Identification of Ophiocordyceps sinensis and Its Artificially Cultured Ophiocordyceps Mycelia by Ultra-Performance Liquid Chromatography/Orbitrap Fusion Mass Spectrometry and Chemometrics
Abstract
:1. Introduction
2. Results
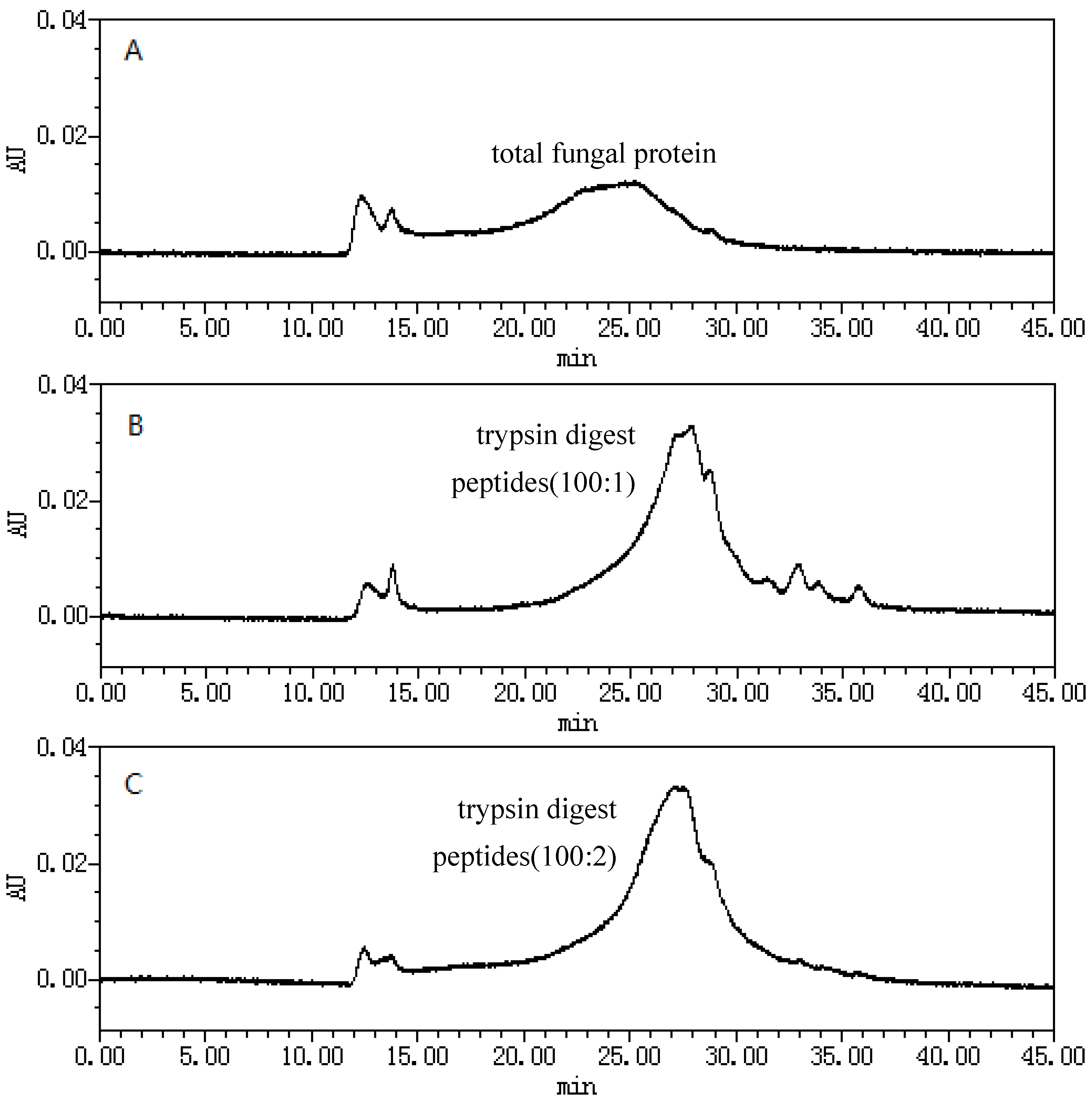
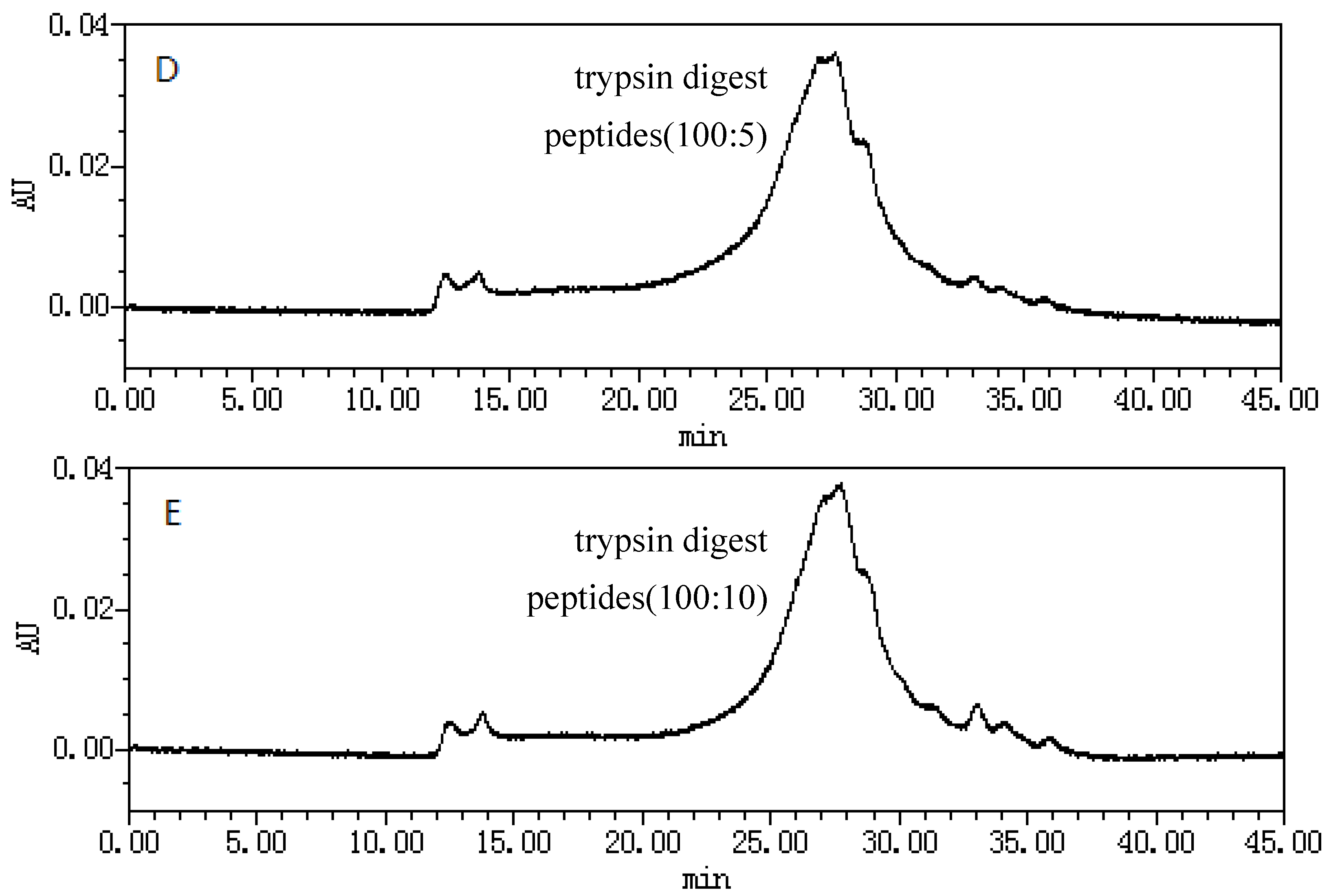
2.1. Size-Exclusion Chromatographic Analysis ofFungal Proteinsand Their Tryptic Digest Mixtures
2.2. Multivariate Data Analysis
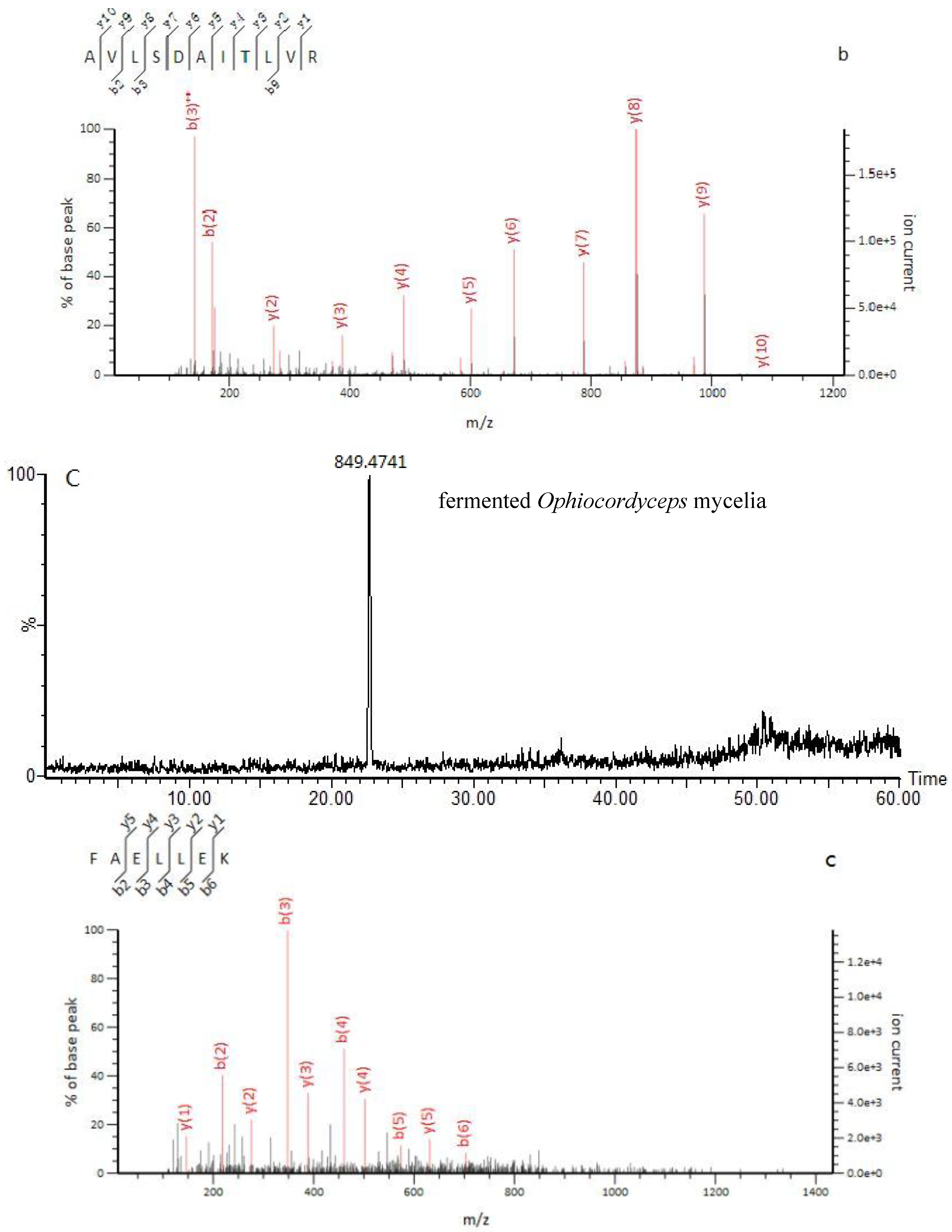
2.3. Identification of Marker Peptides in Digested Mixtures
3. Discussion
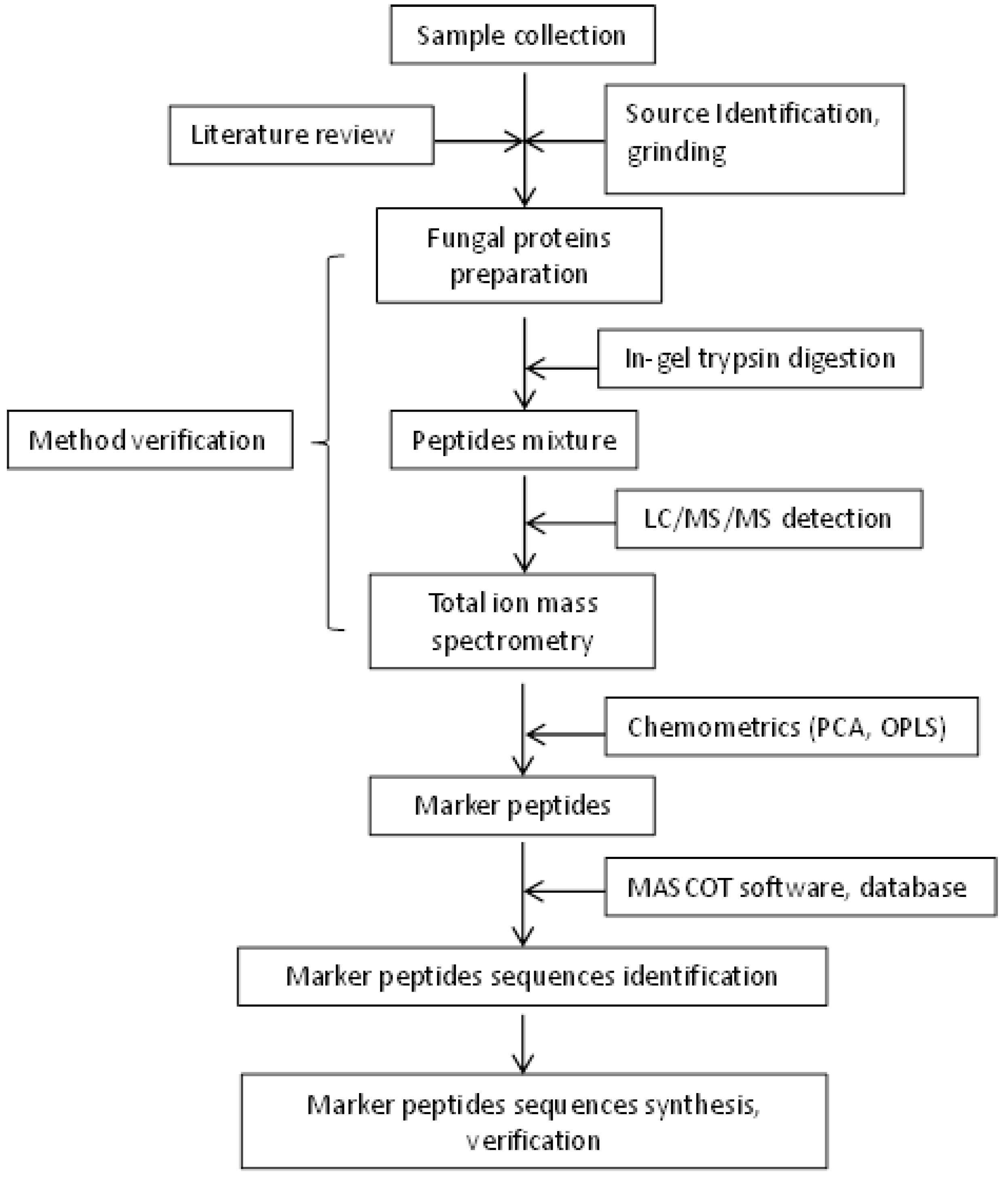
4. Materials and Methods
4.1. Materials and Reagents
4.2. Extraction of Ophiocordyceps Fungal Proteins
4.3. Purification and Tryptic Digestion
4.4. Size Exclusion Chromatography of the Digest Mixture
4.5. Chromatographic Separation and Mass Spectrometry
4.6. Multivariate Data Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, L.; Hao, Q.X.; Wang, S.; Kang, C.Z.; Yang, W.Z.; Guo, L.P. Study on distribution of five heavy metal elements in different parts of Cordyceps sinensis by microwave digestion ICP-MS. China J. Chin. Mater. Med. 2017, 42, 2934–2938. [Google Scholar] [CrossRef]
- Lee, E.J.; Jang, K.H.; Im, S.Y.; Farooq, M.; Farhoudi, R.; Lee, D.J. Physico-chemical properties and cytotoxic potential of Cordyceps sinensis metabolites. Nat. Prod. Res. 2015, 29, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.K.; Swain, K.C. Traditional uses and medicinal potential of Cordyceps sinensis of Sikkim. J. Ayurveda Integr. Med. 2011, 2, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.D.; Wu, Z.G.; Zheng, F. Amelioration of aminoglycoside nephrotoxicity by Cordyceps sinensis in old patients. Chin. J. Integr. Tradit. West. Med. 1994, 14, 271–273. [Google Scholar]
- Bao, T.T.; Wang, G.F.; Yang, J.L. Pharmacological actions of Cordyceps sinensis. Chin. J. Mod. Dev. Tradit. Med. 1988, 8, 325–326, 352–354. [Google Scholar]
- Buenz, E.J.; Bauer, B.A.; Osmundson, T.W.; Motley, T.J. The traditional Chinese medicine Cordyceps sinensis and its effects on apoptotic homeostasis. J. Ethnopharmacol. 2005, 96, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Buenz, E.J.; Weaver, J.G.; Bauer, B.A.; Chalpin, S.D.; Badley, A.D. Cordyceps sinensis extracts do not prevent Fas-receptor and hydrogen peroxide-induced T-cell apoptosis. J. Ethnopharmacol. 2004, 90, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, L. Cordyceps sinensis in protection of the kidney from cyclosporine A nephrotoxicity. Chin. Med. J. 1993, 73, 410–412, 447. [Google Scholar]
- Illana Esteban, C. Cordyceps sinensis, a fungi used in the Chinese traditional medicine. Rev. Iberoam. Micol. 2007, 24, 259–262. [Google Scholar] [CrossRef]
- Jahan-Abad, A.J.; Morteza-Zadeh, P.; Negah, S.S.; Gorji, A. Curcumin attenuates harmful effects of arsenic on neural stem/progenitor cells. Avicenna J. Phytomed. 2017, 7, 376–388. [Google Scholar]
