.P.; Mampuru, L.J.; Howard, R.L. Antimycobacterial evaluation of fifteen medicinal plants in South Africa. Afr. J. Trad. Complement. Altern. Med. 2010, 7, 34–39. [Google Scholar] [CrossRef]
- Aro, A.O.; Dzoyem, J.P.; Hlokwe, T.M.; Madoroba, E.; Eloff, J.N.; McGaw, L.J. Some South African Rubiaceae tree leaf extracts have antimycobacterial activity against pathogenic and non-pathogenic Mycobacterium Species. Phytother. Res. 2015, 29, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Mahlo, S.M.; McGaw, L.J.; Eloff, J.N. Antifungal activity of leaf extracts from South African trees against plant pathogens. Crop Prot. 2010, 29, 1529–1533. [Google Scholar] [CrossRef]
- Mahlo, S.M.; Chauke, H.R.; McGaw, L.J.; Eloff, J.N. Antioxidant and antifungal activity of selected medicinal plant extracts against phytopathogenic fungi. Afr. J. Trad. Complement. Altern. Med. 2016, 13, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Chowe, L. Anti-Inflammatory Activity of Crude Extracts, Flavonoids and Unsaponifiable Fraction of Selected Edible Wild Fruits from Zimbabwe. Master’s Thesis, Bindura University of Science Education, Bindura, Zimbabwe, 2017. [Google Scholar]
- Bapela, M.J.; Kaiser, M.; Meyer, J.J.M. Antileishmanial activity of selected South African plant species. S. Afr. J. Bot. 2017, 108, 342–345. [Google Scholar] [CrossRef]
- Weenen, H.; Nkunya, M.H.H.; Bray, D.H.; Mwasumbi, L.B.; Kinabo, L.S.; Kilimali, V.A.E.B. Antimalarial activity of Tanzanian medicinal plants. Planta Med. 1990, 56, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, C.; Maharaj, V.J.; Crouch, N.R.; Grace, O.M.; Pillay, P.; Matsabisa, M.G.; Bhagwandin, N.; Smith, P.J.; Folb, P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004, 92, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Kaoneka, B.S.K.; Mollel, M. Antifeedant activity of twenty six plant extracts and pure compounds from the root bark of Toddalia asiatica (L) (Rutaceae) against the anomalous emperor moth Nudaurelia belina (Saturnidae). Huria J. Open Univ. Tanzan. 2012, 12, 102–109. [Google Scholar]
- Lindsey, K.; Jäger, A.K.; Raidoo, D.M.; van Staden, J. Screening of plants used by southern African traditional healers in the treatment of dysmenorrhoea for prostaglandin-synthesis inhibitors and uterine relaxing activity. J. Ethnopharmacol. 1999, 64, 9–14. [Google Scholar] [CrossRef]
