Pectin and Pectin-Based Composite Materials: Beyond Food Texture
Abstract
:1. Introduction
1.1. Chemical Structure
1.1.1. Homogalacturonan
1.1.2. Rhamnogalacturonan-I (RG-I)
1.1.3. Rhamnogalacturonan-II (RG-II)
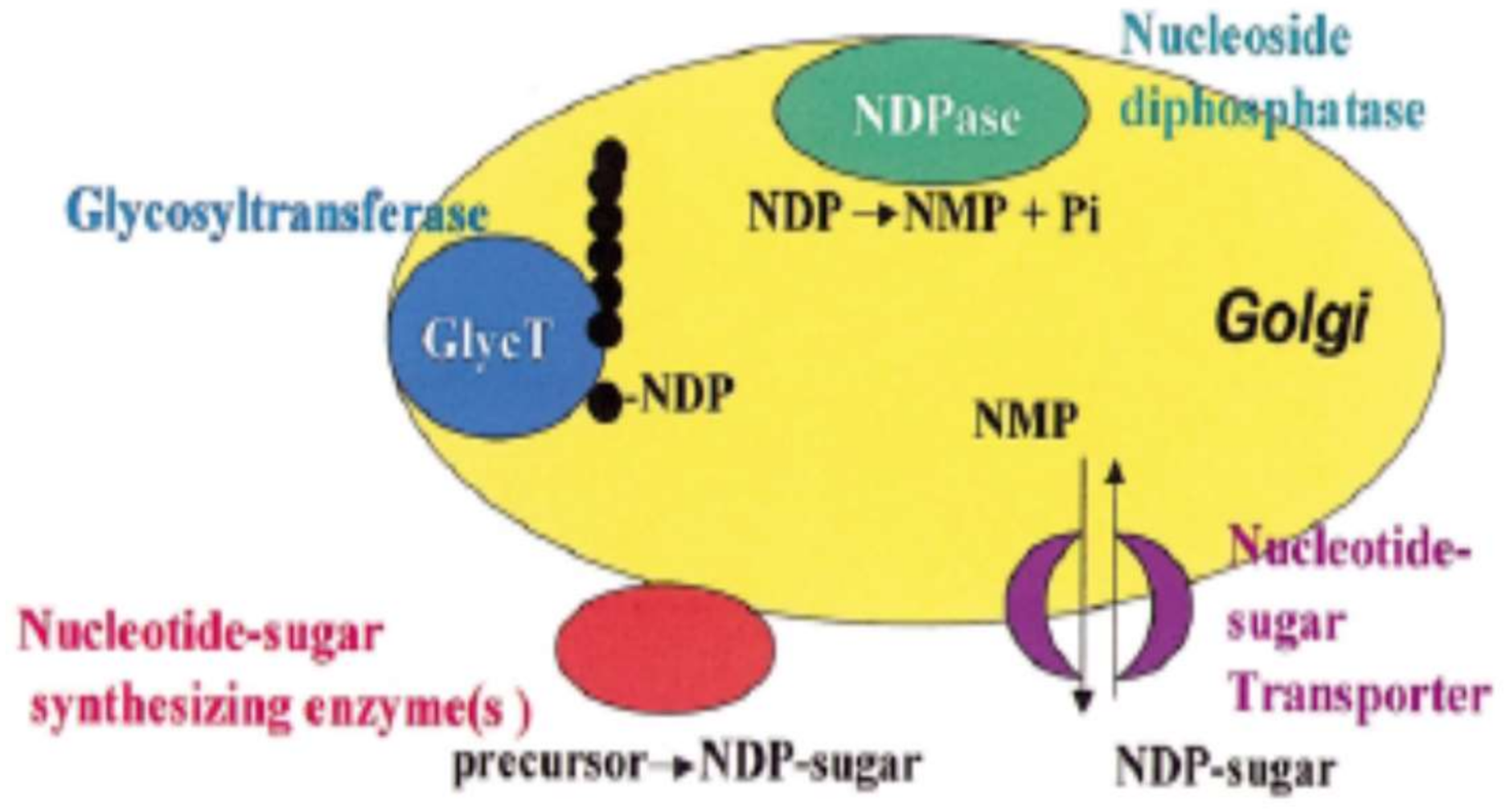
1.2. Biosynthesis of Pectin
1.3. Sources
1.3.1. Traditional Sources
1.3.2. Unconventional Sources
1.3.3. Ferulated Pectins
1.4. Gelling Mechanisms
1.4.1. HM Pectins
1.4.2. LM Pectins
1.4.3. Ferulated Pectins Crosslinking
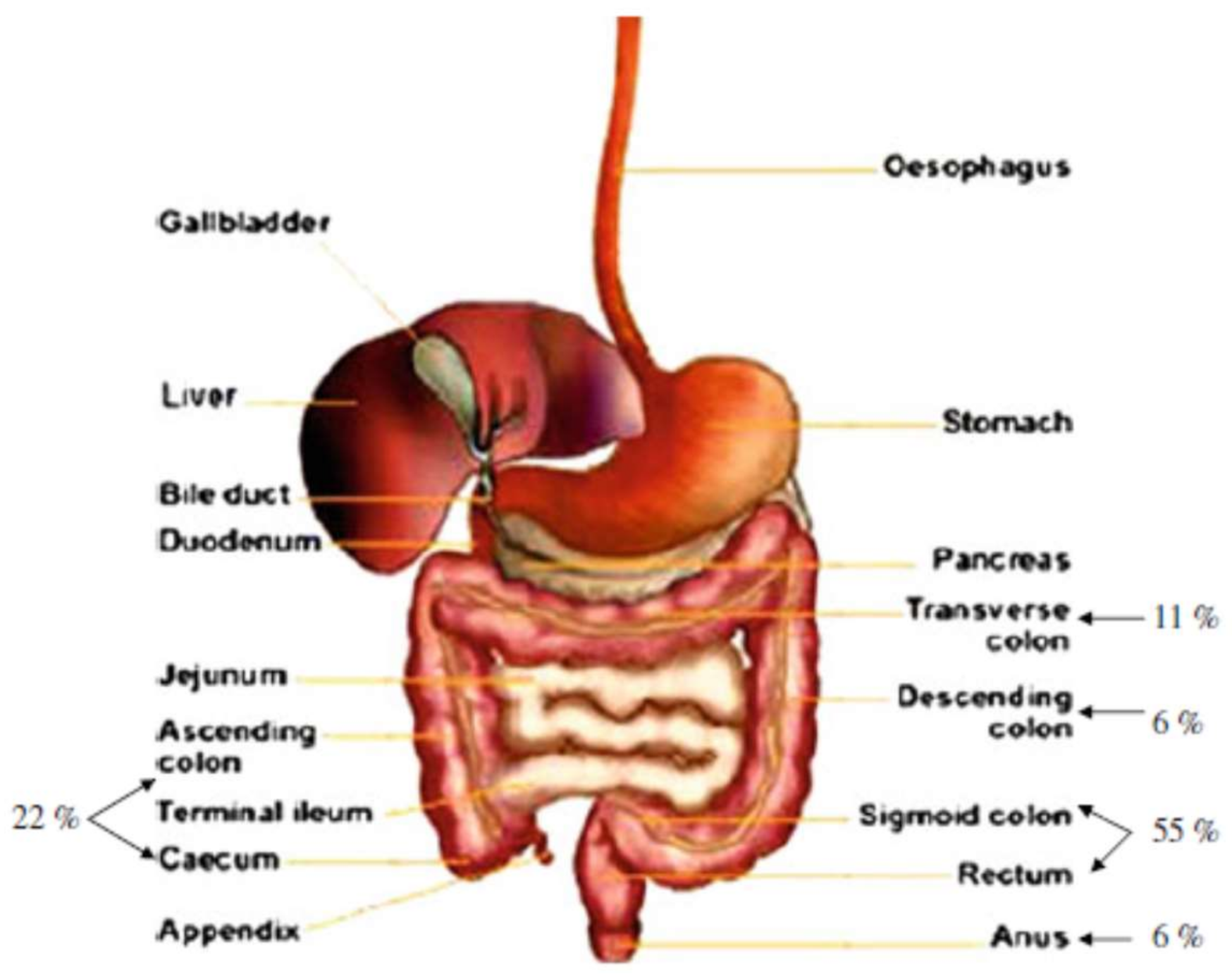
2. Potential Applications of Pectin in Pharmaceutical and Biomedical Industry
2.1. Prebiotic Effect
2.2. Effect on Glucose Levels
2.3. Effect on Cholesterol Levels
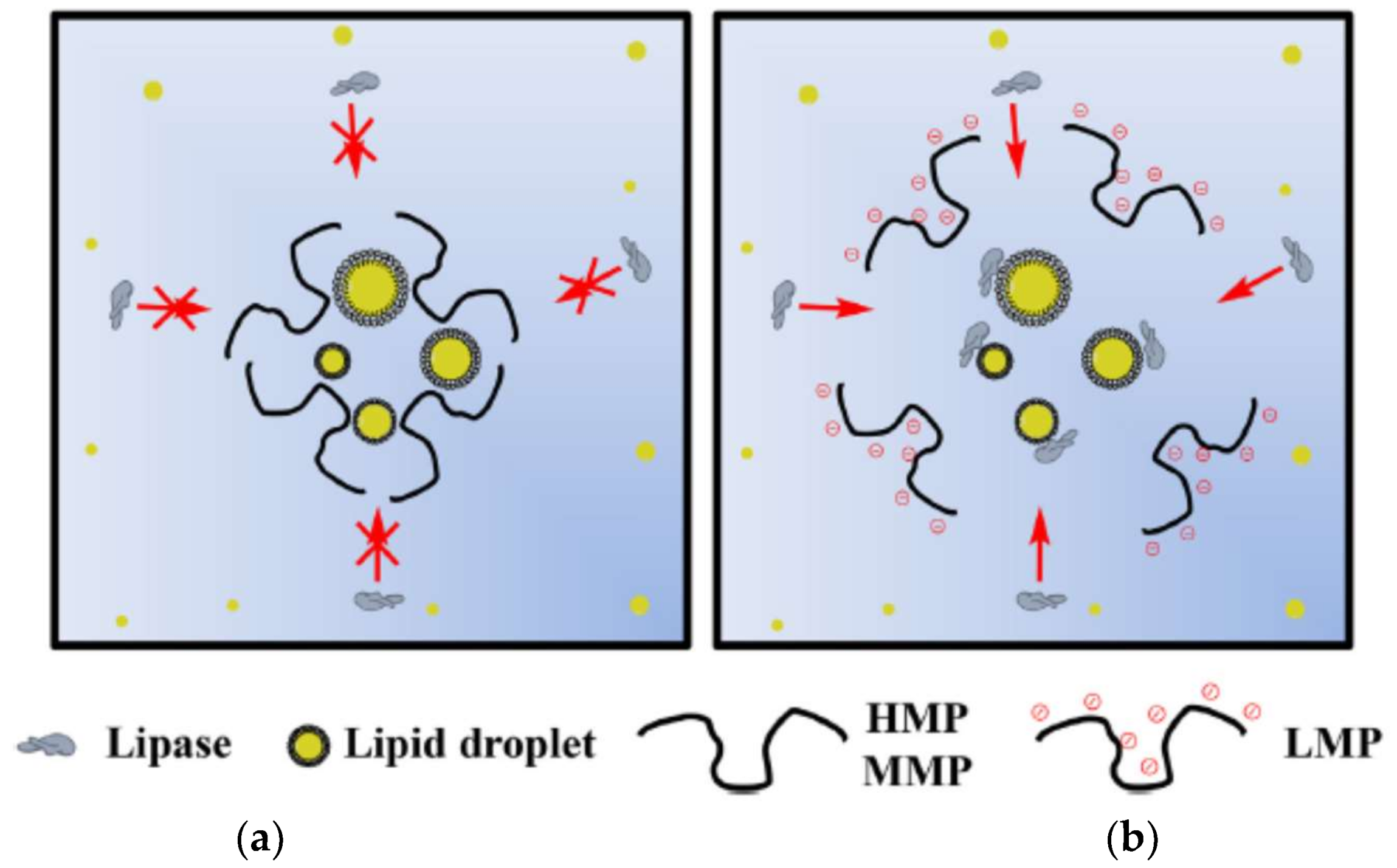
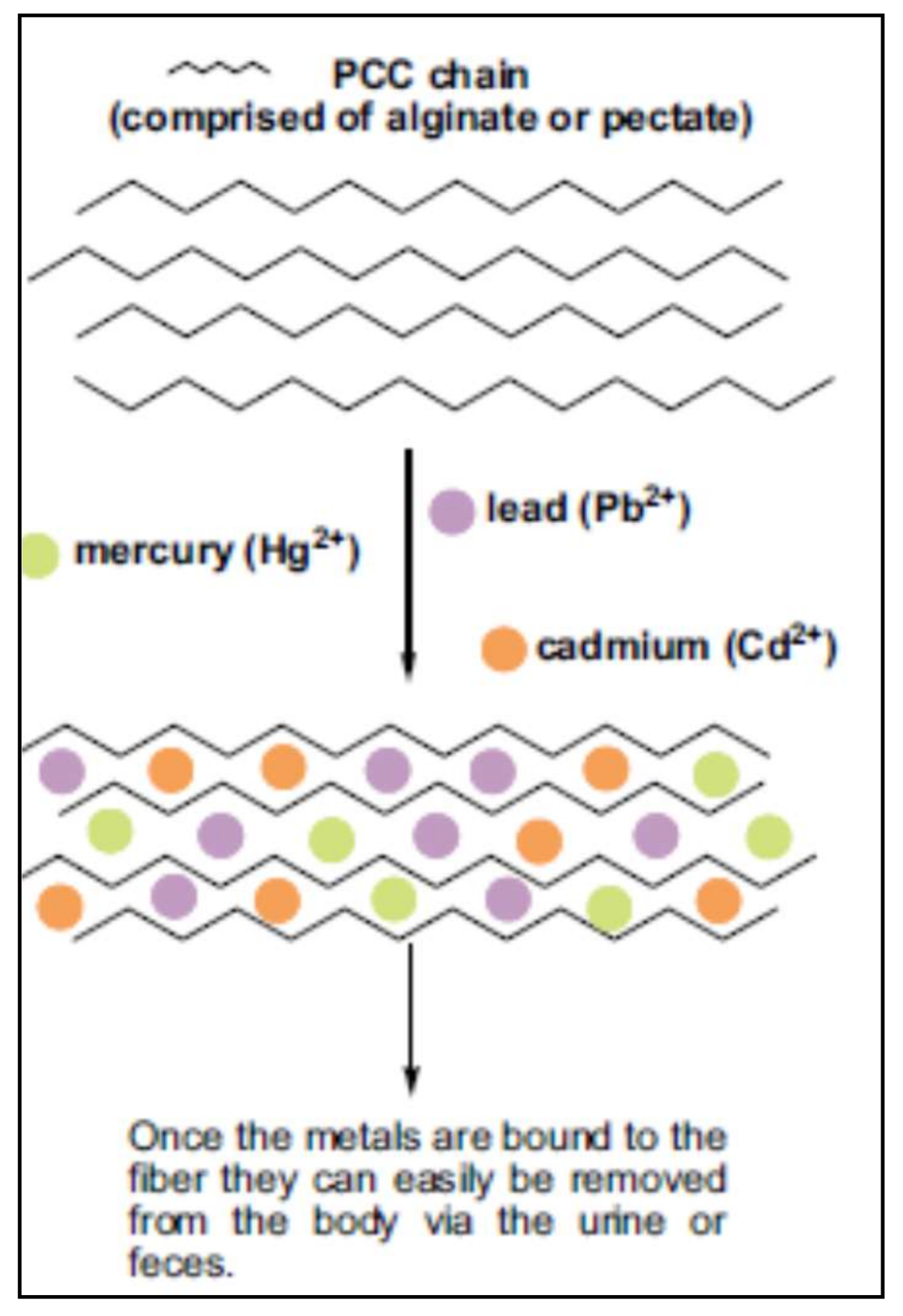
2.4. Removal of Metal Ions
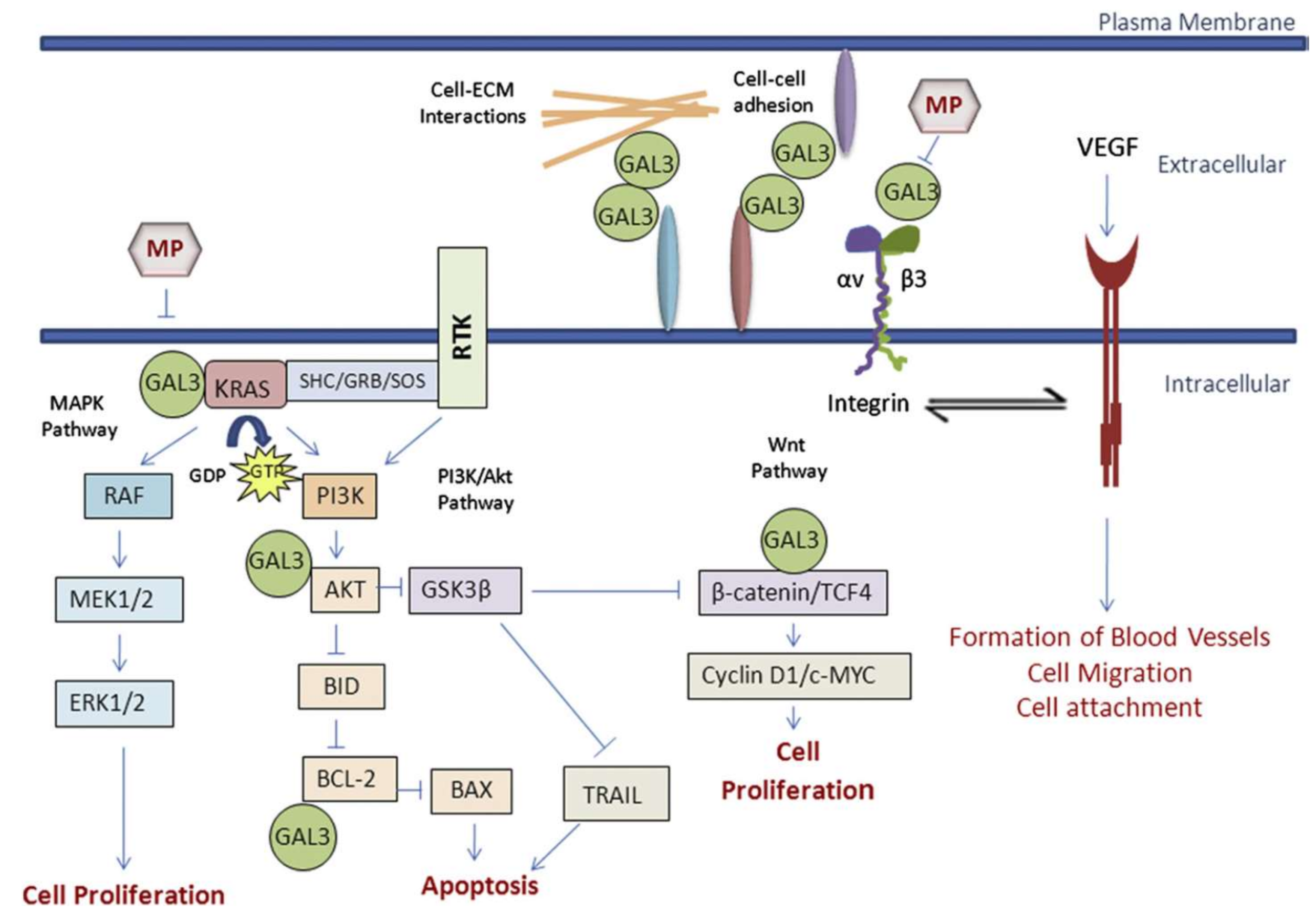
2.5. Cancer Prevention
2.6. Pectin as a Drug Controlled Release Matrix
3. Pectin Composites
3.1. Pectin/Alginate
3.2. Pectin/Chitosan
3.3. Pectin/Protein
3.4. Pectin/Gelatin
3.5. Pectin/Starch
3.6. Pectin/Arabinoxylan
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harholt, J.; Suttangkakul, A.; Scheller, H.V. Biosynthesis of Pectin. Plant Physiol. 2010, 153, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Willats, W.T.; McCartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell Biologyand Prospects for Functional Analysis. Plant Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Mellerowicz, E.J.; Sundberg, B. Wood Cell Walls: Biosynthesis, Developmental Dynamics and their Implications for Wood Properties. Curr. Opin. Plant Biol. 2008, 11, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Van Buren, J.P. Function of Pectin in Plant Tissue Structure and Firmness. In The Chemistry and Technology of Pectin; Walter, R.H., Ed.; Academic Press, Inc.: San Diego, CA, USA, 1991. [Google Scholar]
- Northcote, D.H. Chemistry of the Plant Cell Wall. Annu. Rev. Plant Physiol. 1972, 23, 113–132. [Google Scholar] [CrossRef]
- Parre, E.; Geitmann, A. Pectin and the Role of the Physical Properties of the Cell Wall in Pollen Tube Growth of Solanum chacoense. Planta 2005, 220, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Crombie, H.; Scott, C.; Reid, J. Advances in Pectin and Pectinase Research; Voragen, A.G.J., Schols, H.A., Visser, R.G.F., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 35–45. [Google Scholar]
- Rascón-Chu, A.; Díaz-Baca, J.A.; Carvajal-Millán, E.; López-Franco, Y.; Lizardi-Mendoza, J. New Use for an “Old” Polysaccharide: Pectin-Based Composite Materials. In Handbook of Sustainable Polymers: Structure and Chemistry; Thakur, V.K., Thakur, M.K., Eds.; Pan Stanford Publishing Pte. Ltd.: Singapore, 2016; pp. 72–107. [Google Scholar]
- Jin, D.; West, C. Characteristics of Galacturonic Acid Oligomers as Elicitors of Casbene Synthetase Activity in Castor Bean Seedlings. Plant Physiol. 1984, 74, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Sundar, A.; Rubila, S.; Jayabalan, R.; Ranganathan, T.V. A Review on Pectin: Chemistry due to General Properties of Pectin and its Pharmaceutical Uses. Sci. Rep. 2012, 1, 1–4. [Google Scholar]
