Talarodiolide, a New 12-Membered Macrodiolide, and GC/MS Investigation of Culture Filtrate and Mycelial Extracts of Talaromyces pinophilus
Abstract
:1. Introduction
2. Results
2.1. Isolation and Identification of Metabolites
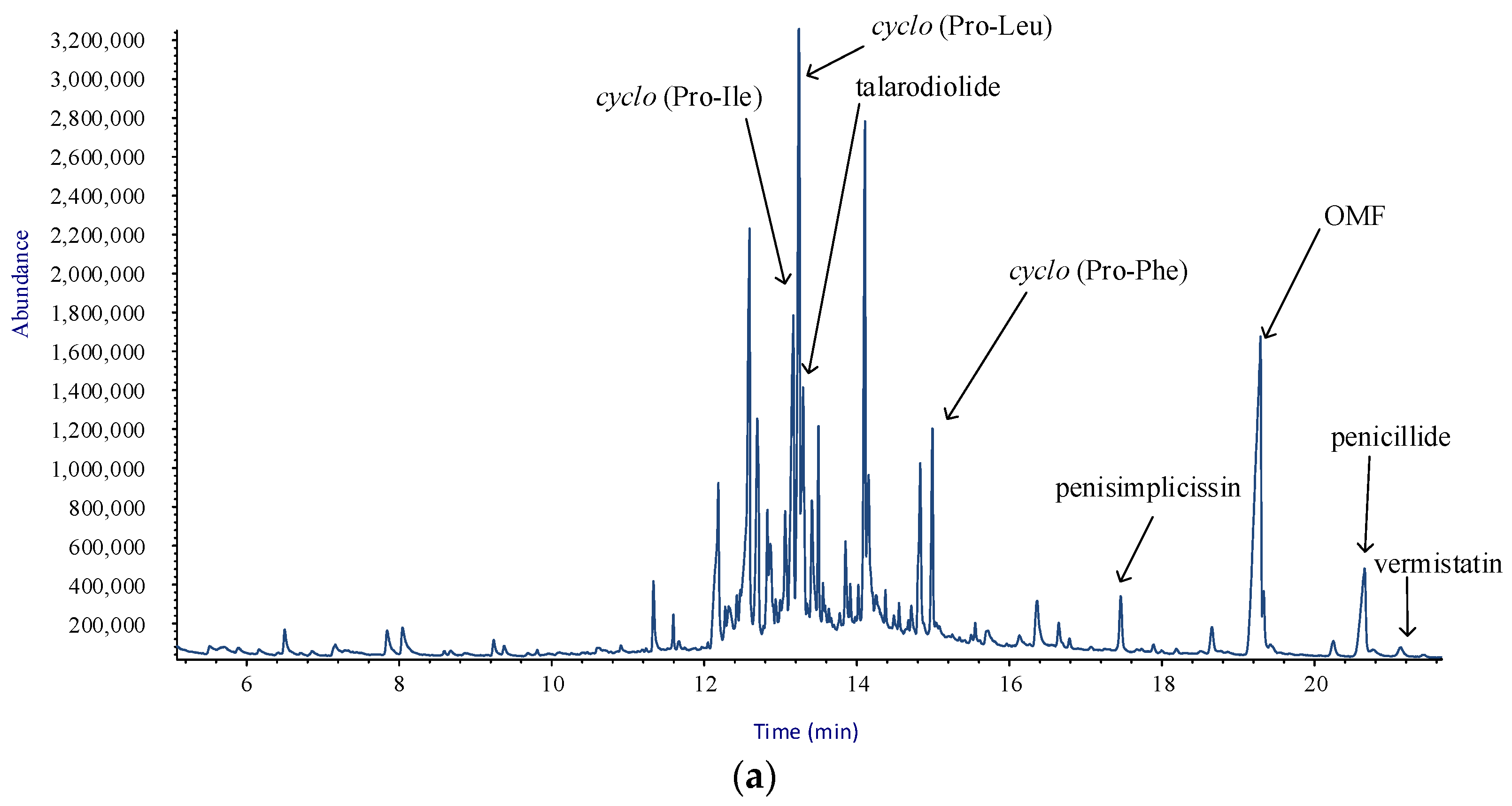
2.2. GC/MS Analysis
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Culture Filtrate Preparation
3.3. Extraction and Isolation of Metabolites from Liquid Cultures
3.4. Extraction and Isolation of Metabolites from Mycelium
3.5. GC/MS Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nicoletti, R.; De Stefano, M.; De Stefano, S.; Trincone, A.; Marziano, F. Antagonism against Rhizoctonia solani and fungitoxic metabolite production by some Penicillium isolates. Mycopathologia 2004, 158, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Trincone, A. Bioactive compounds produced by strains of Penicillium and Talaromyces of marine origin. Mar. Drugs 2016, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Salvatore, M.M.; Andolfi, A. Secondary matabolites of mangrove-associated strains of Talaromyces. Mar. Drugs 2018, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Das, N.; Kumar, B.; Rinu, K.; Trivedi, P. Phosphate solubilization by Penicillium spp. isolated from soil samples of Indian Himalayan region. World J. Microbiol. Biotechnol. 2008, 24, 97–102. [Google Scholar] [CrossRef]
- Wani, Z.A.; Mirza, D.N.; Arora, P.; Riyaz-Ul-Hassan, S. Molecular phylogeny, diversity, community structure, and plant growth promoting properties of fungal endophytes associated with the corms of saffron plant: An insight into the microbiome of Crocus sativus Linn. Fungal Biol. 2016, 120, 1509–1524. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.H.; Lübeck, M.; Frisvad, J.C.; Lübeck, P.S.; Andersen, B. Production of cellulolytic enzymes from ascomycetes: Comparison of solid state and submerged fermentation. Process Biochem. 2015, 50, 1327–1341. [Google Scholar] [CrossRef]
- Li, C.X.; Zhao, S.; Zhang, T.; Xian, L.; Liao, L.S.; Liu, J.L.; Feng, J.X. Genome sequencing and analysis of Talaromyces pinophilus provide insights into biotechnological applications. Sci. Rep. 2017, 7, 490. [Google Scholar] [CrossRef] [PubMed]
- Ohte, S.; Matsuda, D.; Uchida, R.; Nonaka, K.; Masuma, R.; Ōmura, S.; Tomoda, H. Dinapinones, novel inhibitors of triacylglycerol synthesis in mammalian cells, produced by Penicillium pinophilum FKI-3864. J. Antibiot. 2011, 64, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Scognamiglio, M.; Fiorentino, A. Structural and bioactive properties of 3-O-methylfunicone. Mini Rev. Med. Chem. 2014, 14, 1043–1047. [Google Scholar] [CrossRef]