- Cao, L.; Ye, Y.; Han, R. Fruiting body production of the medicinal Chinese caterpillar mushroom, Ophiocordyceps sinensis (Ascomycetes), in artificial medium. Int. J. Med. Mushrooms 2015, 17, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.W.; Li, L.J.; Tian, E.W. Advances in research of the artificial cultivation of Ophiocordyceps sinensis in China. Crit. Rev. Biotechnol. 2014, 34, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.S.; Zhu, J.S. Indiscriminate use of Latin name for natural Cordyceps sinensis insect-fungi complex and multiple Ophiocordyceps sinensis fungi. China J. Chin. Mater. Med. 2016, 41, 1361–1366. [Google Scholar] [CrossRef]
- Zhu, J.S.; Halpern, G.M.; Jones, K. The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis: Part I. J. Altern. Complement. Med. 1998, 4, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Wang, N.; Qu, L.; Li, T.; Zhang, W. Determination of the anamorph of Cordyceps sinensis inferred from the analysis of the ribosomal DNA internal transcribed spacers and 5.8S rDNA. Biochem. Syst. Ecol. 2001, 29, 597–607. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Lin, S.; Baker, P.J.; Wu, L.F.; Wang, X.R.; Wu, H.; Xu, F.; Wang, H.Y.; Brathwaite, M.E.; Zheng, Y.G. Transcriptome sequencing and analysis of the entomopathogenic fungus Hirsutella sinensis isolated from Ophiocordycepssinensis. BMC Genom. 2015, 16, 106. [Google Scholar] [CrossRef]
- Chen, S.J.; Yin, D.H.; Li, L.; Zhou, X.L.; Za, X. Studies on anamorph of Cordyceps sinensis (Berk) from Naqu Tibet. China J. Chin. Mater. Med. 2001, 26, 453–454. [Google Scholar]
- Yang, J.L.; Xiao, W.; He, H.X.; Zhu, H.X.; Wang, S.F.; Cheng, K.D.; Zhu, P. Molecular phylogenetic analysis of Paecilomyces hepiali and Cordyceps sinensis. Acta Pharm. Sin. 2008, 43, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.H.; Zhang, Q.X.; Wu, J.Y. Mycelium cultivation, chemical composition and antitumour activity of a Tolypocladium sp. fungus isolated from wild Cordyceps sinensis. J. Appl. Microbiol. 2006, 101, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Balon, T.W.; Jasman, A.P.; Zhu, J.S. A fermentation product of Cordyceps sinensis increases whole-body insulin sensitivity in rats. J. Altern. Complement. Med. 2002, 8, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Zan, K.; Huang, L.L.; Guo, L.N.; Liu, J.; Zheng, J.; Ma, S.C.; Qian, Z.M.; Li, W.J. Comparative study on specific chromatograms and main nucleosides of cultivated and wild Cordyceps sinensis. China J. Chin. Mater. Med. 2017, 42, 3957–3962. [Google Scholar] [CrossRef]
- Xie, C.; Xu, N.; Shao, Y.; He, Y. Using FT-NIR spectroscopy technique to determine arginine content in fermented Cordyceps sinensis mycelium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Yang, F.Q.; Tsim, K.W. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J. Pharm. Biomed. Anal. 2006, 41, 1571–1584. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.F.; Liau, J.C.; Lee, C.S.; Chiu, C.Y.; Martel, J.; Lin, C.S.; Tseng, S.F.; Ojcius, D.M.; Lu, C.C.; Lai, H.C.; et al. Isolation, culture and characterization of Hirsutella sinensis mycelium from caterpillar fungus fruiting body. PLoS ONE 2017, 12, e0168734. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Ye, M.; Lin, X.; Zhou, Z. The artificial cultivation of medicinal caterpillar fungus, Ophiocordyceps sinensis (Ascomycetes): A review. Int. J. Med. Mushrooms 2013, 15, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.X.; Xu, X.M.; Hong, Y.H.; Li, Y.; Wang, J.H. Stable carbon isotope composition of the lipids in natural Ophiocordyceps sinensis from major habitats in China and its substitutes. Molecules 2017, 22, 1567. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Zhou, J.; Liu, J. Identification of Chinese medicinal fungus Cordyceps sinensis by depth-profiling mid-infrared photoacoustic spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Au, D.; Wang, L.; Yang, D.; Mok, D.K.; Chan, A.S.; Xu, H. Application of microscopy in authentication of valuable Chinese medicine I—Cordyceps sinensis, its counterfeits, and related products. Microsc. Res. Tech. 2012, 75, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.Z.; Song, J.; Meng, F.X.; Meng, Q.F.; Lu, J.H.; Hu, S.; Teng, L.R.; Wang, D.; Xie, J. Study on the detection of active ingredient contents of Paecilomyces hepiali mycelium via near infrared spectroscopy. Spectrosc. Spectr. Anal. 2014, 34, 2645–2651. [Google Scholar]
- Hou, X.R.; Luan, L.J.; Cheng, Y.Y. Quantitative analysis of the nucleosides in Cordyceps sinensis with capillary zone electrophoresis. China J. Chin. Mater. Med. 2005, 30, 447–449. [Google Scholar]
- Huang, L.F.; Guo, F.Q.; Liang, Y.Z.; Chen, B.M. Determination of adenosine and cordycepin in Cordyceps sinensis and C. militarris with HPLC-ESI-MS. China J. Chin. Mater. Med. 2004, 29, 762–764. [Google Scholar]
- Li, S.P.; Li, P.; Dong, T.T.; Tsim, K.W. Determination of nucleosides in natural Cordyceps sinensis and cultured Cordyceps mycelia by capillary electrophoresis. Electrophoresis 2001, 22, 144–150. [Google Scholar] [CrossRef]
- Xie, J.W.; Huang, L.F.; Hu, W.; He, Y.B.; Wong, K.P. Analysis of the main nucleosides in Cordyceps sinensis by LC/ESI-MS. Molecules 2010, 15, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.Q.; Li, A.; Huang, L.F.; Liang, Y.Z.; Chen, B.M. Identification and determination of nucleosides in Cordyceps sinensis and its substitutes by high performance liquid chromatography with mass spectrometric detection. J. Pharm. Biomed. Anal. 2006, 40, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, L.; Zhang, S.; Liu, X.; An, Z.; Wang, M.; Guo, Y. Genetic diversity of Ophiocordyceps sinensis, a medicinal fungus endemic to the Tibetan Plateau: Implications for its evolution and conservation. BMC Evol. Biol. 2009, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.Y.C.; Chan, G.K.L.; Xin, G.Z.; Xu, H.; Ku, C.F.; Chen, J.P.; Yao, P.; Lin, H.Q.; Dong, T.T.X.; Tsim, K.W.K. Authentication of Cordyceps sinensis by DNA analyses: Comparison of ITS sequence analysis and RAPD-derived molecular markers. Molecules 2015, 20, 22454–22462. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.H.; Cheng, Z.; Yang, X.L.; Li, S.; Ding, Z.Q.; Zhou, T.S.; Zhang, W.J.; Chen, J.K. Genetic diversity and structure of Cordyceps sinensis populations from extensive geographical regions in China as revealed by inter-simple sequence repeat markers. J. Microbiol. 2008, 46, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, N.M.; Sarver, S.A.; Sun, L.; Wojcik, R.; Dovichi, N.J. High speed capillary zone electrophoresis-mass spectrometry via an electrokinetically pumped sheath flow interface for rapid analysis of amino acids and a protein digest. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 991, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Matsushima, K.; Miyamoto, K.; Oe, T. UV irradiation-induced methionine oxidation in human skin keratins: Mass spectrometry-based non-invasive proteomic analysis. J. Proteom. 2016, 133, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Zuo, H.L.; Zhang, Q.; Wang, F.Q.; Hu, Y.J.; Qian, Z.M.; Li, W.J.; Xia, Z.N.; Yang, F.Q. Analysis of soluble proteins in natural Cordyceps sinensis from different producing areas by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and two-dimensional electrophoresis. Pharmacogn. Res. 2017, 9, 34–38. [Google Scholar] [CrossRef]
- Hu, S.; Qiu, N.; Liu, Y.; Zhao, H.; Gao, D.; Song, R.; Ma, M. Identification and comparative proteomic study of quail and duck egg white protein using 2-dimensional gel electrophoresis and matrix-assisted laser desorption/ionization time-of-flight tandem mass spectrometry analysis. Poult. Sci. 2016, 95, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gan, J.; Shu, Y.Z.; Humphreys, W.G. High-resolution mass spectrometry-based background subtraction for identifying protein modifications in a complex biological system: Detection of acetaminophen-bound microsomal proteins including argininosuccinate synthetase. Chem. Res. Toxicol. 2015, 28, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Legg, K.M.; Powell, R.; Reisdorph, N.; Reisdorph, R.; Danielson, P.B. Verification of protein biomarker specificity for the identification of biological stains by quadrupole time-of-flight mass spectrometry. Electrophoresis 2017, 38, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Wang, D.; Gooden, D.M.; Ball, C.H.; Fitzgerald, M.C. Targeted mass spectrometry-based approach for protein-ligand binding analyses in complex biological mixtures using a phenacyl bromide modification strategy. Anal. Chem. 2016, 88, 10987–10993. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, Q.; Hu, D.; Xu, H.; Wang, H. Identification of a new protein from silkworm pupas by biological mass spectrometry. In Proceedings of the International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Shanghai, China, 1–4 September 2005; Volume 6, pp. 5709–5711. [Google Scholar]
