Evaluation of Manganese Chloride’s Effect on Biosynthetic Properties of In Vitro Cultures of Eschscholzia californica Cham.
Abstract
:1. Introduction
2. Results
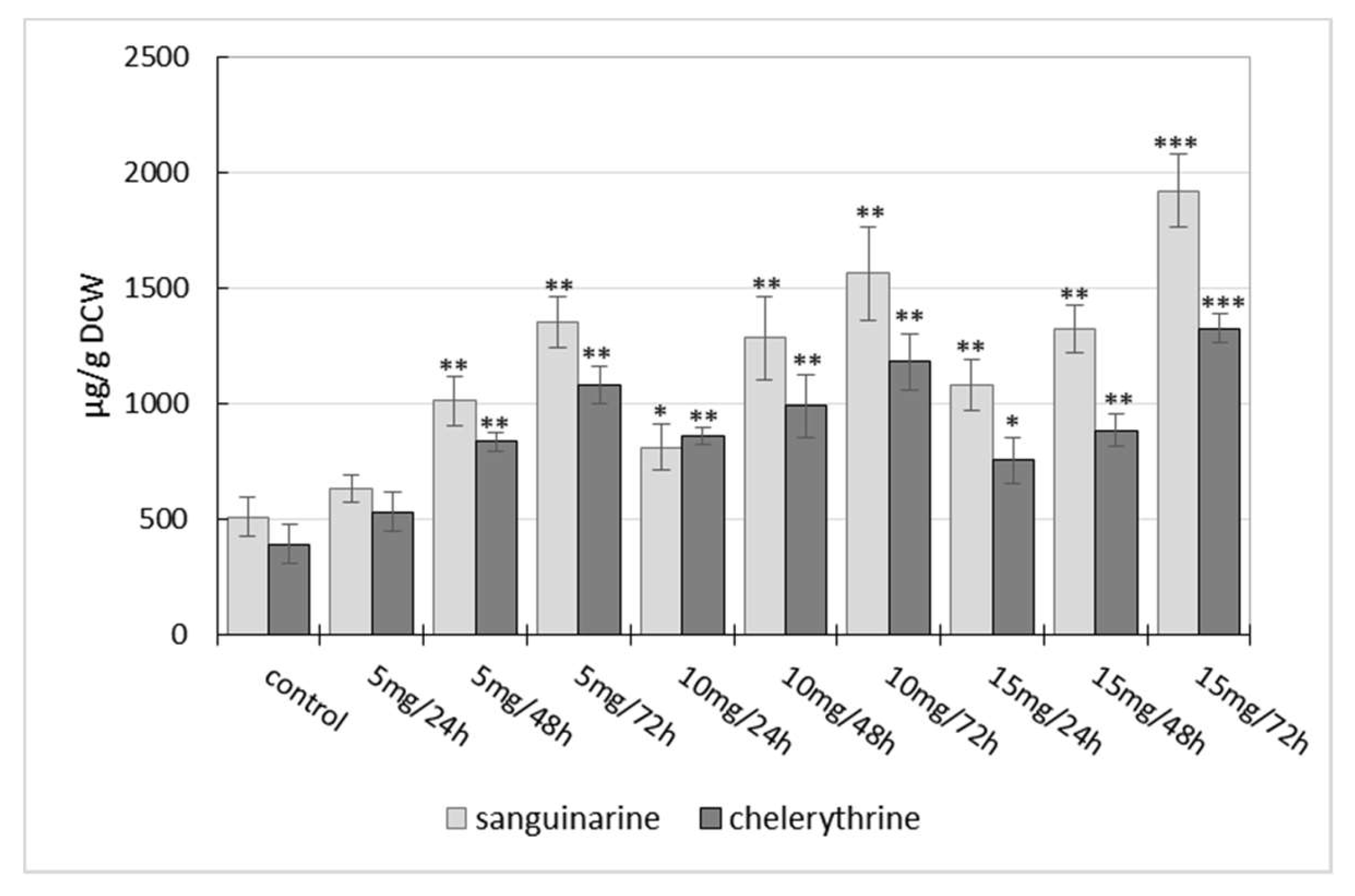
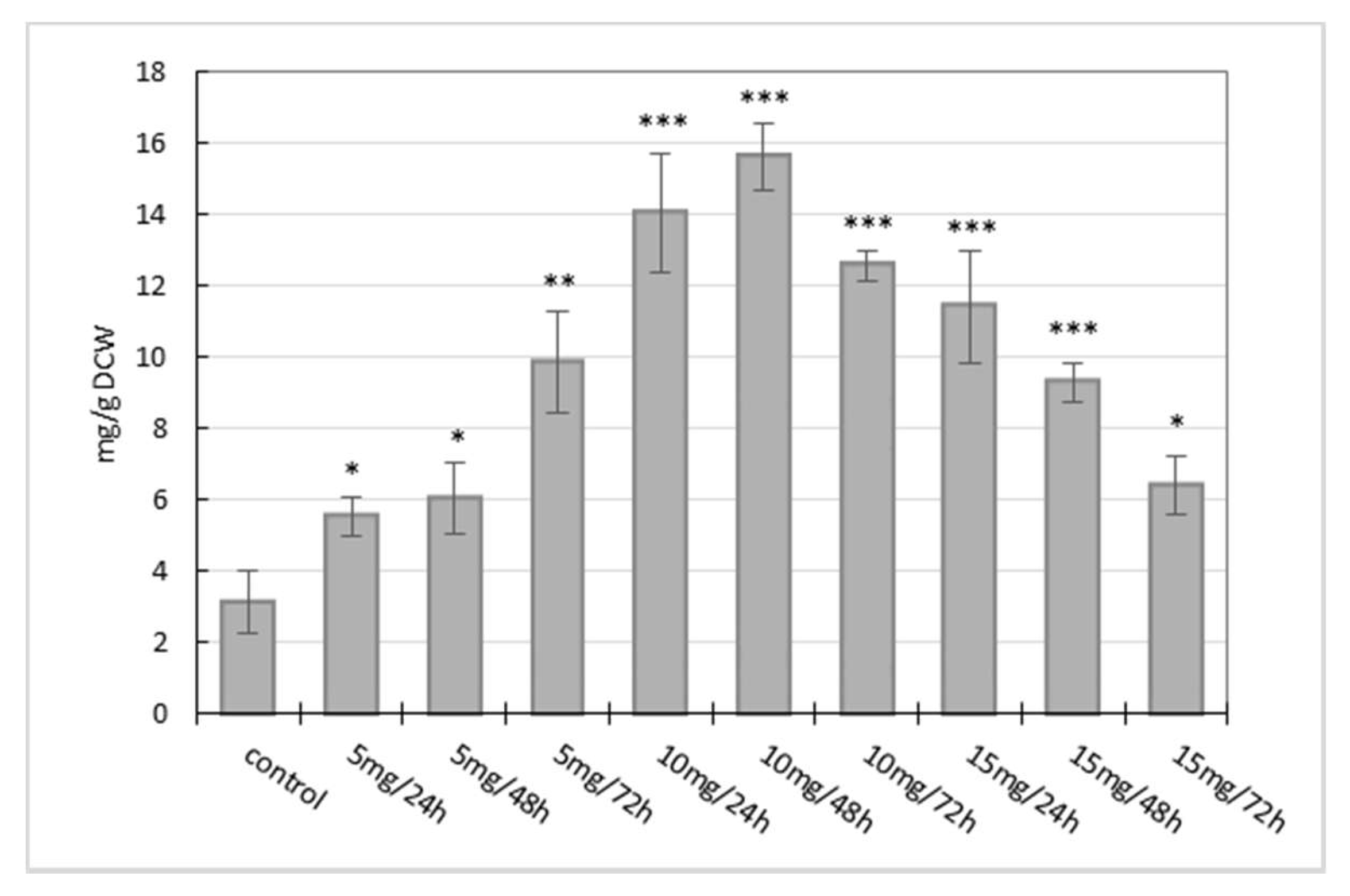
2.1. Influence of Manganese Chloride Elicitation on Benzophenanthridine Alkaloids Production
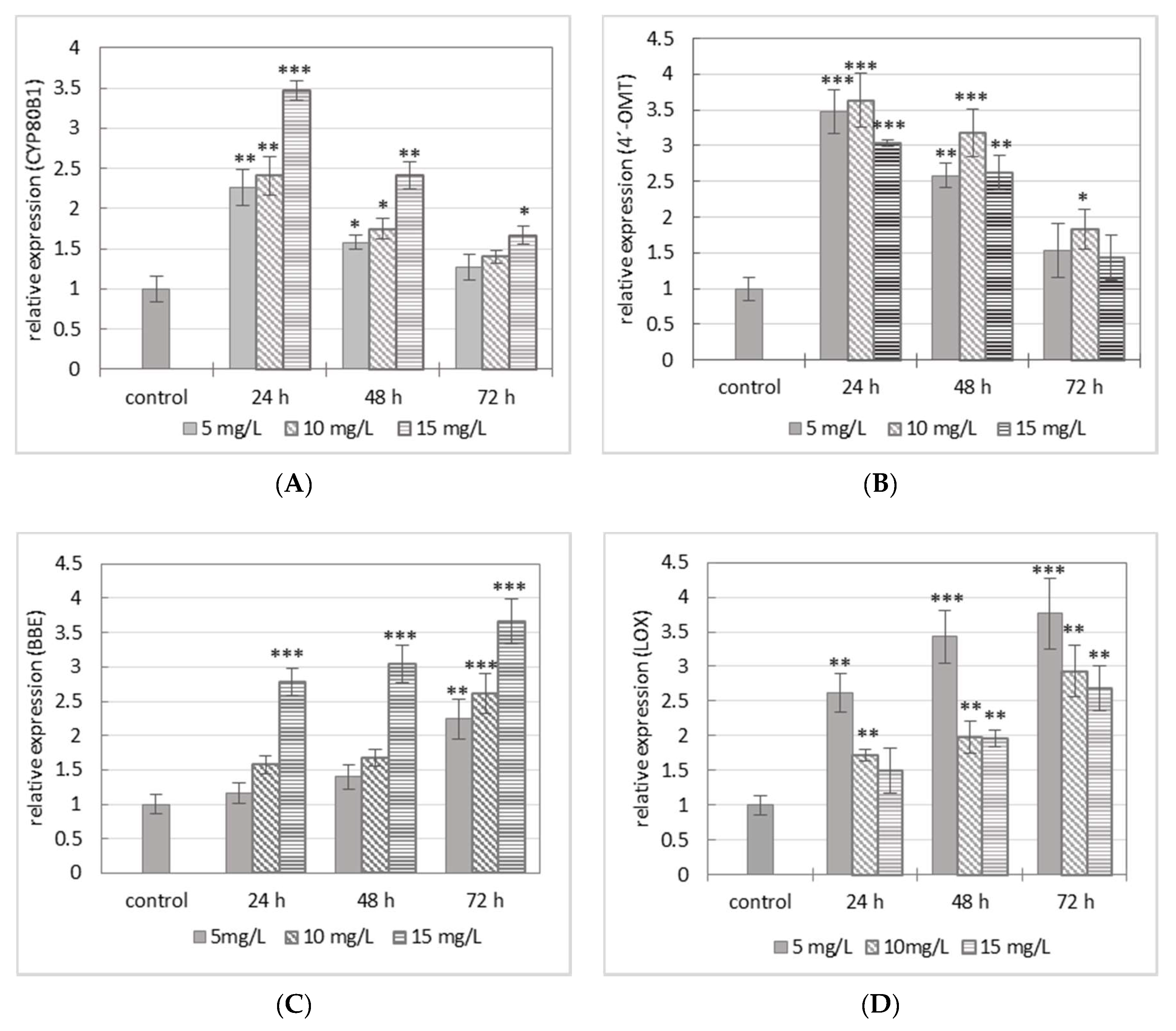
2.2. Evaluation of Gene Expression
3. Discussion
4. Materials and Methods
4.1. Preparation of Suspension Cultures and Elicitation with MnCl2·4H2O
4.2. Elicitor Preparation
4.3. Alkaloids Determination and Quantification
4.4. Total RNA Isolation and Quantitative RT-PCR
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of plant secondary metabolites: A historical perspective. Plant Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Edreva, A.; Velikova, V.; Tsonev, T.; Dagnon, S.; Gürel, A.; Aktaş, L.; Gesheva, E. Stress-protective role of secondary metabolites: Diversity of functions and mechanisms. Gen. Appl. Plant Physiol. 2008, 34, 67–78. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Petrovska, B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Eva-Maria Pferschy-Wenzig, T.L.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; Rollinger, J.M.; et al. Discovery and resupply of pharmacologically active plant- derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Namdeo, A.G. Plant Cell Elicitation for Production of Secondary Metabolites: A Review. Pharmacogn. Rev. 2007, 1, 69–79. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.S.; Fareed, S.; Ansari, S.; Rahman, M.A.; Ahmad, I.Z.; Saeed, M. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied Sci. 2012, 4, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Shakya, P.; Marslin, G.; Siram, K.; Beerhues, L.; Franklin, G. Elicitation as a tool to improve the profiles of high-value secondary metabolites and pharmacological properties of Hypericum perforatum. J. Pharm. Pharmacol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ch, B.; Rao, K.; Gandi, S.; Giri, A. Abiotic elicitation of gymnemic acid in the suspension cultures of Gymnema sylvestre. World J. Microbiol. Biotechnol. 