- Endress, H. Nonfood Uses of Pectin. In The Chemistry and Technology of Pectin; Walter, R., Ed.; Academic Press: San Diego, CA, USA, 1991; pp. 251–268. [Google Scholar]
- Sánchez, D.; Muguerza, B.; Moulay, L.; Hernandez, R.; Miguel, M.; Aleixandre, A. Highly Methoxylated Pectin Improves Insulin Resistance and other Cardiometabolic Risk Factors in Zucker Fatty Rats. J. Agric. Food Chem. 2008, 56, 3574–3581. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, E.G.; Belshaw, N.J.; Waldron, K.W.; Morris, V.J. Pectin—An Emerging New Bioactive Food Polysaccharide. Trends Food Sci. Technol. 2012, 24, 64–73. [Google Scholar] [CrossRef]
- Liu, L.; Fishman, M.L.; Hicks, K.B. Pectin in Controlled Drug Delivery—A Review. Cellulose 2007, 14, 15–24. [Google Scholar] [CrossRef]
- Luppi, B.; Bigucci, F.; Abruzzo, A.; Corace, G.; Cerchiara, T.; Zecchi, V. Freeze-Dried Chitosan/Pectin Nasal Inserts for Antipsychotic Drug Delivery. Eur. J. Pharm. Biopharm. 2010, 75, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Sriamornsak, P. Application of Pectin in Oral Drug Delivery. Expert Opin. Drug Deliv. 2011, 8, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Ahuja, M.; Kaur, H. Thiolated Pectin Nanoparticles: Preparation, Characterization and ex vivo Corneal Permeation Study. Carbohydr. Polym. 2012, 87, 1606–1610. [Google Scholar] [CrossRef]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in Biomedical Applications of Pectin Gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Govender, P.P.; Mamo, M.A.; Tamulevicius, S.; Thakur, V.K. Recent Progress in Gelatin Hydrogel Nanocomposites for Water Purification and Beyond. Vacuum 2017, 146, 396–408. [Google Scholar] [CrossRef]
- Miculescu, F.; Maidaniuc, A.; Voicu, S.I.; Thakur, V.K.; Stan, G.E.; Ciocan, L.T. Progress in Hydroxyapatite–Starch Based Sustainable Biomaterials for Biomedical Bone Substitution Applications. ACS Sustain. Chem. Eng. 2017, 5, 8491–8512. [Google Scholar] [CrossRef]
- Cheung, R.C.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Sahari, M.A.; Ali, A.M.; Manuchehr, H. Effect of Variety and Acid Washing Method on Extraction Yield and Quality of Sunflower Head Pectin. Food Chem. 2003, 83, 43–47. [Google Scholar] [CrossRef]
- Geerkens, C.H.; Nagel, A.; Meike, K.; Miller-Rostek, P.; Rolf, D.; Schweiggert, R.; Carle, R. Mango Pectin Quality as Influenced by Cultivar, Ripeness, Peel Particle Size, Blanching, Drying, and Irradiation. Food Hidrocoll. 2015, 51, 241–251. [Google Scholar] [CrossRef]
- Mohnen, D. Biosynthesis of Pectins and Galactomannans. In Comprehensive Natural Products Chemistry; Barton, D., Nakanishi, K., Meth-Cohn, O., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1999; Volume 3, pp. 497–527. [Google Scholar]
