A Highly Selective and Strong Anti-Interference Host-Guest Complex as Fluorescent Probe for Detection of Amantadine by Indicator Displacement Assay
Abstract
:1. Introduction
2. Results and Discussion
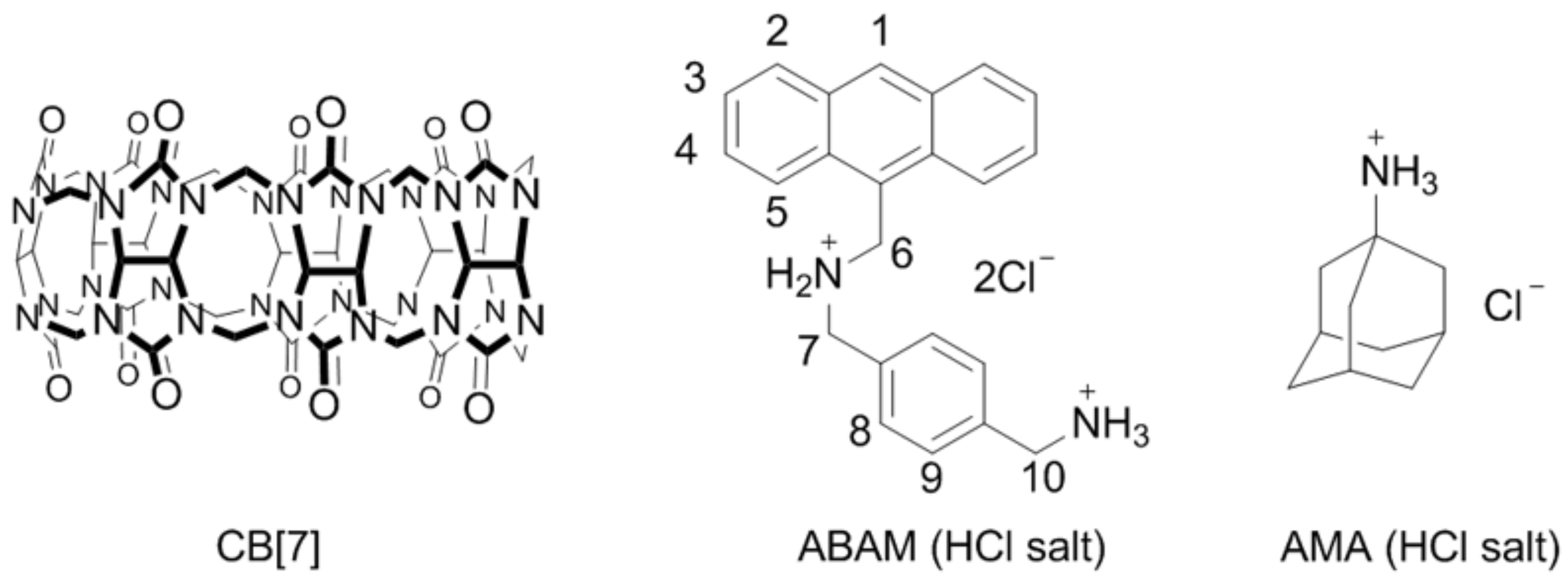
2.1. Binding Studies of the Fluorescent Indicator with CB[7]
2.2. Plots of Standard Curve
2.3. Detection of AMA in Different Samples
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
3.3. Determination of Krel
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Davies, W.L.; Grunert, R.R.; Haff, R.F.; Mcgahen, J.W.; Neumayer, E.M.; Paulshock, M.; Watts, J.C.; Wood, T.R.; Hermann, E.C.; Hoffmann, C.E. Antiviral activity of 1-adamantanamine (amantadine). Science 1964, 144, 862–863. [Google Scholar] [CrossRef] [PubMed]
- Oxford, J.S.; Galbraith, A. Antiviral activity of amantadine: a review of laboratory and clinical data. Pharmacol. Ther. 1980, 11, 181–262. [Google Scholar] [CrossRef]
- Dobrovolny, H.M.; Gieschke, R.; Davies, B.E.; Jumbe, N.L.; Beauchemin, C.A. Neuraminidase inhibitors for treatment of human and avian strain influenza: A comparative modeling study. J. Theor. Biol. 2011, 269, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Dal, P.F.; Thiry, E. Antiviral chemotherapy in veterinary medicine: current applications and perspectives. Rev. Sci. Tech. 2014, 33, 791–801. [Google Scholar]
- Hayden, F.G. Antivirals for influenza: Historical perspectives and lessons learned. Antivir. Res. 2006, 71, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Tarbin, J.; Sharman, M.; Carson, M.; Smith, M.; Smith, S. Screening method for the analysis of antiviral drugs in poultry tissues using zwitterionic hydrophilic interaction liquid chromatography/tandem mass spectrometry. Anal. Chim. Acta 2011, 700, 194–200. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Qiao, J.; Dong, C.; He, C.; Zhao, L.; Tian, Y. Amantadine resistance among H5N1 avian influenza viruses isolated in Northern China. Antivir. Res. 2008, 77, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Sun, Y.; Ren, Y.; Ju, P.; Ren, Y. Simultaneous non-destructive determination of two components of combined paracetamol and amantadine hydrochloride in tablets and powder by NIR spectroscopy and artificial neural networks. J. Pharm. Biomed. 2005, 37, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Titova, A.V.; Arzamastsev, A.P.; Gretskii, S.V. Analysis of rimantadine hydrochloride by near-infrared spectroscopy. Pharm. Chem. J. 2009, 43, 534–537. [Google Scholar] [CrossRef]
- Tsuruoka, Y.; Nakajima, T.; Kanda, M.; Hayashi, H.; Matsushima, Y.; Yoshikawa, S.; Nagata, M.; Koike, H.; Nagano, C.; Sekimura, K.; et al. Simultaneous determination of amantadine, rimantadine, andmemantine in processed products, chicken tissues, and eggs by liquidchromatography with tandem mass spectrometry. J. Chromatogr. B 2017, 1044–1045, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Farajzadeh, M.A.; Nouri, N.; Nabil, A.A.A. Determination of amantadine in biological fluids using simultaneousderivatization and dispersive liquid–liquid microextraction followedby gas chromatography-flame ionization detection. J. Chromatog. B 