2012, 28, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Villarreal, M.; Howat, S.; Hong, S.; Jang, M.O.; Jin, Y.-W.; Lee, E.-K.; Loake, G.J. Plant cell culture strategies for the production of natural products. BMB Rep. 2016, 49, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Szymanowska, U.; Złotek, U.; Karaś, M.; Baraniak, B. Anti-inflammatory and antioxidative activity of anthocyanins from purple basil leaves induced by selected abiotic elicitors. Food Chem. 2015, 172, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Złotek, U.; Szymanowska, U.; Karaś, M.; Świeca, M. Antioxidative and anti-inflammatory potential of phenolics from purple basil (Ocimum basilicum L.) leaves induced by jasmonic, arachidonic and β-aminobutyric acid elicitation. Int. J. Food Sci. Technol. 2016, 51, 163–170. [Google Scholar] [CrossRef]
- Kim, H.J.; Chen, F.; Wang, X.; Choi, J.H. Effect of methyl jasmonate on phenolics, isothiocyanate, and metabolic enzymes in radish sprout (Raphanus sativus L.). J. Agric. Food Chem. 2006, 54, 7263–7269. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef] [PubMed]
- Holková, I.; Bezáková, L.; Bilka, F.; Balažová, A.; Vanko, M.; Blanáriková, V. Involvement of lipoxygenase in elicitor-stimulated sanguinarine accumulation in Papaver somniferum suspension cultures. Plant Physiol. Biochem. 2010, 48, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Kollárová, R.; Obložinsḱy, M.; Kováčiková, V.; Holková, I.; Balažová, A.; Pekárová, M.; Hoffman, P.; Bezáková, L. Lipoxygenase activity and sanguinarine production in cell suspension cultures of California poppy (Eschscholtzia californica CHAM.). Pharmazie 2014, 69, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, H.; Fatima, N.; Ahmad, I.Z. Evaluation of Antioxidant and Antibacterial Potentials of Nigella sativa L. Suspension Cultures under Elicitation. BioMed Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Godowski, K.C. Antimicrobial action of sanguinarine. J. Clin. Dent. 1989, 1, 96–101. [Google Scholar] [PubMed]