- Liu, J.; Willför, S.; Xu, C. A Review of Bioactive Plant Polysaccharides: Biological Activities, Functionalization, and Biomedical Applications. Bioact. Carbohydr. Dietary Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Ridley, B.; O’Neill, M.; Mohnen, D. Pectins: Structure, Biosynthesis, and Oligogalacturonide-related Signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Coenen, G.J.; Verhoef, R.P.; Schols, H.A. Pectin, a Versatile Polysaccharide Present in Plant Cell Walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Mukhiddinov, Z.K.; Khalikov, D.K.; Abdusamiev, F.T.; Avloev, C.C. Isolation and Structural Characterization of a Pectin Homo and Ramnogalacturonan. Talanta 2000, 53, 171–176. [Google Scholar] [CrossRef]
- Sriamornsak, P. Chemistry of Pectin and its Pharmaceutical Uses: A Review. Univ. Int. J. 2003, 206–223. [Google Scholar]
- Voragen, F.; Beldman, G.; Schols, H. Chemistry and Enzymology of Pectins. In Advanced Dietary Fibre Technology; McCleary, B.V., Prosky, L., Eds.; Blackwell Science Ltd.: Oxford, UK, 2001; pp. 379–398. [Google Scholar]
- Oakenfull, D.G. The Chemistry of High-Methoxylpectins. In The Chemistry and Technology of Pectin; Walter, R.H., Ed.; Academic Press: San Diego, CA, USA, 1991; pp. 87–108. [Google Scholar]
- Thakur, B.R.; Singh, R.K.; Handa, A.K. Chemistry and Uses of Pectin—A Review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef] [PubMed]
- Besson, V.; Yapo, B.; Maxwell, G.A.; Koffi, K.; Gnakri, D. Cinnamon Apple Pectins: Structural and Rheological Properties. J. Food Stud. 2014, 3, 1–14. [Google Scholar]
- Axelos, M.A.V.; Thibault, J.F. The Chemistry of Low-Methoxyl Pectin Gelation. In The Chemistry and Technology of Pectin; Walter, R.H., Ed.; Academic Press: San Diego, CA, USA, 1991; pp. 109–118. [Google Scholar]
- Nawirska, A.; Kwasniewska, M. Dietary Fiber Fractions from Fruit and Vegetable Processing Waste. Food Chem. 2005, 91, 221–225. [Google Scholar] [CrossRef]
- Albersheim, P.; Darvill, A.; O’Neill, M.; Schols, H.; Voragen, A. Pectin and Pectinases. In Progress in Biotechnology, 1st ed.; Visser, J., Voragen, A.G.J., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1996; pp. 1–987. [Google Scholar]
- Zandleven, J.; Beldman, G.; Bosveld, M.; Schols, H.A.; Voragen, A.G.J. Enzymatic Degradation Studies of Xylogalacturonans from Apple and Potato, using Xylogalacturonan Hydrolase. Carbohydr. Polym. 2006, 65, 495–503. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin Structure and Biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Schols, H.A.; Voragen, A.G.J. Complex pectins: Structure Elucidation using Enzymes. In Pectins and Pectinases; Visser, J., Voragen, A.G.J., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1996; pp. 3–19. [Google Scholar]
- Yu, L.; Mort, A.J. Pectins and Pectinases; Visser, J., Voragen, A.G.J., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1996; pp. 79–88. [Google Scholar]
- Schols, H.A.; Bakx, E.J.; Schipper, D.; Voragen, A.G.J. A Xylogalacturonan Subunit Present in the Modified Hairy Regions of Apple Pectin. Carbohydr. Res. 1995, 279, 265–279. [Google Scholar] [CrossRef]
