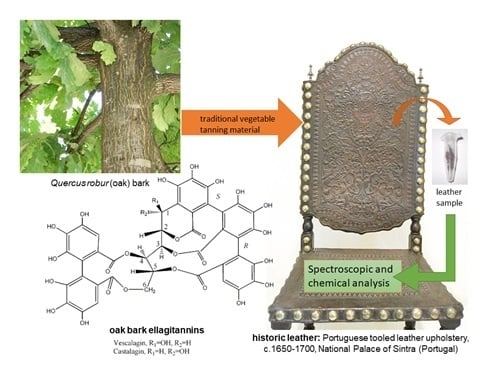
Vegetable Tannins Used in the Manufacture of Historic Leathers
Abstract
:1. Introduction
2. Vegetable Tanning Materials: The Sources of Tannins
2.1. Barks
2.2. Wood
2.3. Leaves
2.4. Fruits
2.5. Galls
3. Detection and Characterization of Tannins in Historic Vegetable Tanned Leathers
3.1. Colorimetric Tests
3.1.1. Ferric Test
3.1.2. Acidified Vanillin Test
3.1.3. Acidified Butanol Test
3.1.4. Nitrous Acid Test
3.1.5. Rhodanine Test
3.2. Spectroscopic Techniques
3.2.1. UV-Vis
3.2.2. FTIR
3.2.3. Other Spectroscopic Techniques: Fluorescence Spectroscopy and Solid State 13C-NMR
Funding
Acknowledgments
Conflicts of Interest
References
- Covington, A.D. Tanning Chemistry: The Science of Leather; Royal Society of Chemistry: Cambridge, UK, 2009; ISBN 978-1-84973-434-9. [Google Scholar]
- Forbes, R.J. Studies in Ancient Technology, 2nd ed.; Brill Publishers: Leiden, The Netherlands, 1966; Volume V. [Google Scholar]
- Reed, R. Ancient Skins, Parchments and Leathers; Seminar Press: London, UK; New York, NY, USA, 1972; ISBN 978-0-129-03550-3. [Google Scholar]
- Haslam, E. Vegetable tannage: Where do the tannins go? J. Soc. Leather Technol. Chem. 1997, 81, 45–51. [Google Scholar]
- Goffer, Z. Archaeological Chemistry, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 978-0-471-25288-7. [Google Scholar]
- Van Driel-Murray, C. Tanning and leather. In The Oxford Handbook of Engineering and Technology in the Classical World; Oleson, J.P., Ed.; Oxford University Press: New York, NY, USA, 2009; pp. 483–495. [Google Scholar]
- Chahine, C. Cuir et Parchemin ou la Métamorphose de la Peau; CNRS Éditions: Paris, France, 2013; ISBN 978-2-271-07686-1. [Google Scholar]
- Sharphouse, J.H. Leather Technician’s Handbook, 75th ed.; Leather Producers’ Association: Northampton, UK, 1995; ISBN 978-0950228518. [Google Scholar]
- Florian, M.-L. Protein Facts: Fibrous Proteins in Cultural Artifacts; Archetype Publications: London, UK, 2008; ISBN 978-1873132340. [Google Scholar]
- Thomson, R. Skin, leather and tanning: Some definitions. In Leather Tanneries: The Archaeological Evidence; Thomson, R., Mould, Q., Eds.; Archetype Publications—Archaeological Leather Group: London, UK, 2011; pp. 3–7. ISBN 978-1904982616. [Google Scholar]
- Seymour-Jones, F.L. The beginnings of leather chemistry. J. Chem. Educ. 1927, 4, 831–835. [Google Scholar] [CrossRef]
- Azéma, J.-P. Moulins du Cuir et de la Peau: Moulins a Tan et à Chamoiser en France; Éditions Creer: Nonette, France, 2004; ISBN 978-2848190143. [Google Scholar]
- Waterer, J.H. Leather Craftsmanship; G. Bell and Sons: London, UK, 1968. [Google Scholar]
- Thomson, R. Leather manufacture in the postmedieval period with special reference to Northamptonshire. Post Med. Archaeol. 1981, 15, 161–175. [Google Scholar] [CrossRef]
- Clarkson, L.A. Developments in tanning methods during the Post-Medieval Period (1500–1850). In Leather Manufacture through the Ages, Proceedings of the 27th East Midlands Industrial Archaeology Conference; Thomson, R., Beswick, J.A., Eds.; Arkle Print Ltd.: Northampton, UK, 1983; pp. 11–21. [Google Scholar]
- Sol, B. L’évolution des procédés de tannage végétal. In Environnement et Conservation de l’Écrit, de l’Image et du Son; Association pour la Recherche Scientifique sur les Arts Graphiques-ARSAG: Paris, France, 1994; pp. 89–95. [Google Scholar]
- Miguelez, C. Arte de Curtir ó Instruccion General de Curtidos; Imprenta Real: Madrid, Spain, 1805. [Google Scholar]