- Magni, F.; Van Der Burgt, Y.E.M.; Chinello, C.; Mainini, V.; Gianazza, E.; Squeo, V.; Deelder, A.M.; Kienle, M.G. Biomarkers discovery by peptide and protein profiling in biological fluids based on functionalized magnetic beads purification and mass spectrometry. Blood Transfus. 2010, 8, s92–s97. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.C.; Sheu, F. A novel protein from edible fungi Cordyceps militaris that induces apoptosis. J. Food Drug Anal. 2018, 26, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Gong, Z.; Su, Y.; Lin, J.; Tang, K. Cordyceps fungi: Natural products, pharmacological functions and developmental products. J. Pharm. Pharmacol. 2009, 61, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Wang, S.; Hu, D.J.; Yang, F.Q.; Wang, H.X.; Li, S.P. Random amplified polymorphic DNA (RAPD) analysis and the nucleosides assessment of fungal strains isolated from natural Cordyceps sinensis. J. Pharm. Biomed. Anal. 2009, 50, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Wang, W.; Zhang, H.; Zhang, X.; Han, C. The chemical constituents and pharmacological actions of Cordyceps sinensis. Evid. Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef]
- Hu, H.; Xiao, L.; Zheng, B.; Wei, X.; Ellis, A.; Liu, Y.M. Identification of chemical markers in Cordyceps sinensis by HPLC-MS/MS. Anal. Bioanal. Chem. 2015, 407, 8059–8066. [Google Scholar] [CrossRef] [PubMed]
- Bedlovičová, Z.; Ungvarská, L.M.; Salayová, A.; Harvanová, J.; Očenáš, P. Determination of biologically active compounds in the fungi of the genus Cordyceps sinensis by HPLC and NMR. CeskaSlov. Farm. 2015, 64, 202–205. [Google Scholar]
- Zhang, L.; Ge, Y.; Li, J.; Hao, J.; Wang, H.; He, J.; Gao, X.M.; Chang, Y.X. Simultaneous determination of columbianetin-β-d-glucopyranoside and columbianetin in a biological sample by high-performance liquid chromatography with fluorescence detection and identification of other columbianetin-β-d-glucopyranoside metabolites by ultra high-performance liquid chromatography coupled with quadrupole-time of flight mass spectrometry. J. Pharm. Biomed. Anal. 2018, 153, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Woolman, M.; Gribble, A.; Bluemke, E.; Zou, J.; Ventura, M.; Bernards, N.; Wu, M.; Ginsberg, H.; Das, S.; Vitkin, A.; et al. Optimized Mass Spectrometry Analysis Workflow with Polarimetric Guidance for ex vivo and in situ Sampling of Biological Tissues. Sci. Rep. 2017, 7, 468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lai, X.; Wu, C.; Wang, S.; Chen, X.; Huang, J.; Yang, G. Application of Differential Proteomic Analysis to Authenticate Ophiocordyceps sinensis. Curr. Microbiol. 2016, 72, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Qian, Z.M.; Yao, S.U.; Liu, X.Z.; Wen, J.L.; Dong, C.H. Comparative analyses of proteomic profile at different development stages of Chinese cordyceps by iTRAQ-coupled 2D LC-MSMS. Microbiology 2012, 39, 853–864. [Google Scholar]