- Le Goff, A.L.; Renard, C.; Bonnin, E.; Thibault, J.-F. Extraction, Purification and Chemical Characterisation of Xylogalacturonans from Pea Hulls. Carbohydr. Polym. 2001, 45, 325–334. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Crépeau, M.-J.; Thibault, J.-F. Structure of the Repeating Units in the Rhamnogalacturonic Backbone of Apple, Beet and Citrus Pectins. Carbohydr. Res. 1995, 275, 155–165. [Google Scholar] [CrossRef]
- Yapo, B.M.; Lerouge, P.; Thibault, J.-F.; Ralet, M.-C. Pectins from Citrus Peel Cell Walls Contain Homogalacturonans Homogenous with Respect to Molar Mass, Rhamnogalacturonan I and Rhamnogalacturonan II. Carbohydr. Polym. 2007, 69, 426–435. [Google Scholar] [CrossRef]
- Lerouge, P.; Oneill, M.A.; Darvill, A.G.; Albersheim, P. Structural Characterization of endo-Glycanase-Generated Oligoglycosyl Side Chains of Rhamnogalacturonan I. Carbohydr. Res. 1993, 243, 359–371. [Google Scholar] [CrossRef]
- Yapo, B.M. Pectin Substances: From Simple Pectic Polysaccharides to Complex Pectins—A New Hypothetical Model. Carbohydr. Polym. 2011, 86, 373–385. [Google Scholar] [CrossRef]
- Huisman, M.M.; Brüll, L.P.; Thomas-Oates, J.E.; Haverkamp, J.; Schols, H.A.; Voragen, A.G. The Occurrence of Internal (1→5)-Linked Arabinofuranose and Arabinopyranose Residues in Arabinogalactan Side Chains from Soybean Pectic Substances. Carbohydr. Res. 2001, 330, 103–114. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Pilnik, W.; Thibault, J.-F.; Axelos, M.A.V.; Renard, C.M.C.G. Pectins. In Food Polysaccharides and Their Applications; Stephen, A.M., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1995; pp. 287–339. [Google Scholar]
- Renard, C.; Voragen, A.; Thibault, J.-F.; Pilnik, W. Studies on Apple Protopectin V: Structural Studies on Enzymatically Extracted Pectins. Carbohydr. Polym. 1991, 16, 137–154. [Google Scholar] [CrossRef]
- Basic, A.; Harris, P.J.; Stone, B.A. Carbohydrates. In The Biochemistry of Plants; Stumpf, P.K., Conn, E.E., Preiss, J., Eds.; Academic Press: London, UK, 1988; pp. 297–369. [Google Scholar]
- Nothnagel, E. Proteoglycans and Related Components in Plant Cells. Int. Rev. Cytol. 1997, 174, 195–291. [Google Scholar] [PubMed]
- Gaspar, Y.; Johnson, K.L.; Mckenna, J.A.; Bacic, A.; Schultz, C.J. The Complex Structures of Arabinogalactan-Proteins and the Journey towards Understanding Function. Plant Mol. Biol. 2001, 47, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Beldman, G.; Schols, H.; Pitson, S.; Leeuwen, M.S.-V.; Voragen, A. Arabinans and Arabinan Degrading Enzymes. In AdvancesMacromolecular Carbohydrate Research; Sturgeon, R.J., Ed.; JAI Press Inc.: London, UK, 1997; pp. 1–64. [Google Scholar]