2013, 940, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Duh, T.H.; Wu, H.L.; Pan, C.W.; Kou, H.S. Fluorimetric liquid chromatographic analysis of amantadine in urine and pharmaceutical formulation. J. Chromatogr. A 2005, 1088, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Nakamura, S.; Matsumura, H.; Fujii, Y. Simultaneous liquid chromatographic assay of amantadine and its four related compounds in phosphate-buffered saline using 4-fluoro-7-nitro-2,1,3-benzoxadiazole as a fluorescent derivatization reagent. Biomed. Chromatogr. 2006, 20, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Ju, W.; Xia, X.; Tan, H.; Chen, M.; Zhang, J.; Xiong, N.; Jiang, M.; Chen, L.; Gong, L. Determination of rimantadine in rat plasma by liquid chromatography/electrospray mass spectrometry and its application in a pharmacokinetic study. J. Chromatogr. B 2008, 864, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.J.; Fang, F.; Han, L.; Ming, M. New method for high-performance liquid chromatographic determination of amantadine and its analogues in rat plasma. J. Pharm. Biomed. 2007, 44, 1100–1105. [Google Scholar]
- Askal, H.F.; Khedr, A.S.; Darwish, I.A.; Mahmoud, R.M. Quantitative thin-layer chromatographic method for determination of amantadine hydrochloride. Int. J. Biomed. Sci. 2008, 4, 155–160. [Google Scholar] [PubMed]
- Yeh, H.H.; Yang, Y.H.; Chen, S.H. Simultaneous determination of memantine and amantadine in human plasma as fluorescein derivatives by micellar electrokinetic chromatography with laser-induced fluorescence detection and its clinical application. Electrophoresis 2010, 31, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Pazourek, J.; Revilla, A.L.; Gajdosová, D.; Havel, J. Validation of a capillary zone electrophoresis method for determination of rimantadine hydrochloride in rimantadin100 Tablets and the method application to dissolution test monitoring. Drug. Dev. Ind. Pharm. 2004, 30, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Reichová, N.; Pazourek, J.; Polásková, P.; Havel, J. Electrophoretic behavior of adamantane derivatives possessing antiviral activity and their determination by capillary zone electrophoresis with indirect detection. Electrophoresis 2002, 23, 259–262. [Google Scholar] [CrossRef]
- Abdel-Ghani, N.T.; Shoukry, A.F.; Hussein, S.H. Flow injection potentiometric determination of amantadine HCl. J. Pharm. Biomed. 2002, 30, 601–611. [Google Scholar] [CrossRef]
- Jalali, F.; Maghooli, R. Potentiometric Determination of trace amounts of amantadine using a modified carbon-paste electrode. Anal. Sci. 2009, 25, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Khalil, N.Y.; Darwish, I.A.; Aboul-Fadl, T. Selective spectrophotometric and spectrofluorometric methods for the determination of amantadine hydrochloride in capsules and plasma via derivatization with 1,2-Naphthoquinone-4-sulphonate. Int. J. Anal. Chem. 2009, 2009, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Darwish, I.A.; Khedr, A.S.; Askal, H.F.; Mahmoud, R.M. Simple fluorimetric method for determination of certain antiviral drugs via their oxidation with cerium (IV). Rev. Bras. Farmacogn. 2005, 60, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.A.; Abdel-Fattah, S.A.; Toubar, S.S.; Sultan, M.A. Spectrophotometric determination of acyclovir and amantadine hydrochloride through metals complexation. J. Anal. Chem. 2004, 59, 33–38. [Google Scholar] [CrossRef]