- Procter, H.R. A Text-Book of Tanning: A Treatise on the Conversion of Skins into Leather; E. & F. N. Spon: London, UK, 1885. [Google Scholar]
- Villon, A.-M. Traité Pratique de la Fabrication des Cuirs et du Travail des Peaux; Librairie Polytechnique: Paris, France, 1889. [Google Scholar]
- Bennett, H.G. The Manufacture of Leather; Constable & Company Ltd.: London, UK, 1920. [Google Scholar]
- Mould, Q.; Carlisle, I.; Cameron, E. Leather and leatherworking in Anglo-Scandinavian and Medieval York; Council for the British Archaeology: York, UK, 2003; Volume 17, ISBN 978-1902771366. [Google Scholar]
- Departament de Cultura. L'Art en la Pell. Cordovans i Guadamassils de la Collecció Colomer Munmany; Departament de Cultura: Vich, Spain, 1992. [Google Scholar]
- Nenno, R.; Rathke, C. Lederlust: Meisterwerke der Angewandten Kunst aus dem Deutschen Ledermuseum Offenbach; Kerber Christof Verlag: Darmstadt, Germany, 2006; ISBN 978-3-938025-67-3. [Google Scholar]
- Thomson, R. Leather. In Conservation Science: Heritage Materials; The Royal Society of Chemistry: Cambridge, UK, 2006; pp. 92–120. [Google Scholar]
- Larsen, R. The chemical degradation of leather. Chimia 2008, 62, 899–902. [Google Scholar] [CrossRef]
- Hagerman, A.E. The Tannin Handbook. 2018. Available online: http://chemistry.muohio.edu/hagerman (accessed on 28 March 2018).
- Khanbabaee, K.; van Ree, T. Tannins: Classification and definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar] [PubMed]
- Arbenz, A.; Avérous, L. Chemical modification of tannins to elaborate aromatic biobased macromolecular architectures. Green Chem. 2015, 17, 2626–2646. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R. The manufacture of leather. In Conservation of Leather and Related Materials; Kite, M., Thomson, R., Eds.; Butterworth Heinemann-Elsevier: Oxford, UK, 2006; pp. 121–129. [Google Scholar]
- Trommer, B. Archaölogisches Leder: Herkunft, Gerbstoffe, Technologien, Alterungs–und Abbauverhalten; VDM: Saarbrücken, Germany, 2008; ISBN 978-3836496292. [Google Scholar]
- Hilbert, F.L. Tanning materials (vegetable). In Encyclopedia of Chemical Technology; Kirk, R.E., Othmer, D., Eds.; The Interscience Encyclopedia: New York, NY, USA, 1954; Volume 13, pp. 578–586. [Google Scholar]
- Howes, F.N. Vegetable Tanning Materials; Butterworths Scientific Publications: London, UK, 1953. [Google Scholar]
- Kuliev, Z.A.; Vdovin, A.D.; Abdullaev, N.D.; Makhmatkulov, A.B.; Malikov, V.M. Study of the catechins and proanthocyanidins of Quercus robur. Chem. Nat. Compd. 1997, 33, 642–652. [Google Scholar] [CrossRef]
- Pallenbach, E.; Scholz, E.; König, M.; Rimpler, H. Proanthocyanidins from Quercus petraea Bark. Planta Med. 1993, 59, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Falcão, L.; Araújo, M.E.M. Application of ATR–FTIR spectroscopy to the analysis of tannins in historic leathers: The case study of the upholstery from the 19th century Portuguese Royal Train. Vib. Spectrosc. 2014, 74, 98–103. [Google Scholar] [CrossRef]
- Matsuo, Y.; Wakamatsu, H.; Omar, M.; Tanaka, T. Reinvestigation of the stereochemistry of the C-glycosidic ellagitannins, vescalagin and castalagin. Org. Lett. 2015, 17, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.M.C. A indústria no distrito de Évora, 1836-90. Anal. Soc. 1991, 26, 561–581. [Google Scholar]
- Conde, E.; Cadahía, E.; García-Vallejo, M.C.; Fernández de Simón, B. Polyphenolic Composition of Quercus suber Cork from Different Spanish Provenances. J. Agric. Food Chem. 1998, 46, 3166–3171. [Google Scholar] [CrossRef]