- Palin, R.; Geitmann, A. The Role of Pectin in Plant Morphogenesis. BioSystems 2012, 109, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Matsunaga, T.; Pellerin, P.; O’Neill, M.A.; Darvill, A.; Albersheim, P. The Plant Cell Wall Polysaccharide Rhamnogalacturonan II Self-Assembles into a Covalently Cross-Linked Dimer. J. Biol. Chem. 1999, 274, 13098–13104. [Google Scholar] [CrossRef] [PubMed]
- Geshi, N.; Jorgensen, B.; Ulvskov, P. Subcellular Localization and Topology of β(1→4)Galactosyltransferase that Elongates β(1→4) Galactan Side Chains in Rhamnogalacturonan I in Potato. Planta 2004, 218, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Andeme-Onzighi, C.; Lhuissier, F.; Vicre, M.; Yamada, H.; Driouich, A. A (1→3,6)-β-d-galactosyl Epitope Containing Uronic Acids Associated with Bioactive Pectins Occurs in Discrete Cell Wall Domains in Hypocotyls and Root Tissues of Flax Seedlings. Histochem. Cell Biol. 2000, 113, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Nebenführ, A.; Staehelin, L.A. Mobile factories: Golgi Dynamics in Plant Cells. Trends Plant Sci. 2001, 6, 160–167. [Google Scholar] [CrossRef]
- Ibar, C.; Orellana, A. The Import of S-adenosylmethionine into the Golgi Apparatus is Required for the Methylation of Homogalacturonan. Plant Physiol. 2007, 145, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Mohnen, D.; Bar-Peled, L.; Somerville, C. Cell wall synthesis. In Biomass Recalcitrance: Deconstruction the Plant Cell Wall for Bioenergy; Himmel, M., Ed.; Wiley-Blackwell: Oxford, UK, 2008; pp. 94–187. [Google Scholar]
- Pelloux, J.; Rustérucci, C.; Mellerowicz, E.J. New Insights into Pectin Methylesterase Structure and Function. Trends Plant Sci. 2008, 12, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, P.; McCann, M.C.; Roberts, K. Restricted Cell Elongation in Arabidopsis Hypocotyls is Associated with a Reduced Average Pectin Esterification Level. BMC Plant Biol. 2007, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, M.C.; Briggs, S.P.H.; Knox, J.P. Intercellular Adhesion and Cell Separation in Plants. Plant Cell Environ. 2003, 26, 977–989. [Google Scholar] [CrossRef]
- Rascón-Chu, A.; Martínez-López, A.; Carvajal-Millán, E.; Ponce de León-Renova, N.; Márquez-Escalante, J.; Romo-Chacón, A. Pectin from Low Quality “Golden Delicious” Apples: Composition and Gelling Capability. Food Chem. 2009, 116, 101–113. [Google Scholar] [CrossRef]
- Masmoudi, M.; Besbes, S.; Abbes, F.; Robert, C.; Paquot, M.; Blecker, C.; Attia, H. Pectin Extraction from Lemon By-Product with Acidified Date Juice: Effect of Extraction Conditions on Chemical Composition of Pectins. Food Bioprocess Technol. 2012, 5, 687–695. [Google Scholar] [CrossRef]
- May, C.D. Industrial Pectins: Sources, Production and Applications. Carbohydr. Polym. 1990, 12, 79–99. [Google Scholar] [CrossRef]
- Nelson, D.; Smit, C.; Wiles, R. Commercially Important Pectic Substances. In Food Colloids; Graham, H.D., Ed.; The Avi Publishing Company: Westport, CT, USA, 1977; pp. 418–437. [Google Scholar]
- Srivastava, P.; Malviya, R. Sources of Pectin, Extraction and its Applications in Pharmaceutical Industry-An Overview. Indian J. Nat. Prod. Resour. 2011, 2, 10–18. [Google Scholar]