- Orhan, I.; Özçelik, B.; Karaoǧlu, T.; Şener, B. Antiviral and antimicrobial profiles of selected isoquinoline alkaloids from Fumaria and Corydalis species. Z. Naturforsch. 2007, 62c, 19–26. [Google Scholar] [CrossRef]
- Lenfeld, J.; Kroutil, M.; Maršálek, E.; Slavík, J.; Preininger, V.; Šimánek, V. Antiinflammatory Activity of Quaternary Benzophenanthridine Alkaloids from Chelidonoum majus. Planta Med. 1981, 43, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Fan, T.; Li, W.; Xing, W.; Huang, H. The anti-inflammatory effects of sanguinarine and its modulation of inflammatory mediators from peritoneal macrophages. Eur. J. Pharmacol. 2012, 689, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Hagel, J.M.; Facchini, P.J. Benzylisoquinoline alkaloid metabolism: A century of discovery and a brave new world. Plant Cell Physiol. 2013, 54, 647–672. [Google Scholar] [CrossRef] [PubMed]
- Slaninová, I.; Slunská, Z.; Šinkora, J.; Vlková, M.; Táborská, E. Screening of minor benzo(c)phenanthridine alkaloids for antiproliferative and apoptotic activities. Pharm. Biol. 2007, 45, 131–139. [Google Scholar] [CrossRef]
- Pica, F.; Balestrieri, E.; Serafino, A.; Sorrentino, R.; Gaziano, R.; Moroni, G.; Moroni, N.; Palmieri, G.; Mattei, M.; Garaci, E.; et al. Antitumor effects of the benzophenanthridine alkaloid sanguinarine in a rat syngeneic model of colorectal cancer. Anticancer. Drugs 2012, 23, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, R.; Moroni, G.; Buè, C.; Miele, M.T.; Sinibaldi-Vallebona, P.; Pica, F. Antitumor effects of the benzophenanthridine alkaloid sanguinarine: Evidence and perspectives. World J. Gastrointest. Oncol. 2016, 8, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, J.; Bird, D.A.; Franceschi, V.R.; Facchini, P.J. Sanguinarine Biosynthesis Is Associated with the Endoplasmic Reticulum in Cultured Opium Poppy Cells after Elicitor Treatment. Plant Physiol. 