- Palo, R.T. Distribution of birch (Betula spp.), willow (Salix spp.), and poplar (Populus spp.) secondary metabolites and their potential role as chemical defense against herbivores. J. Chem. Ecol. 1984, 10, 499–520. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, K.; Siikaahoa, M.; Pattathil, S.; Giovando, S.; Kruus, K. Spruce bark as an industrial source of condensed tannins and non-cellulosic sugars. Ind. Crops Prod. 2014, 52, 158–168. [Google Scholar] [CrossRef]
- No II. Extract of Mimosa-Bark, for the Use of Tanners. 1823. Available online: http://www.jstor.org/stable/41325900 (accessed on 28 March 2018).
- Reid, D.G.; Bonnet, S.L.; Kemp, G.; van der Westhuizen, J.H. Analysis of commercial proanthocyanidins. Part 4: Solid state 13C NMR as a tool for in situ analysis of proanthocyanidin tannins, in heartwood and bark of quebracho and acacia, and related species. Phytochemistry 2013, 94, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Crestini, C.; Lange, H.; Bianchetti, G. Detailed Chemical Composition of Condensed Tannins via Quantitative 31P NMR and HSQC Analyses: Acacia catechu, Schinopsis balansae, and Acacia mearnsii. J. Nat. Prod. 2016, 79, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Smout, T.C. Oak as a commercial crop in the eighteenth and nineteenth centuries. Bot. J. Scotl. 2005, 57, 107–114. [Google Scholar] [CrossRef]
- Zhang, B.; Cai, J.; Duan, C.-Q.; Reeves, M.J.; He, F. A Review of Polyphenolics in Oak Woods. Int. J. Mol. Sci. 2015, 16, 6978–7014. [Google Scholar] [CrossRef] [PubMed]
- Jumelle, H. Le Quebracho. Rev. Bot. Appl. Agric. Colon. 1924, 30, 88–98. [Google Scholar] [CrossRef]
- Venter, P.B.; Sisa, M.; van der Merwe, M.J.; Bonnet, S.L.; van der Westhuizen, J.H. Analysis of commercial proanthocyanidins. Part 1: The chemical composition of quebracho (Schinopsis lorentzii and Schinopsis balansae) heartwood extract. Phytochemistry 2012, 73, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Kardel, M.; Taube, F.; Schulz, H.; Schutze, W.; Gierus, M. Different approaches to evaluate tannin content and structure of selected plant extracts—Review and new aspects. J. Appl. Bot. Food Qual. 2013, 86, 154–166. [Google Scholar] [CrossRef]
- Córdoba de la Llave, R. Cuatro textos sobre literatura técnica medieval sobre el trabajo del cuero. Merid. Rev. Hist. Med. 2002, 5–6, 171–204. [Google Scholar]
- Cardon, D.; Pinto, A. Le redoul, herbe des tanneurs et des teinturiers. Collecte, commercialisation et utilisations d’une plante sauvage dans l’espace meridional (XIIIe-XVe siècles). Médiévales 2007, 53, 51–64. [Google Scholar] [CrossRef]
- Zalacain, A.; Prodanov, M.; Carmona, M.; Alonso, G.L. Optimisation of extraction and identification of gallotannins from sumac leaves. Biosyst. Eng. 2003, 84, 211–216. [Google Scholar] [CrossRef]
- Regazzonia, L.; Arlandinia, E.; Garzona, D.; Santagatib, N.A.; Berettaa, G.; Facinoa, R.M. A rapid profiling of gallotannins and flavonoids of the aqueous extract of Rhus coriaria L. by flow injection analysis with high-resolution mass spectrometry assisted with database searching. J. Pharm. Biomed. Anal. 2013, 72, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Romeo, F.V.; Ballistreri, G.; Fabroni, S.; Pangallo, S.; Nicosia, M.G.L.D.; Schena, L.; Rapisarda, P. Chemical Characterization of Different Sumac and Pomegranate Extracts Effective against Botrytis cinereal Rots. Molecules 2015, 20, 11941–11958. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Amakura, Y.; Tokuhara, M.; Yoshida, T. Polyphenolic compounds isolated from the leaves of Myrtus communis. J. Nat. Med. 2008, 62, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, S.; Pizzi, A.; Bahabri, F.; Ganash, A. Analysis of Valonia Oak (Quercus aegylops) Acorn Tannin and Wood Adhesives Application. BioResources 2015, 10, 7165–7177. [Google Scholar] [CrossRef]