- Rolin, C. Pectin. Industrial Gums. Polysaccharides and Their Derivatives, 3rd ed.; Whistler, R.L., BeMiller, J.N., Eds.; Academic Press: New York, NY, USA, 1993; pp. 1–642. [Google Scholar]
- Ishii, T. Feruloyl Oligosaccharides from Cell Walls of Suspension-Cultured Spinach Cells and Sugar Beet Pulp. Plant Cell Physiol. 1994, 35, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Bunzel, M.; Ralph, J.; Lu, F.; Hatfield, R.D.; Steinhart, H. Lignins and Ferulate-Coniferyl Alcohol Cross-Coupling Products in Cereal Grains. J. Agric. Food Chem. 2004, 52, 6496–6502. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Dua, G.; Jinga, W.; Lia, J.; Yana, J.; Liu, Z. Combined Effects of Independent Variables on Yield and Protein Content of Pectin Extracted from Sugar Beet Pulp by Citric Acid. Carbohydr. Polym. 2015, 129, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, F.M.; Thibault, J.-F. Feruloylated Pectic Substances from Sugar-beet Pulp. Carbohydr. Res. 1986, 154, 177–188. [Google Scholar] [CrossRef]
- Michel, F.; Thibault, J.F.; Mereier, C.; Heitz, F.; Pouillaude, F. Extraction and Characterization of Pectins from Sugar Beet Pulp. J. Food Sci. 1985, 50, 1499–1500. [Google Scholar] [CrossRef]
- Dea, I.; Madden, J. Acetylated Pectic Polysaccharides of Sugar Beet. Food Hydrocoll. 1986, 1, 71–88. [Google Scholar] [CrossRef]
- Renard, C.; Thibault, J.F. Structure and Properties of Apple and Sugar Beet Pectins Extracted by Chelating Agents. Carbohydr. Res. 1993, 244, 99–114. [Google Scholar] [CrossRef]
- Sun, R.C.; Hughes, S. Extraction and Physico-chemical Characterization of Pectins from Sugar Beet Pulp. Polym. J. 1998, 30, 671–677. [Google Scholar] [CrossRef]
- Funami, T.; Zhang, G.; Hiroe, M.; Noda, S.; Nakauma, M.; Asai, I.; Cowman, M.K.; Al-Assaf, S.; Phillips, G.O. Effects of the Proteinaceous Moiety on the Emulsifying Properties of Sugar Beet Pectin. Food Hydrocoll. 2007, 21, 1319–1329. [Google Scholar] [CrossRef]
- Drusch, S. Sugar Beet Pectin: A Novel Emulsifying Wall Component for Microencapsulation of Lipophilic Food Ingredients by Spray-Drying. Food Hydrocoll. 2007, 21, 1223–1228. [Google Scholar] [CrossRef]
- Williams, P.A.; Sayers, C.; Viebke, C.; Senan, C. Elucidation of the Emulsification Properties of Sugar Beet Pectin. J. Agric. Food Chem. 2005, 53, 3592–3597. [Google Scholar] [CrossRef] [PubMed]
- Funami, T.; Nakauma, M.; Ishihara, S.; Tanaka, R.; Inoue, T.; Phillips, G.O. Structural Modifications of Sugar Beet Pectin and the Relationship of Structure to Functionality. Food Hydrocoll. 2011, 25, 221–229. [Google Scholar] [CrossRef]
- Norsker, M.; Jensen, M.; Adler-Nissen, J. Enzymatic Gelation of Sugar Beet Pectin in Food Products. Food Hydrocoll. 2000, 14, 237–243. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Coimbra, M.A.; Silva, J.L.D. Calcium-Mediated Gelation of an Olive Pomace Pectic Extract. Carbohydr. Polym. 2003, 52, 125–133. [Google Scholar] [CrossRef]