- Kim, J.; Jung, I.S.; Kim, S.Y.; Lee, E.; Kang, J.K.; Sakamoto, S.; Yamaguchi, K.; Kim, K. New Cucurbituril Homologues: Syntheses, Isolation, Characterization, and X-ray Crystal Structures of Cucurbit[n]uril (n = 5, 7, and 8). J. Am. Chem. Soc. 2000, 122, 540–541. [Google Scholar] [CrossRef]
- Day, A.; Arnold, A.P.; Blanch, R.J.; Snushall, B. Controlling factors in the synthesis of cucurbituril and its homologues. J. Org. Chem. 2001, 66, 8094–8100. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qiu, S.C.; Zhang, J.; Chen, K.; Huang, Y.; Xiao, X.; Zhang, Y.; Li, F.; Zhang, Y.Q.; Xue, S.F.; et al. Twisted Cucurbit[n]urils. Org. Lett. 2016, 18, 4020–4023. [Google Scholar] [CrossRef] [PubMed]
- Lagona, J.; Mukhopadhyay, P.; Chakrabarti, S.; Isaacs, L. The Cucurbit[n]uril Family. Angew. Chem. Int. Ed. 2005, 44, 4844–4870. [Google Scholar] [CrossRef] [PubMed]
- Masson, E.; Ling, X.; Joseph, R.; Kyeremeh, M.L.; Lu, X. Cucurbituril chemistry: A tale of supramolecular success. RSC Adv. 2012, 2, 1213–1247. [Google Scholar] [CrossRef]
- Assaf, K.I.; Nau, W.M. Cucurbiturils: from synthesis to high-affinity binding and catalysis. Chem. Soc. Rev. 2015, 44, 394–418. [Google Scholar] [CrossRef] [PubMed]
- Barrow, S.J.; Kasera, S.; Rowland, M.J.; Barrio, J.D.; Scherman, O.A. Cucurbituril-Based Molecular Recognition. Chem. Rev. 2015, 115, 12320–12406. [Google Scholar] [CrossRef] [PubMed]
- Shetty, D.; Khedkar, J.K.; Park, K.M.; Kim, K. Can we beat the biotin-avidin pair? cucurbit[7]uril-based ultrahigh affinity host-guest complexes and their applications. Chem. Soc. Rev. 2015, 44, 8747–8761. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wang, H.; Zhang, D.; Liu, Y.; Li, Z. Supramolecular organic frameworks (SOFs): Homogeneous regular 2D and 3D pores in water. Natl. Sci. Rev. 2017, 4, 426–436. [Google Scholar] [CrossRef]
- Gao, R.H.; Chen, L.X.; Chen, K.; Tao, Z.; Xiao, X. Development of hydroxylated cucurbit[n]urils, their derivatives and potential applications. Coord. Chem. Rev. 2017, 348, 1–24. [Google Scholar] [CrossRef]
- Kuok, K.I.; Li, S.; Wyman, I.W.; Wang, R. Cucurbit[7]uril: An emerging candidate for pharmaceutical excipients. Ann. N.Y. Acad. Sci. 2017, 1398, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, F.; Zhao, Z.; Liang, F.; Zhang, H.; Liu, S. Cucurbit[10]uril-based chemistry. Chin. Chem. Lett. 2018. [Google Scholar] [CrossRef]
- Liu, S.; Ruspic, C.; Mukhopadhyay, P.; Chakrabarti, S.; Zavalij, P.Y.; Isaacs, L. The cucurbit[n]uril family: Prime components for self-sorting systems. J. Am. Chem. Soc. 2005, 127, 15959–15967. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, S.; Yang, C.; Rekharsky, M.; Ko, Y.H.; Kim, K.; Inoue, Y.; Gilson, M.K. New ultrahigh affinity host-guest complexes of cucurbit[7]uril with bicyclo[2.2.2]octane and adamantane guests: Thermodynamic analysis and evaluation of M2 affinity calculations. J. Am. Chem. Soc. 2011, 133, 3570–3581. [Google Scholar] [CrossRef] [PubMed]