- Pérez, M.A.; Rengifo, R.; Pereira, C.; Hernández, V. Dividivi tannins: An ecological product for water-based drilling fluids. Environ. Dev. Sustain. 2017, 19, 1815–1829. [Google Scholar] [CrossRef]
- Florian, M.-L. Vegetable tannins. Leather Conserv. News 1984, 1, 37–38. [Google Scholar]
- Larsen, R.; Wouters, J.; Chahine, C.; Brimblecombe, P.; Calnan, C. Recommendations on the production, artificial ageing, assessment, storage and conservation of vegetable tanned leather. In Environment Leather Project: Deterioration and Conservation of Vegetable Tanned Leather—Research Report No. 6; Larsen, R., Ed.; The Royal Danish Academy of Fine Arts: Copenhagen, Denmark, 1996; pp. 189–202. [Google Scholar]
- Van Driel-Murray, C. Practical evaluation of a field test for the identification of ancient vegetable tanned leathers. J. Archaeol. Sci. 2002, 29, 17–21. [Google Scholar] [CrossRef]
- Poulsen, D.V. Presentation and evaluation of spot tests for identification of the tannin type in vegetable tanned leather. In Proceedings of the 13th Triennal Meeting ICOM Committee for Conservation, Rio de Janeiro, Brazil, 22–27 September 2002; Vontobel, R., Ed.; James and James: London, UK, 2002; pp. 792–797. [Google Scholar]
- Falcão, L.; Araújo, M.E.M. Tannins characterisation in new and historic vegetable tanned leathers fibres by spot tests. J. Cult. Herit. 2011, 12, 149–156. [Google Scholar] [CrossRef]
- Johnson, A. Evaluation of the use of SC6000 in conjunction with Klucel G as a conservation treatment for bookbinding leather: Notes on a preliminary study. J. Inst. Conserv. 2013, 36, 125–144. [Google Scholar] [CrossRef]
- Wouters, J. High-performance liquid chromatography of vegetable tannins extracted from new and old leathers. In Proceedings of the 10th Triennal Meeting ICOM Committee for Conservation, Washington, DC, USA, 22–27 August 1993; James & James for ICOM-CC: London, UK, 1993; pp. 669–673. [Google Scholar]
- Odegaard, N.; Carroll, S.; Zimmt, W.S. Material Characterization Tests for Objects of Art and Archaeology, 2nd ed.; Archetype Publications: London, UK, 2005; ISBN 1904982093. [Google Scholar]
- Falcão, L.; Araújo, M.E.M. Tannins characterization in historic leathers by complementary analytical techniques ATR-FTIR, UV-Vis and chemical tests. J. Cult. Herit. 2013, 14, 499–508. [Google Scholar] [CrossRef]
- Scalbert, A. Quantitative methods for the estimation of tannins in plant tissues, In Plant. Polyphenols: Synthesis, Properties, Significance; Hemingway, R.W., Laks, P.E., Eds.; Plenum Press: New York, NY, USA, 1992; pp. 259–278. [Google Scholar]
- Nikolova, D.; Goshev, I. Modification of the method of Broadhurst and Jones for proving of tannins in cultural-historical objects. C. R. Acad. Bulg. Sci. 2009, 62, 825–830. [Google Scholar]
- Wilson, T.C.; Hagerman, A.E. Quantitative determination of ellagic acid. J. Agric. Food Chem. 1990, 38, 1678–1683. [Google Scholar] [CrossRef]
- Inoue, K.H.; Hagerman, A.E. Determination of gallotannins with rhodanine. Anal. Biochem. 1988, 169, 363–369. [Google Scholar] [CrossRef]
- Nakagawa, K.; Sugita, M. Spectroscopic characterization and molecular weight of vegetable tannins. J. Soc. Leather Technol. Chem. 1999, 83, 261–264. [Google Scholar]