- Jiménez, A.; Rodríguez, R.; Fernández-Caro, I.; Guillén, R.; Fernández-Bolaños, J.; Heredia, A. Olive Fruit Cell Wall:Degradation of Pectic Polysaccharides during Ripening. J. Agric. Food Chem. 2001, 49, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Vierhuis, E.; Korver, M.; Schols, H.A.; Voragen, A. Structural Characteristics of Pectic Polysaccharides from Olive Fruit (Olea europaea cv moraiolo) in Relation to Processing for Oil Extraction. Carbohydr. Polym. 2003, 51, 135–148. [Google Scholar] [CrossRef]
- Shi, X.Q.; Chang, K.C.; Schwarz, J.G.; Wiesenborn, D.P.; Shih, M.C. Optimizing Pectin Extraction from Sunflower Heads by Alkaline Washing. Bioresour. Technol. 1996, 58, 291–297. [Google Scholar] [CrossRef]
- Li, G.; Chang, K.C. Viscosity and Gelling Characteristics of Sunflower Pectin As Affected by Chemical and Physical Factors. J. Agric. Food Chem. 1997, 45, 4785–4789. [Google Scholar] [CrossRef]
- Iglesias, M.; Lozano, J. Extraction and Characterization of Sunflower Pectin. J. Food Eng. 2004, 62, 215–223. [Google Scholar] [CrossRef]
- Lin, M.J.Y.; Humbert, E.S.; Sosulski, F.W.; Downey, R.K. Distribution and Composition of Pectins in Sunflower Plants. Can. J. Plant Sci. 1975, 55, 507–513. [Google Scholar] [CrossRef]
- Lin, M.; Sosulski, F.; Humbert, E. Acidic Isolation of Sunflower Pectin. Can. Inst. Food Sci. Technol. J. 1978, 11, 75–77. [Google Scholar] [CrossRef]
- Turquois, T.; Rinaudo, M.; Taravel, F.R.; Heyraud, A. Extraction of Highly Gelling Pectic Substances from Sugar Beet Pulp and Potato Pulp: Influence of Extrinsic Parameters on their Gelling Properties. Food Hydrocoll. 1999, 13, 255–262. [Google Scholar] [CrossRef]
- Ptitchkina, N.M.; Danilova, I.A.; Doxastakis, G.; Kasapis, S.; Morris, E.R. Pumpkin Pectin: Gel Formation at Unusually Low Concentration. Carbohydr. Polym. 1994, 23, 265–273. [Google Scholar] [CrossRef]
- Pagán, J.; Ibarz, A.; Llorca, M.; Coll, L. Quality of Industrial Pectin Extracted from Peach Pomace at Different pH and Temperatures. J. Sci. Food Agric. 1999, 79, 1038–1042. [Google Scholar] [CrossRef]
- Pagán, J.; Ibarz, A.; Llorca, M.; Barbosa-Cánovas, G.V. Extraction and Characterization of Pectin from Stored Peach Pomace. Food Res. Int. 2001, 34, 605–612. [Google Scholar] [CrossRef]
- Dı́az-Rojas, E.; Pacheco-Aguilar, R.; Lizardi, J.; Argüelles-Monal, W.; Valdez, M.; Rinaudo, M.; Goycoolea, F. Linseed Pectin: Gelling Properties and Performance as an Encapsulation Matrix for Shark Liver Oil. Food Hydrocoll. 2004, 18, 293–304. [Google Scholar] [CrossRef]
- Colquhoun, I.J.; Ralet, M.-C.; Thibault, J.-F.; Faulds, C.B.; Williamson, G. Structure identification of feruloylated oligosaccharides from sugar-Beet pulp by NMR spectroscopy. Carbohydr. Res. 1994, 263, 243–256. [Google Scholar] [CrossRef]
- Harris, P.J.; Hartley, R.D. Detection of bound ferulic acid in cell walls of the Gramineae by ultraviolet fluorescence microscopy. Nature 1976, 259, 508–510. [Google Scholar] [CrossRef]