2005, 138, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, H.; Kutchan, T.M. Molecular cloning, expression, and induction of berberine bridge enzyme, an enzyme essential to the formation of benzophenanthridine alkaloids in the response of plants to pathogenic attack. Proc. Natl. Acad. Sci. USA 1991, 88, 9969–9973. [Google Scholar] [CrossRef] [PubMed]
- Ikezawa, N.; Iwasa, K.; Sato, F. Molecular cloning and characterization of methylenedioxy bridge-forming enzymes involved in stylopine biosynthesis in Eschscholzia californica. FEBS J. 2007, 274, 1019–1035. [Google Scholar] [CrossRef] [PubMed]
- Liscombe, D.K.; Facchini, P.J. Molecular cloning and characterization of tetrahydroprotoberberine cis-N-methyltransferase, an enzyme involved in alkaloid biosynthesis in opium poppy. J. Biol. Chem. 2007, 282, 14741–14751. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.-T.T.; Facchini, P.J. Characterization of Three O-Methyltransferases Involved in Noscapine Biosynthesis in Opium Poppy. Plant Physiol. 2012, 159, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Purwanto, R.; Hori, K.; Yamada, Y.; Sato, F. Unraveling Additional O-Methylation Steps in Benzylisoquinoline Alkaloid Biosynthesis in California Poppy (Eschscholzia californica). Plant Cell Physiol. 2017, 58, 1528–1540. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Dai, P.; Bao, H.; Liang, P.; Wang, W.; Xing, A.; Sun, J. Anti-inflammatory and neuroprotective effects of sanguinarine following cerebral ischemia in rats. Exp. Ther. Med. 2017, 13, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Villegas, M.; Sommarin, M.; Brodelius, P.E. Effects of sodium orthovanadate on benzophenanthridine alkaloid formation and distribution in cell suspension cultures of Eschscholtzia californica. Plant Physiol. Biochem. 2000, 38, 233–241. [Google Scholar] [CrossRef]
- Bilková, A.; Bilka, F.; Blanáriková, V.; Bezáková, L. Effect of excess of cupric sulfate on sanguinarine formation and activities of amine oxidase and polyphenol oxidase in cell suspension cultures of Papaver somniferum. Biologia 2005, 60, 661–664. [Google Scholar]