- Laghi, L.; Parpinello, G.P.; Del Rio, D.; Calani, L.; Mattioli, A.U.; Versari, A. Fingerprint of enological tannins by multiple techniques approach. Food Chem. 2010, 121, 783–788. [Google Scholar] [CrossRef]
- Giurginca, M.; Badea, N.; Miu, L.; Meghea, A. Spectral technics for identifying tanning agents in the heritage leather items. Rev. Chim. 2007, 58, 923–927. [Google Scholar]
- Derrick, M.R.; Stulik, D.C.; Landry, J.M. Infrared Spectroscopy in Conservation Science; The Getty Conservation Institute: Los Angeles, CA, USA, 1999. [Google Scholar]
- Stuart, B.H. Analytical Techniques in Materials Conservation; John Wiley & Sons: Chichester, UK, 2007; ISBN 978-0470012802. [Google Scholar]
- Bonnot-Diconne, C.; Robinet, L.; Pacheco, C.; Ioele, M.; Paris, M. Multi-technique analysis of gilt-leather wall coverings (16th–18th centuries). In Proceedings of the ICOM-CC 17th Triennial Conference Preprints, Melbourne, Australia, 15–19 September 2014; International Council of Museums: Paris, France, 2014. ISBN 978-92-9012-410-8. [Google Scholar]
- Falcão, L.; Pereira, F.A.B.; Araújo, M.E.M. Caracterização de cabedais adamascados e guadamecis dos séculos XVII e XVIII por ATR. Conserv. Patrim. 2018, 27, 49–61. [Google Scholar] [CrossRef]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Kilmartin, P.A.; Versari, A. Application of Fourier transform infrared (FTIR) spectroscopy in the characterization of tannins. Appl. Spectrosc. Rev. 2015, 50, 407–442. [Google Scholar] [CrossRef]
- Tondi, G.; Petutschnigg, A. Middle infrared (ATR FT-MIR) characterization of industrial tannin extracts. Ind. Crops Prod. 2015, 65, 422–428. [Google Scholar] [CrossRef]
- Romer, F.H.; Underwood, A.P.; Senekal, N.D.; Bonnet, S.L.; Duer, M.J.; Reid, D.G.; van der Westhuizen, J.H. Tannin fingerprinting in vegetable tanned leather by solid state NMR spectroscopy and comparison with leathers tanned by other processes. Molecules 2011, 16, 1240–1252. [Google Scholar] [CrossRef] [PubMed]







| Botanical Name | Common Name | Origin and Distribution | Part of the Plant Used | Main Tannins | Geographical Uses | Observations |
|---|---|---|---|---|---|---|
| Acacia mearnsii | mimosa, wattle | Australia, cultivated in South Africa since 1864 and South America | barks | condensed | imported since second decade of 19th century, commercial extracts | |
| Betula spp. | birch | northern Europe, Russia | barks | condensed | northern Europe, Russia | used to produce Russia leather |
| Caesalpinia coriaria | divi-divi | Central and South America | pods | hydrolysable: gallotannins | imported since late 18th century | |
| Castanea sativa | chestnut, sweet chestnut | Mediterranean region | wood | hydrolysable: ellagitannins | since 19th century, commercial extracts | used mixed with other vegetable materials to produce firm leather |
| Coriaria myrtifolia | Mediterrenean coriaria (emborrachacabras, redoul, roldor, rodor) | southern France and Mediterranean coastal Spain | leaves (redoul) | hydrolysable | southern France and Mediterranean coastal Spain | |
| Cotinus coggygria (syn Rhus cotinus) | smoke tree | southern Europe, Mediterranean region | leaves (Venetian or Turkish sumac) | hydrolysable: gallotannins | southern Europe | |
| Larix | larch | northern Europe | bark | condensed | northern Europe | |
| Mirtus communis | myrtle | southern Europe | leaves | hydrolysable: ellagitannins | Italic Peninsula | |
| Picea abies | Norway spruce | Alps, Pyrenees, Germany, Scandinavia | barks | condensed | northern and central Europe | |
| Pinus halepensis | Aleppo pine | coastal areas of the western Mediterranean region | barks | condensed | northern Europe | yields a reddish leather |