- Dsouza, R.N.; Pischel, U.; Nau, W.M. Fluorescent dyes and their supramolecular host/guest complexes with macrocycles in aqueous solution. Chem. Rev. 2011, 111, 7941–7980. [Google Scholar] [CrossRef] [PubMed]
- Ghale, G.; Nau, W.M. Dynamically analyte-responsive macrocyclic host−fluorophore systems. Acc. Chem. Res. 2014, 47, 2150–2159. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Q.; Qin, Y.F.; Du, L.M.; Li, J.F.; Jing, X.; Chang, Y.X.; Wu, H. Determination of amantadine and rimantadine using a sensitive fluorescent probe. Spectrochem. Acta A 2012, 98, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, Y.L.; Yang, L.L.; Liu, K.; Wang, Z.Q.; Zhang, X. Cucurbit[7]uril as a ‘‘protective agent’’: Controlling photochemistry and detecting 1-adamantanamine. Chem. Commun. 2013, 49, 3905–3907. [Google Scholar] [CrossRef] [PubMed]
- Kuang, S.; Hu, Z.; Zhang, H.; Zhang, X.; Liang, F.; Zhao, Z.; Liu, S. Enhancement of metal–metal interactions inside a large-cavity synthetic host in water. Chem. Commun. 2018, 54, 2169–2172. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, I.; Nau, W.M. The strategic use of supramolecular pKa shifts to enhance the bioavailability of drugs. Adv. Drug. Deliv. Rev. 2012, 64, 764–783. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds ABAM and CB[7] are available from the authors. |





| Technique | Sample | Linear Range (μg/mL) | Minimum Working Level (μg/mL) | Refs |
|---|---|---|---|---|
| HPLC | Biological samples | 0.025~2.5 | 0.02 | [13] |
| LC-MS/MS | Rat plasma | 0.002~0.75 | 0.002 | [14] |
| MEKC | human plasma | 0.002~0.06 | 0.0005 | [17] |
| DLLME | urine | 0.0087~5 | 0.0027 | [11] |
| potentiometry | blood-serum | 0.000118~0.013 | 1.18 × 10−4 | [21] |
| Spectrofluorimerty | Tabellae urine | 0.004~0.9 | 0.0012 | [41] |
| Spectrofluorimerty | Tabellae | 0.000188~0.375 | 6.5 × 10−5 | This work 1 |
| Setting Concentration of AMA (μg/mL) | Δ Int | Calculate Value (μg/mL) | Recovery (%) ± S.D 1 |
|---|---|---|---|
| 0.0282 (150 nM) | −144.06 | 0.028 | 99.3 ± 0.34 |
| 0.00469 (25 nM) | −24.86 | 0.00468 | 99.8 ± 0.78 |
| 0.000939 (5 nM) | −5.26 | 0.00089 | 94.8 ± 1.09 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Zhao, Z.; Zhang, X.; Zhang, H.; Liang, F.; Liu, S. A Highly Selective and Strong Anti-Interference Host-Guest Complex as Fluorescent Probe for Detection of Amantadine by Indicator Displacement Assay. Molecules 2018, 23, 947. https://doi.org/10.3390/molecules23040947
Zhu L, Zhao Z, Zhang X, Zhang H, Liang F, Liu S. A Highly Selective and Strong Anti-Interference Host-Guest Complex as Fluorescent Probe for Detection of Amantadine by Indicator Displacement Assay. Molecules. 2018; 23(4):947. https://doi.org/10.3390/molecules23040947
Chicago/Turabian StyleZhu, Linzhao, Zhiyong Zhao, Xiongzhi Zhang, Haijun Zhang, Feng Liang, and Simin Liu. 2018. "A Highly Selective and Strong Anti-Interference Host-Guest Complex as Fluorescent Probe for Detection of Amantadine by Indicator Displacement Assay" Molecules 23, no. 4: 947. https://doi.org/10.3390/molecules23040947