- Moshi, M.J.; Innocent, E.; Magadula, J.J.; Otieno, D.F.; Weisheit, A.; Mbabazi, P.K.; Nondo, R.S.O. Brine shrimp toxicity of some plants used as traditional medicines in Kagera Region, north western Tanzania. Tanzan. J. Health Res. 2010, 12, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Munodawafa, T.; Moyo, S.; Chipurura, B.; Chagonda, L. Brine shrimp lethality bioassay of some selected Zimbabwean traditional medicinal plants. Int. J. Phytopharmacol. 2016, 7, 229–232. [Google Scholar]


| Vernacular Name(s), Ethnic Group or Geographical Region in Brackets | Country | References |
|---|---|---|
| Wild meldlar (English), mmilo, monyonyana, mothwane, nzwigwa (Setswana) | Botswana | [9,10] |
| Mibiru (Kimîîru), orgomei (Maa) | Kenya | [11,12] |
| Mumzwiro (Chindau, Chitewe), African medlar, wild medlar (English), maphilwa, n’pfilwa (Ronga) | Mozambique | [13,14,15,16,17] |
| Omundjenja (Herero), oshimbu (Oshiwambo) | Namibia | [6,18,19,20] |
| Grootmispel, mispel, wildemispel (Afrikaans), velvet wild medlar, wild medlar (English), umbizo, umviyo (Ndebele), mmilo (Northern Sotho), mpfilwa (Shangaan, Tsonga), xinyathelo (Tsonga), amantulwane, umntuli, umntulwa, umvile (Swati), mmilo, mothwanyê (Tswana), mavelo, muzwilo, muzwilu (Venda), umvilo, umviyo (Xhosa), idulumuthwa, inkhabayomtwana, isantulu-tshwana, umfilwa, umgana, umsunuwengane, umtulwa, umviki, umviyo (Zulu) | South Africa | [6,7,8,21,22,23,24,25,26,27,28,29,30] |
| Wild medlar (English), imadnulu, imandulu, infahlo, infaylo, infaylo, infayo, limandvulo, mantulwa, mavelo, santudlevane, santulwan, santulwana, santulwane, umfilwa, umgana, umntudlwana, umntuli, umntulu, umntulwa, umvigo, umvile, umviyo (Siswati) | Swaziland | [31,32] |
| Amabungo, mtugunda (Kagera, Lindi), mviru (Kiluguru), mdaria (Kipare), msada, mvilu (Zigua) | Tanzania | [33,34,35,36] |
| False medlar, velvet wild medlar (English), umthofu, umviyo (Ndebele), mudzvirungombe, munjiro, munzviro, munzirwa, munzvirwa, mutsviru (Shona) | Zimbabwe | [2,20,37,38,39] |
| Use | Plant Parts Used | Country Practiced | References |
|---|---|---|---|
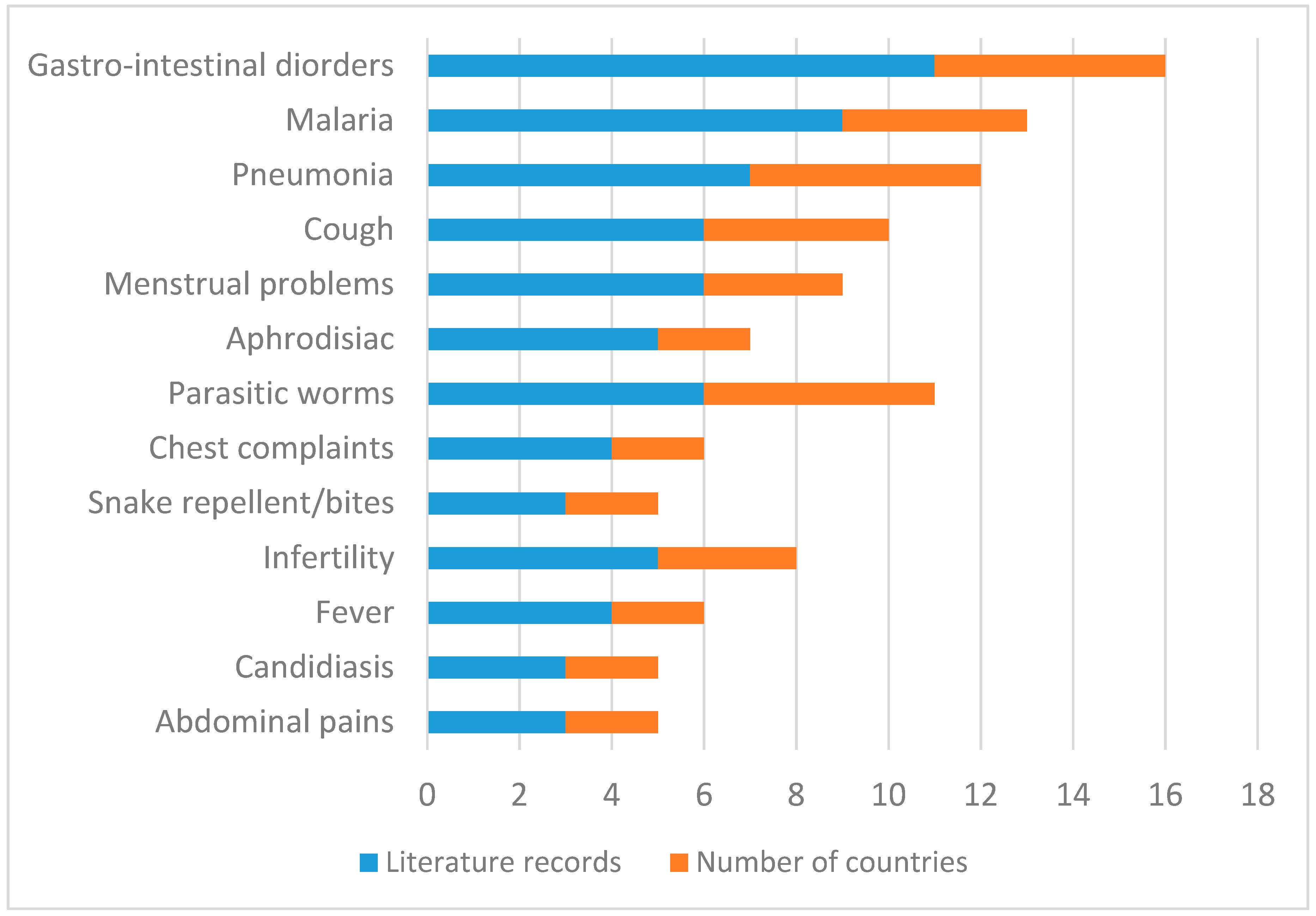
| Abdominal pains | Root infusion taken orally | Kenya, Zimbabwe | [37,39,47] |
| Abscesses | After abscess is drained, leaf infusion is applied topically | Malawi | [55] |
| Anaesthetic | Smoke from burnt roots inhailed mixed with roots of Bridelia micrantha (Hochst.) Baill. and Dichrostachys cinerea (L.) Wight & Arn. | South Africa | [7] |
| Aphrodisiac | Leaf, root decoction taken orally | South Africa, Tanzania | [6,24,33,40,50] |
| Asthma | Bark, leaf, root infusion taken orally | Malawi, Mozambique | [13,55] |
| Bewitchment | Root decoction taken orally | Tanzania | [51] |