- Bilka, F.; Balažová, A.; Bilková, A.; Holková, I. Effect of abiotic elicitation on the sanguinarine production and polyphenol oxidase activity in the suspension culture of Eschscholtzia californica CHAM. Čes. Slov. Farm. 2013, 62, 169–173. [Google Scholar]
- Gangopadhyay, M.; Dewanjee, S.; Bhattacharya, S. Enhanced plumbagin production in elicited Plumbago indica hairy root cultures. J. Biosci. Bioeng. 2011, 111, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Son, S.Y.; Rhee, H.S.; Yoon, S.Y.H.; Lee-Parsons, C.W.T.; Park, J.M. Synergistic effects of sequential treatment with methyl jasmonate, salicylic acid and yeast extract on benzophenanthridine alkaloid accumulation and protein expression in Eschscholtzia californica suspension cultures. J. Biotechnol. 2008, 135, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Tamás, L.; Dudíková, J.; Ďurčeková, K.; Halušková, L.; Huttová, J.; Mistrík, I. Effect of cadmium and temperature on the lipoxygenase activity in barley root tip. Protoplasma 2009, 235, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, M.; Cho, W.J.; Yoshida, S.; Fueki, T.; Mukai, C. Chemical Transformation of Protoberberines. XVI. Regioselective Introduction of an Oxy Functionality at the C12 Position of the Benzo[c]phenanthridine Skeleton: A Convenient Synthesis of Macarpine from Oxychelirubine. Chem. Pharm. Bull. 1990, 38, 3335–3340. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Guo, M.; Luo, X.; Yao, S. A simple and sensitive method of nonaqueous capillary electrophoresis with laser-induced native fluorescence detection for the analysis of chelerythrine and sanguinarine in Chinese herbal medicines. Talanta 2006, 70, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Winer, J.; Jung, C.K.S.; Shackel, I.; Williams, P.M. Development and Validation of Real-Time Quantitative Reverse Transcriptase–Polymerase Chain Reaction for Monitoring Gene Expression in Cardiac Myocytesin Vitro. Anal. Biochem. 