- Ashoub, A.; Berberich, T.; Beckhaus, T.; Bruggemann, W. A competent extraction method of plant proteins for 2-D gel electrophoresis. Electrophoresis 2011, 32, 2975–2978. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.; Bernach, M.; Bajdzeienko, K.; Giavalisco, P. A Simple Fractionated Extraction Method for the Comprehensive Analysis of Metabolites, Lipids, and Proteins from a Single Sample. J. Vis. Exp. 2017, 124. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.J.; Daim, L.D.; Jamil, A.A.; Mohtarrudin, N.; Thilakavathy, K. An effective placental cotyledons proteins extraction method for 2D gel electrophoresis. Electrophoresis 2017, 38, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Huynh, M.L.; Russell, P.; Walsh, B. Tryptic digestion of in-gel proteins for mass spectrometry analysis. Methods Mol. Biol. 2009, 519, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Albright, J.C.; Dassenko, D.J.; Mohamed, E.A.; Beussman, D.J. Identifying gel-separated proteins using in-gel digestion, mass spectrometry, and database searching: Consider the chemistry. Biochem. Mol. Biol. Educ. 2009, 37, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Lill, J.R.; Nesatyy, V.J. Microwave-assisted protein staining, destaining, and in-gel/in-solution digestion of proteins. Methods Mol. Biol. 2012, 869, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, Q.; Weng, L.; Hauser, L.A.; Strawser, C.J.; Rocha, A.G.; Dancis, A.; Mesaros, C.; Lynch, D.R.; Blair, I.A. Liquid Chromatography-High Resolution Mass Spectrometry Analysis of Platelet Frataxin as a Protein Biomarker for the Rare Disease Friedreich’s Ataxia. Anal Chem. 2018, 90, 2216–2223. [Google Scholar] [CrossRef] [PubMed]
- Randall, D.R.; Park, P.S.; Chau, J.K. Identification of altered protein abundances in cholesteatoma matrix via mass spectrometry-based proteomic analysis. J. Otolaryngol. Head Neck Surg. 2015, 44, 50. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds marker peptides of TLLEAIDSIEPPK, AVLSDAITLVR, FAELLEK, and LESVVTSFTK are available from the authors. |

 ): wild O. sinensis fruiting body, (
): wild O. sinensis fruiting body, (  ): fermented O.S. mycelia powder, (
): fermented O.S. mycelia powder, (  ): artificial Ophiocordyceps mycelia powder, (
): artificial Ophiocordyceps mycelia powder, (  ): fermented Ophiocordyceps mycelia powder, (
): fermented Ophiocordyceps mycelia powder, (  ): O. mortierella mycelia powder, and (b) the loading plot of wild O. sinensis and four cultured Ophiocordyceps mycelia.
): O. mortierella mycelia powder, and (b) the loading plot of wild O. sinensis and four cultured Ophiocordyceps mycelia.
 ): wild O. sinensis fruiting body, (
): wild O. sinensis fruiting body, (  ): fermented O.S. mycelia powder, (
): fermented O.S. mycelia powder, (  ): artificial Ophiocordyceps mycelia powder, (
): artificial Ophiocordyceps mycelia powder, (  ): fermented Ophiocordyceps mycelia powder, (
): fermented Ophiocordyceps mycelia powder, (  ): O. mortierella mycelia powder, and (b) the loading plot of wild O. sinensis and four cultured Ophiocordyceps mycelia.