| Blood pressure | Bark, leaf, root decoction taken orally | Tanzania | [51] |
| Bloody stool | Bark infusion taken orally | Swaziland | [31,70] |
| Chest pain | Bark, leaf, root decoction taken orally | South Africa, Swaziland | [24,32,40,60] |
| Cold | Leaf, root, shoots infusion taken orally | Namibia | [53,54] |
| Cough | Bark, leaf, root infusion taken orally | Malawi, Mozambique, Namibia, South Africa | [13,49,53,54,55,61] |
| Dermatitis | Leaf infusion applied topically | Namibia | [19,20] |
| Diabetes | Leaf, root decoction taken orally | Tanzania | [51] |
| Diarrhoea and stomach problems | Bark, leaf, root decoction taken orally | Malawi, Mozambique, South Africa, Tanzania, Zimbabwe | [13,20,37,38,39,51,52,55,62,64] |
| Epilepsy | Root decoction taken orally | Malawi | [55] |
| Fever | Leaf, root, shoots decoction taken orally | Namibia, Tanzania | [51,52,53,54] |
| Headache | Leaf, root, shoot decoction taken orally | Namibia | [53,54] |
| Hernia | Leaf, root decoction taken orally | Tanzania | [52] |
| Induce labour | Bark, leaf, root decoction taken orally | Mozambique | [13] |
| Infertility | Root decoction taken orally mixed with roots of Helinus integrifolius (Lam.) Kuntze | South Africa | [26] |
| Infertility | Bark, root decoction taken orally | Malawi, South Africa, Tanzania | [51,55,66,67] |
| Inflammation of umbilical cord | Root decoction taken orally | Zimbabwe | [37,39] |
| Malaria | Leaf, root decoction taken orally | Malawi, South Africa, Swaziland, Tanzania, Zimbabwe | [6,24,31,36,39,40,55,56,57,58] |
| Male virility | Root decoction taken orally | Tanzania | [52] |
| Measles | Root decoction applied topically | Malawi | [55] |
| Menstrual problems | Fruit, root, seed decoction taken orally | South Africa, Tanzania, Zimbabwe | [30,35,37,39,52,69] |
| Nervous system disorders | Root decoction taken orally | Malawi | [55] |
| Oral candidiasis or candidiasis | Bark, leaf, root decoction taken orally | Namibia, South Africa | [27,59] |
| Parasitic worms | Fruit, leaf, root decoction taken orally | Malawi, South Africa, Swaziland, Tanzania, Zimbabwe | [28,31,35,39,51,55] |
| Pleurisy | Leaf decoction taken orally | Malawi | [55] |
| Pneumonia | Leaf, root, seed decoction taken orally | South Africa, Swaziland, Tanzania, Zimbabwe | [6,28,31,35,39,52,56] |
| Pre-natal care | Root decoction taken orally | South Africa | [68] |
| Protective wash against sorcery | Leaf decoction taken orally | Malawi | [55] |
| Purgative | Leaf, root decoction taken orally | South Africa, Swaziland | [28,31] |