- Zhai, M.M.; Li, J.; Jiang, C.X.; Shi, Y.P.; Di, D.L.; Crews, P.; Wu, Q.X. The bioactive secondary metabolites from Talaromyces species. Nat. Prod. Bioprospect. 2016, 6, 1–24. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, S.; Nicoletti, R.; Milone, A.; Zambardino, S. 3-O-Methylfunicone, a fungitoxic metabolite produced by the fungus Penicillium pinophilum. Phytochemistry 1999, 52, 1399–1401. [Google Scholar] [CrossRef]
- Nicoletti, R.; Manzo, E.; Ciavatta, L. Occurence and bioactivities of funicone-related compounds. Int. J. Mol. Sci. 2009, 10, 1430–1444. [Google Scholar] [CrossRef] [PubMed]
- Buommino, E.; Paoletti, I.; De Filippis, A.; Nicoletti, R.; Ciavatta, M.L.; Menegozzo, S.; Menegozzo, M.; Tufano, M.A. 3-O-Methylfunicone, a metabolite produced by Penicillium pinophilum, modulates ERK1/2 activity, affecting cell motility of human mesothelioma cells. Cell Prolif. 2010, 43, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Buommino, E.; Tirino, V.; De Filippis, A.; Silvestri, F.; Nicoletti, R.; Ciavatta, M.L.; Pirozzi, G.; Tufano, M.A. 3-O-Methylfunicone, from Penicillium pinophilum, is a selective inhibitor of breast cancer stem cells. Cell Prolif. 2011, 44, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Buommino, E.; De Filippis, A.; Nicoletti, R.; Menegozzo, M.; Menegozzo, S.; Ciavatta, M.L.; Rizzo, A.; Brancato, V.; Tufano, M.A.; Donnarumma, G. Cell-growth and migration inhibition of human mesothelioma cells induced by 3-O-methylfunicone from Penicillium pinophilum and cisplatin. Investig. New Drugs 2012, 30, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Buommino, E.; De Filippis, A.; Lopez-Gresa, M.P.; Manzo, E.; Carella, A.; Petrazzuolo, M.; Tufano, M.A. Bioprospecting for antagonistic Penicillium strains as a resource of new antitumor compounds. World J. Microbiol. Biotechnol. 2008, 24, 189–195. [Google Scholar] [CrossRef]
- Baroni, A.; De Luca, A.; De Filippis, A.; Petrazzuolo, M.; Manente, L.; Nicoletti, R.; Tufano, M.A.; Buommino, E. 3-O-methylfunicone, a metabolite of Penicillium pinophilum, inhibits proliferation of human melanoma cells by causing G2 + M arrest and inducing apoptosis. Cell Prolif. 2009, 42, 541–553. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, S.; Nicoletti, R.; Zambardino, S.; Milone, A. Structure elucidation of a novel funicone-like compound produced by Penicillium pinophilum. Nat. Prod. Lett. 2002, 16, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Ciavatta, M.L.; Manzo, E.; Contillo, R.; Nicoletti, R. Methoxyvermistatin production by Penicillium pinophilum isolate LT4. In Proceedings of the 4th Congress of European Microbiologists (FEMS 2011), Geneva, Switzerland, 26–30 June 2011. [Google Scholar]
- Adamczeski, M.; Reed, A.R.; Crews, P. New and known diketopiperazines from the Caribbean sponge, Calyx cf. podatypa. J. Nat. Prod. 1995, 58, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Dai, S.; Chen, M.; Wu, H.; Xie, L.; Luo, X.; Li, X. Two diketopiperazine cyclo-(Pro-Phe) isomers from marine bacteria Bacillus subtilis sp. 13–2. Chem. Nat. Compd. 2010, 46, 583–585. [Google Scholar] [CrossRef]
- Mazri, R.; Belaidi, S.; Kerassa, A.; Lanez, T. Conformational analysis, substituent effect and structure activity relationships of 16-membered macrodiolides. Int. Lett. Chem. Phys. Astron. 2014, 14, 146–167. [Google Scholar] [CrossRef]
- Fuska, J.; Uhrin, D.; Proksa, B.; Votický, Z.; Ruppeldt, J. The structure of vermistatin, a new metabolite from Penicillium vermiculatum. J. Antibiot. 1986, 39, 1605–1608. [Google Scholar] [CrossRef] [PubMed]
- Komai, S.I.; Hosoe, T.; Itabashi, T.; Nozawa, K.; Yaguchi, T.; Fukushima, K.; Kawai, K. New vermistatin derivatives isolated from Penicillium simplicissimum. Heterocycles 2005, 11, 2771–2776. [Google Scholar]
- Komai, S.I.; Hosoe, T.; Itabashi, T.; Nozawa, K.; Yaguchi, T.; Fukushima, K.; Kawai, K.I. New penicillide derivatives isolated from Penicillium simplicissimum. J. Nat. Med. 2006, 60, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Nieva-Echevarría, B.; Goicoechea, E.; Manzanos, M.J.; Guillén, M.D. A method based on 1H-NMR spectral data useful to evaluate the hydrolysis level in complex lipid mixtures. Food Res. Int. 2014, 66, 379–387. [Google Scholar] [CrossRef]
- NIST Standard Reference Data. Available online: http://www.nist.gov/srd/nist1a.cfm (accessed on 20 March 2018).
- Vinale, F.; Nicoletti, R.; Lacatena, F.; Marra, R.; Sacco, A.; Lombardi, N.; d’Errico, G.; Digilio, M.C.; Lorito, M.; Woo, S.L. Secondary metabolites from the endophytic fungus Talaromyces pinophilus. Nat. Prod. Res. 2017, 31, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- AMDIS NET. Available online: http://www.amdis.net/ (accessed on 20 March 2018).