): O. mortierella mycelia powder, and (b) the loading plot of wild O. sinensis and four cultured Ophiocordyceps mycelia.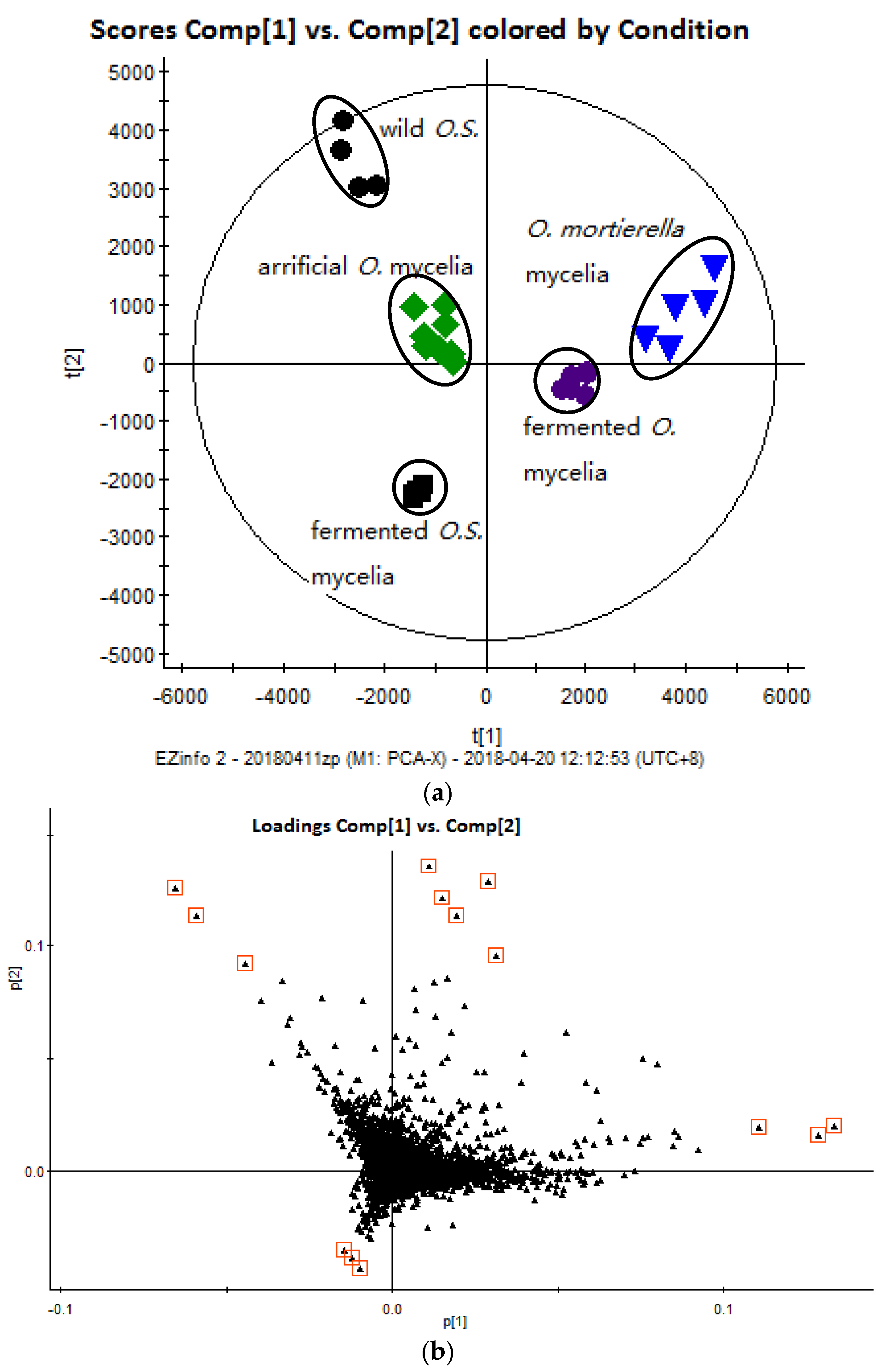


| Marker | Mascot Matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | No.of Ions | Precursor Ion, (m/z) | Charge | Fragment Ion, (m/z) | Time, (min) | Peptide Match | Peptide Fragmentation | Protein Match |
| Ophiocordyceps sinensis | 1 | 713.39 | 2 | 41.1 | TLLEAIDSIEPPK | gi:A4U9H1 | ||
| 969.52 | y | (Ophiocordyceps brunneipunctata) | ||||||
| 898.48 | y | |||||||
| 785.4 | y | |||||||
| 215.13 | b | |||||||
| 452.25 | y0 | |||||||
| 881.46 | y * | |||||||
| 2 | 851.74 | 3 | 46.2 | SVEMHHEQLTEGLPGDNVGFNVK | gi: 0A060IK44 | |||
| 1046.52 | y | (Ophiocordyceps sinensis) | ||||||
| 949.47 | y | |||||||
| 497.7 | b++ | |||||||
| 596.25 | b *++ | |||||||
| 777.42 | y | |||||||
| 1208.54 | b | |||||||
| fermented O. sinensis mycelia | 3 | 579.34 | 2 | 35 | AVLSDAITLVR | gi: T5AC53 | ||
| 987.58 | y | (Hirsutella sinensis) | ||||||
| 874.49 | y | |||||||
| 787.46 | y | |||||||
| 672.44 | y | |||||||
| 488.31 | y | |||||||
| 142.6 | b++ | |||||||
| 4 | 670.3 | 2 | 14.5 | NAGSGCPTYTVGR | gi: T5AC53 | |||
| 1154.52 | y | (Hirsutella sinensis) | ||||||
| 1010.47 | y | |||||||
| 397.21 | y++ | |||||||
| 370.13 | b * | |||||||
| 387.16 | b | |||||||
| 5 | 614.85 | 2 | 54.7 | MVEVLGIIQAR | gi: T5AC53 | |||
| 657.4 | y | (Hirsutella sinensis) | ||||||
| 770.48 | y | |||||||
| 998.59 | y | |||||||
| 231.11 | b | |||||||
| 360.15 | b | |||||||
| artificial Ophiocordyceps mycelia | 6 | 555.8 | 2 | 26 | LESVVTSFTK | gi: 0A0B7JUZ6 | ||
| 868.47 | y | (Gliocladium roseum) | ||||||
| 682.37 | y | |||||||
| 243.13 | b | |||||||
| 215.12 | b++ | |||||||
| 482.75 | b++ | |||||||
| 7 | 637.34 | 2 | 44.9 | HALVIYDDLSK | gi: 0A0B7JUZ6 | |||
| 952.49 | y | (Gliocladium roseum) | ||||||
| 740.34 | y | |||||||
| 577.28 | y | |||||||
| 209.1 | b | |||||||
| 534.33 | b | |||||||