| Skin blisters | Bark, leaf, root decoction applied topically | Mozambique | [13] |
| Slow down heartbeat | Root decoction taken orally | Botswana, South Africa | [48,49] |
| Snake repellent or remedy for snake bites | Root decoction sprayed around homestead or applied topically on the place of the bite | Malawi, South Africa | [29,55] |
| Stomach ulcers | Root decoction decoction taken orally | Tanzania | [33] |
| Swellings | Leaf decoction applied topically | Malawi | [55] |
| Syphilis | Bark decoction taken orally | Tanzania | [51] |
| Toothache | Leaf decoction applied topically | South Africa | [28] |
| Virginal discharge | Root decoction applied topically | Zimbabwe | [37,39] |
| Caloric and Nutritional Composition | Values | Plant Parts | Recommended Dietary Allowance (RDA) | Reference |
|---|---|---|---|---|
| Acid detergent fibre (ADF) (%) | 19.0–39.5 | Fruits, seeds | - | [86,87] |
| Ash (g/100g dry matter) | 2.6–5.5 | Fruits, pulp, seeds | - | [16,86,87,88,89] |
| Ca (mg/100g) | 0.20–186.33 | Fruits, seeds | 1000–1300 | [16,86,87,88,89,90] |
| Carbohydrate (%) | 77.07–78.10 | Fruits | 45–65 | [88,89] |
| Crude fat (g/100g dry matter) | 0.5–7.0 | Fruits, pulp, seeds | 300 | [16,86,88] |
| Crude fibre (g/100g dry matter) | 10.20–10.29 | Fruits | 25–38 | [88,89] |
| Crude protein (g/100g dry matter) | 3.01–21.30 | Fruits, pulp, seeds | 34 | [16,86,88,89,90] |
| Cu (mg/100g) | 5.91–10.1 | Fruits, seeds | 1–2 | [86] |
| Dry matter (%) | 19.30–97.10 | Fruits, pulp, seeds | - | [16,86,87,88,89,90] |
| Energy value (Kj/100g | 1445 | Fruits | - | [88] |
| Fe (mg/100g) | 0.09–21.60 | Fruits, seeds | 8–15 | [86,87,88,89,90] |
| Fructose (g/100g) | 1.4 | Pulp | 130 | [16] |
| Glucose (g/100g) | 1.4 | Pulp | 130 | [16] |
| K (mg/100g) | 1.80–1683.00 | Fruits | 4700 | [87,88,89,90] |
| Mg (mg/100g) | 0.06–99.00 | Fruits, seeds | 310–320 | [86,87,88,89] |
| Mn (mg/100g) | 2.91–47.40 | Fruits, seeds | 5 | [86,89,90] |
| Moisture (%) | 4.16–80.70 | Fruits | - | [89,90] |
| N (mg/100g) | 0.90 | Fruits | - | [90] |
| Na (mg/100g) | 13.70–160.81 | Fruits | 2300 | [87,88,89] |
| Neutral detergent fibre (NDF) (%) | 12.40–39.40 | Fruits, seeds | - | [86,87] |
| P (mg/100g) | 3.50–86.86 | Fruits, seeds | 1250 | [16,86,87,88,89,90] |
| Phenolic content (gallic acid equivalent mg/mL) | 444. 07 | Aerial shoots | - | [54] |
| Sucrose (g/100g) | 2.7 | Pulp | 130 | [16] |
| Tannin (mg/mL gallic acid equivalent) | 4.08 | Leaf | - | [91] |
| Vitamin C (mg/100g) | 11.50–67.70 | Fruits | 46 | [87,89] |
| Zn (mg/100 g) | 0.02–0.16 | Fruits, seeds | 8–11 | [86,87] |