| Quercus aegilops | valonea oak, Turkish oak | eastern Mediterranean region | acorn cups | hydrolysable: ellagitannins | Middle Ages in Turkey, Greece, Italy | |
| Quercus coccifera | garouille | Mediterranean region | husk of root (rusque) | hydrolysable | south of France | |
| Quercus infectoria | Aleppo oak | eastern Mediterranean region | galls (Allepo galls) | hydrolysable: gallotannins | Europe | |
| Quercus ilex | holm oak | central-western part of the Mediterranean | barks | condensed and hydrolysable | Iberian Peninsula | |
| Quercus spp. (Q. ilex, Q. robur, Q. petraea, Q. pyrenaica) | oak | Europe | barks | condensed and hydrolysable: ellagitannins | Europe | |
| wood | hydrolysable: ellagitannins | |||||
| Quercus suber | cork oak | inner bark | condensed and hydrolysable: ellagitannins | Iberian Peninsula | ||
| Rhus coriaria | sumac | Mediterranean region | leaves (Sicilian sumac) | hydrolysable: gallotannins | southern Europe | yields light coloured, soft and supple leathers. Used to produce basil and cordovan leather. |
| Salix spp. | willow | northern Europe, Russia | barks | condensed | northern Europe, Russia | yields a light coloured, yellowish-brown leather that is soft and flexible |
| Schinopsis balansae, S. lorentzii | quebracho | south America | wood | condensed | imported and used in Europe since last decades of 19th century | |
| Terminalia chebula | myrabolans | India | fruits | hydrolysable | British Islands | used in mixed tannages for sole leather |
| Tannins Name | ||||
|---|---|---|---|---|
| Procyanidin | Profisetidin | Prorobinetidin | Prodelphinidin | |
| Chemical structure of the building block |  |  |  |  |
| Coupling position | 4–8 | 4–6 | 4–6 | 4–8 |
| Bands (cm−1) | Assignment | Tannin Identification |
|---|---|---|
| 1731–1704 (m-s) | ν C=O phenolic esters lactones | hydrolysable tannins |
| ν C=O phenolic esters | ||
| 1615–1606 (m-vs) | ν C=C aromatic ring | present in all classes of tannins |
| 1518–1507 (w-m) | ν C=C skeletal ring | present in all classes of tannins |
| 1452–1446 (m-s) | ν C=C aromatic ring | present in all classes of tannins |
| 1325–1317 (m-s) | ν C-O lactones and O-H deformation | hydrolysable tannins |
| 1288–1282 (ms-vs) | ν C-O pyran ring, flavonoids | condensed tannins |
| 1211–1196 (m-vs) | ν aromatic C-OH | present in all classes of tannins |
| 1162–1155 (s) | ν, asymmetric, C-O-C cyclic ether | condensed tannins |
| 1116–1110 (s-vs) | ν, asymmetric, C-O-C cyclic ether | condensed tannins |
| 1088–1082 (m) | ν, symmetric, C-O-C aryl phenolic ester | gallotannins |
| 1043–1030 (m-vs) | β = C-H deformation | present in all classes of tannins |
| 976 (w) | condensed tannins | |
| 844–842 (w) | γ tetrasubsituted aromatic C-H | condensed tannins |
| 872–870 (w) | γ OH and γ tetrasubsituted aromatic C-H | gallotannins |
| 763–758 (w-m) | ν, symmetric skeletal (sugar ring, breathing vibration) | gallotannins |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falcão, L.; Araújo, M.E.M. Vegetable Tannins Used in the Manufacture of Historic Leathers. Molecules 2018, 23, 1081. https://doi.org/10.3390/molecules23051081
Falcão L, Araújo MEM. Vegetable Tannins Used in the Manufacture of Historic Leathers. Molecules. 2018; 23(5):1081. https://doi.org/10.3390/molecules23051081
Chicago/Turabian StyleFalcão, Lina, and Maria Eduarda M. Araújo. 2018. "Vegetable Tannins Used in the Manufacture of Historic Leathers" Molecules 23, no. 5: 1081. https://doi.org/10.3390/molecules23051081