| fermented Ophiocordyceps mycelia | 8 | 849.47 | 1 | 22.6 | (FAELLEK) | Unassigned | ||
| 502.32 | y | |||||||
| 389.23 | y | |||||||
| 348.15 | b | |||||||
| 9 | 799.44 | 2 | 966.52 | 58 | YLEIIKETSNFIK | y | gi: A0A172PXZ9 | |
| 596.85 | y++ | (Ophiostoma pseudocatenulatum) | ||||||
| 406.19 | b | |||||||
| 990.55 | b | |||||||
| 487.26 | b *++ | |||||||
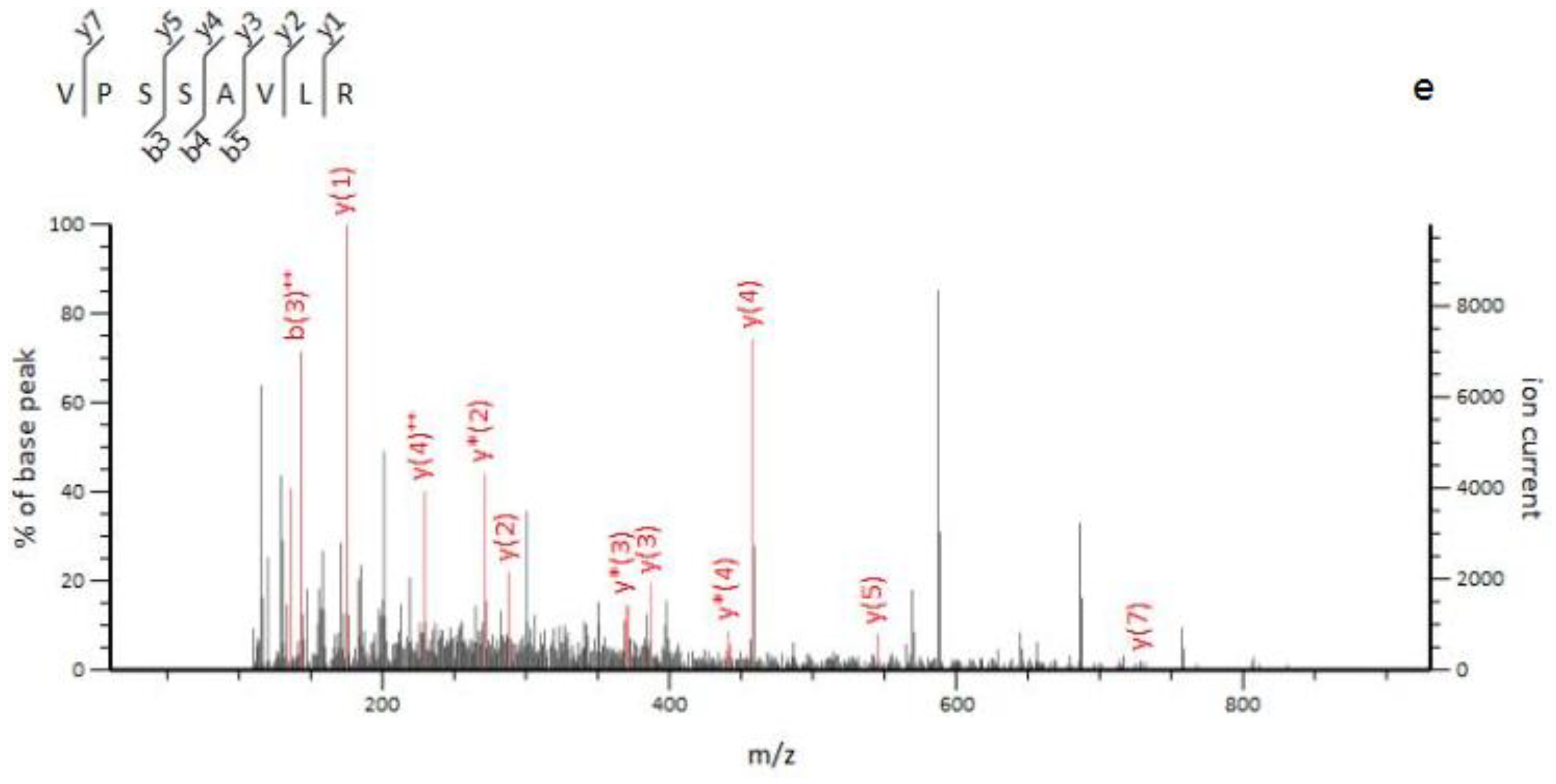
| O. mortierella mycelia | 10 | 414.75 | 2 | 13.6 | (VPSSAVLR) | Unassigned | ||
| 458.3 | y | |||||||
| 370.24 | y * | |||||||
| 387.27 | y | |||||||
| 142.58 | b++ | |||||||
| Sample Status | Claimed Names a | No. of Samples | Locations |
|---|---|---|---|
| Wild fruiting body | Ophiocordyceps sinensis | 4 | Tibet |
| Cultured mycelium powder b | Fermented O.S. mycelia | 5 | Zhejiang |
| Fermented Ophiocordyceps mycelia | 6 | Jiangxi | |
| Artificial Ophiocordyceps mycelia | 8 | Hebei | |
| O. mortierella mycelia | 5 | Zhejiang |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Li, S.; Li, J.; Wei, F.; Cheng, X.; Zhang, G.; Ma, S.; Liu, B. Identification of Ophiocordyceps sinensis and Its Artificially Cultured Ophiocordyceps Mycelia by Ultra-Performance Liquid Chromatography/Orbitrap Fusion Mass Spectrometry and Chemometrics. Molecules 2018, 23, 1013. https://doi.org/10.3390/molecules23051013
Zhang P, Li S, Li J, Wei F, Cheng X, Zhang G, Ma S, Liu B. Identification of Ophiocordyceps sinensis and Its Artificially Cultured Ophiocordyceps Mycelia by Ultra-Performance Liquid Chromatography/Orbitrap Fusion Mass Spectrometry and Chemometrics. Molecules. 2018; 23(5):1013. https://doi.org/10.3390/molecules23051013
Chicago/Turabian StyleZhang, Ping, Saina Li, Juan Li, Feng Wei, Xianlong Cheng, Guifeng Zhang, Shuangcheng Ma, and Bin Liu. 2018. "Identification of Ophiocordyceps sinensis and Its Artificially Cultured Ophiocordyceps Mycelia by Ultra-Performance Liquid Chromatography/Orbitrap Fusion Mass Spectrometry and Chemometrics" Molecules 23, no. 5: 1013. https://doi.org/10.3390/molecules23051013