| Compound | Plant Part | Isolation and Identification Method | References |
|---|---|---|---|
| Biflavonoid | |||
| 5,7,3′,5″,7″,4′′′-hexahydroxy (4′-O-3′′′)-biflavone | Leaves, stem bark | ESI-MS, IR, NMR, UV | [93] |
| Fatty acid | |||
| Hexanoic acid (5600 µg/g of dry matter) | Fruits | GC-MS | [17] |
| Octanoic acid (240 µg/g of dry matter) | Fruits | GC-MS | [17] |
| Fatty acid ethyl and methyl esters | |||
| Ethyl hexanoate (44 µg/g of dry matter) | Fruits | GC-MS | [17] |
| Ethyl octanoate (13 µg/g of dry matter) | Fruits | GC-MS | [17] |
| Methyl hexanoate (15 µg/g of dry matter) | Fruits | GC-MS | [17] |
| Methyl octanoate (12 µg/g of dry matter) | Fruits | GC-MS | [17] |
| Flavonoids | |||
| Apigenin-7-O-rutinoside (22 mg/g of dry matter) | Leaves | NMR, UV | [94] |
| Daidzein | Leaves, stem bark | ESI-MS, IR, NMR, UV | [93] |
| Dihydrokaempferol | Leaves, stem bark | ESI-MS, IR, NMR, UV | [93] |
| Dihydroquercetin-3′-O-glucoside | Leaves, stem bark | ESI-MS, IR, NMR, UV | [93] |
| Epiafzelechin | Leaves, stem bark | ESI-MS, IR, NMR, UV | [93] |
| (−)-epicatechin | Leaves, stem bark | ESI-MS, IR, NMR, UV | [93] |
| Genistein | Leaves, stem bark | ESI-MS, IR, NMR, UV | [93] |
| Luteolin | Leaves, stem bark | ESI-MS, IR, NMR, UV | [93] |
| Luteolin-7-O-rutinoside (25 mg/g of dry matter) | Leaves | NMR, UV | [94] |
| Luteolin-4-O-glucoside (14 mg/g of dry matter) | Leaves | NMR, UV | [94] |
| Quercetin (10 mg/g of dry matter) | Leaves, stem bark | ESI-MS, IR, NMR, UV | [93,94] |
| Quercetin-3-O-glucoside (18 mg/g of dry matter) | Leaves | NMR, UV | [94] |
| Polyketide derivative | |||
| Methylcylohex-1-ene | Leaves, stem bark | ESI-MS, IR, NMR, UV | [93] |
| Iridoid lactone | |||
| Morindolide (4 mg/g of dry matter) | Roots | NMR, TLC | [95] |
| Pentacyclic triterpenoid | |||
| Friedelin (7 mg/g of dry matter) | Roots | NMR, TLC | [95] |
| Triterpenoid acids | |||
| Tomentosolic acid | Roots | TLC | [91] |
| Vanguerolic acid | Roots | TLC | [91] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maroyi, A. Nutraceutical and Ethnopharmacological Properties of Vangueria infausta subsp. infausta. Molecules 2018, 23, 1089. https://doi.org/10.3390/molecules23051089
Maroyi A. Nutraceutical and Ethnopharmacological Properties of Vangueria infausta subsp. infausta. Molecules. 2018; 23(5):1089. https://doi.org/10.3390/molecules23051089
Chicago/Turabian StyleMaroyi, Alfred. 2018. "Nutraceutical and Ethnopharmacological Properties of Vangueria infausta subsp. infausta" Molecules 23, no. 5: 1089. https://doi.org/10.3390/molecules23051089