- Stein, S.E. An integrated method for spectrum extraction and compound identification from GC/MS data. J. Am. Soc. Mass Spectrom. 1999, 10, 770–781. [Google Scholar] [CrossRef]
- Schauer, N.; Steinhauser, D.; Strelkov, S.; Schomburg, D.; Allison, G.; Moritz, T.; Lundgren, K.; Roessner-Tunali, U.; Forbes, M.G.; Willmitzer, L.; et al. GC–MS libraries for the rapid identification of metabolites in complex biological samples. FEBS Lett. 2005, 579, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Hummel, J.; Strehmel, N.; Selbig, J.; Walther, D.; Kopka, J. Decision tree supported substructure prediction of metabolites from GC-MS profiles. Metabolomics 2010, 6, 322–333. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–9 are available from the authors. |


| Position | δC | δH (J in Hz) | HMBC |
|---|---|---|---|
| 2, 8 | 164.6 C | - | |
| 3, 9 | 116.8 CH | 5.84, brs | |
| 4,10 | 157.7 C | - | |
| 5, 11 | 29.2 CH2 | 2.40, brt, 6.3 | |
| 6, 12 | 65.8 CH2 | 4.40, t, 6.3 | C-8/C-2,C-4/C-10, C-5/C-11 |
| 13, 14 | 22.4 CH3 | 2.03, brs | C-3/C-9, C-4/C-10, C-5/C-11 |
| Metabolite | Code | Diagnostic Ions m/z (Abundance) | RI | A% of Total Ion Current | B% of Total Ion Current |
|---|---|---|---|---|---|
| Talarodiolide | 1 | 224 [M]•+ (5), 209 [M − Me]+ (4), 194 [M − 2Me]+ (35), 149 [M − 2Me − CO2 − O]+ (60), 70 [M − C8H9O3]+ (100) | 2064 | 3.55 | |
| 3-O-Methylfunicone | 2 | 388 [M]•+ (40), 373 [M − Me]+ (15), 357 [M − 2Me]+, 223 [M − C9O3H9]+ (65), 192 [M − 2Me − C9O3H9]+ (100) | 3006 | 15.26 | 38.12 |
| Cyclo-(Pro-Leu) | 3 | 195 [M − Me]+ (5), 154 [M − C4H9]+ (100), 125 [M − C6H13]+ (15), 111 [M − C7H15]+ (3), 70 [M − C7NO2H11]+ (75) | 2068 | 11.06 | |
| Cyclo-(Pro-Ile) | 4 | 154 [M − C4H9]+ (100), 125 [M − C6H13]+ (120), 111 [M − C7H15]+ (5), 70 [M − C7NO2H11]+ (65) | 2039 | 6.90 | |
| Cyclo-(Pro-Phe) | 5 | 244 [M]•+ (34), 215 [M − C2H4]+ (3), 153 [M − C6H5 − CH2] (28), 125 [M − C3H6 − C6H5] (100) | 2443 | 2.93 | |
| Vermistatin | 6 | 328 [M]•+ (100), 313 [M − Me]+ (10), 285 [M − Me − C2H4]+ (48), 165 [M − C2H4 − C8O2H8]+ (43) | 3105 | 0.424 | 1.124 |
| Penisimplicissin | 7 | 302 [M]•+ (100), 287 [M − Me]+, 273 [M − 2Me]+ (17), 175 [M − Me − C6H7O2] (14), 165 [M − C8O2H8]+ (47) | 2835 | 1.328 | 0.39 |
| Penicillide | 8 | 372 [M − Me]+ (16), 269 [M − 2Me − C5OH10] (100), 253 [M − Me − OCH3 − C5OH10] (20) | 3103 | 3.64 | 6.71 |
| 1-glycerol-linoleate | 9 | 354 [M]•+ (4), 336 [M − OH]+, 262 [M − C3O3H7]+ (63), 234 [M − C4O4H7]+ (12) | 2076 | 4.19 | |
| Methyl ester of palmitic acid | 10 | [27] | 2020 | 5.73 | |
| Methyl ester of linoleic acid | 11 | [27] | 2146 | 17.211 | |
| Methyl ester of stearic acid | 12 | [27] | 2158 | 1.76 | |
| Linoleic acid | 13 | [27] | 2169 | 6.64 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvatore, M.M.; DellaGreca, M.; Nicoletti, R.; Salvatore, F.; Vinale, F.; Naviglio, D.; Andolfi, A. Talarodiolide, a New 12-Membered Macrodiolide, and GC/MS Investigation of Culture Filtrate and Mycelial Extracts of Talaromyces pinophilus. Molecules 2018, 23, 950. https://doi.org/10.3390/molecules23040950
Salvatore MM, DellaGreca M, Nicoletti R, Salvatore F, Vinale F, Naviglio D, Andolfi A. Talarodiolide, a New 12-Membered Macrodiolide, and GC/MS Investigation of Culture Filtrate and Mycelial Extracts of Talaromyces pinophilus. Molecules. 2018; 23(4):950. https://doi.org/10.3390/molecules23040950
Chicago/Turabian StyleSalvatore, Maria Michela, Marina DellaGreca, Rosario Nicoletti, Francesco Salvatore, Francesco Vinale, Daniele Naviglio, and Anna Andolfi. 2018. "Talarodiolide, a New 12-Membered Macrodiolide, and GC/MS Investigation of Culture Filtrate and Mycelial Extracts of Talaromyces pinophilus" Molecules 23, no. 4: 950. https://doi.org/10.3390/molecules23040950