1999, 270, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Triptahi, V.; Singh, S.; Phukan, U.J.; Gupta, M.M.; Shanker, K.; Shukla, R.K. Wound Induced Tanscriptional Regulation of Benzylisoquinoline Pathway and Characterization of Wound Inducible PsWRKY Transcription Factor from Papaver somniferum. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Macarpine was isolated from elicited plant material and used for experiments and structure elucidation. Sanguinarine and chelerythrine are available commercially. Lyophilized plant material is available from the authors. |
| Primer Name | Oligonucleotide Sequences (5′- to 3′-) |
|---|---|
| CYP80B1 | forward TCAAACAGTGGTAGGCGAGAGA reverse CAATGGAGTTGGTGGGTGAA |
| BBE | forward GAGATTAGTAGGAGTTGGGGTGAGA reverse ATTGGAGGGATACTTTGTGGATG |
| 4′-OMT | forward CCTAGAAGAGGAATCAGAACATCCA reverse TCACTTCTCTCCCTTCCACCA |
| LOX | forward ATTGGGAAAATGACGATGGAAAA reverse CTACGTTATCCCTTGTAAACCATTC |
| β-actin | forward GGTATTGTGCTGGATTCTGGTG reverse GTAGGATTGCGTGGGGTAGTG |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balažová, A.; Urdová, J.; Bilka, F.; Holková, I.; Horváth, B.; Forman, V.; Mučaji, P. Evaluation of Manganese Chloride’s Effect on Biosynthetic Properties of In Vitro Cultures of Eschscholzia californica Cham. Molecules 2018, 23, 971. https://doi.org/10.3390/molecules23040971
Balažová A, Urdová J, Bilka F, Holková I, Horváth B, Forman V, Mučaji P. Evaluation of Manganese Chloride’s Effect on Biosynthetic Properties of In Vitro Cultures of Eschscholzia californica Cham. Molecules. 2018; 23(4):971. https://doi.org/10.3390/molecules23040971
Chicago/Turabian StyleBalažová, Andrea, Júlia Urdová, František Bilka, Ivana Holková, Branislav Horváth, Vladimír Forman, and Pavel Mučaji. 2018. "Evaluation of Manganese Chloride’s Effect on Biosynthetic Properties of In Vitro Cultures of Eschscholzia californica Cham." Molecules 23, no. 4: 971. https://doi.org/10.3390/molecules23040971