- Hartley, R.D.; Jones, E.C. Diferulic Acid as a Component of Cell Walls of Lolium multiflorum. Phytochemistry 1976, 15, 1157–1160. [Google Scholar] [CrossRef]
- Smith, M.M.; Hartley, R.D. Occurrence and Nature of Ferulic Acid Substitution of Cell-wall Polysaccharides in Graminaceous Plants. Carbohydr. Res. 1983, 118, 65–80. [Google Scholar] [CrossRef]
- Saulnier, L.; Vigouroux, J.; Thibault, J.-F. Isolation and Partial Characterization of Feruloylated Oligosaccharides from Maize Bran. Carbohydr. Res. 1995, 272, 241–253. [Google Scholar] [CrossRef]
- Ralet, M.C.; Thibault, J.F.; Faulds, C.B.; Williamson, G. Isolation and Purification of Feruloylated Oligosaccharides from Cell-walls of Sugar-beet Pulp. Carbohydr. Res. 1994, 263, 227–241. [Google Scholar] [CrossRef]
- Ishii, T. Structure and Functions of Feruloylated Polysaccharides. Plant Sci. 1997, 127, 111–127. [Google Scholar] [CrossRef]
- Guillon, F.; Thibault, J.-F. Methylation Analysis and Mild Acid Hydrolysis of the “Hairy” Fragments of Sugar-Beet Pectins. Carbohydr. Res. 1989, 190, 85–96. [Google Scholar] [CrossRef]
- Ralet, M.-C.; Andre-Leroux, G.; Quemener, B.; Thibault, J.-F. Sugar Beet (Beta vulgaris) Pectins are covalently Cross-linked through Diferulic Bridges in the Cell Wall. Phytochemistry 2005, 66, 2800–2814. [Google Scholar] [CrossRef] [PubMed]
- Micard, V.; Grabber, J.H.; Ralph, J.; Renard, C.M.G.C.; Thibault, J.-F. Dehydrodiferulic Acids from Sugar-beet Pulp. Phytochemistry 1997, 44, 1365–1368. [Google Scholar] [CrossRef]
- Fry, S.C. Phenolic Components of the Primary Cell Wall. Biochem. J. 1982, 203, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Tobita, T. Structural Characterization of Feruloyl Oligosaccharides from Spinach-Leaf Cell Walls. Carbohydr. Res. 1993, 248, 179–190. [Google Scholar] [CrossRef]
- Renard, C.; Wende, G.; Booth, E.J. Cell Wall Phenolics and Polysaccharides in Different Tissues of Quinoa (Chenopodium quinoa Willd). J. Sci. Food Agric. 1999, 79, 2029–2034. [Google Scholar] [CrossRef]
- Renard, C.; Champenois, Y.; Thibault, J.-F. Characterization of the Extractable Pectins and Hemicelluloses of the Cell Wall of Glasswort, Salicornia ramosissima. Carbohydr. Polym. 1993, 22, 239–245. [Google Scholar] [CrossRef]
- Bunzel, M.; Ralph, J.; Steinhart, H. Association of non-Starch Polysaccharides and Ferulic Acid in Grain Amaranth (Amaranthus caudatus L.) Dietary Fiber. Mol. Nutr. Food Res. 2005, 49, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Micard, V.; Renard, C.M.; Thibault, J.-F. Studies on Enzymic Release of Ferulic Acid from Sugar-beet Pulp. Lebensm. Wiss. Technol. 1994, 27, 59–66. [Google Scholar] [CrossRef]
- Waldron, K.W.; Smith, A.C.; Parr, A.J.; Ng, A.; Parker, M.L. New Approaches to Understanding and Controlling Cell Separation in Relation to Fruit and Vegetable Texture. Trends Food Sci. Technol. 1997, 8, 213–221. [Google Scholar] [CrossRef]
- Parker, M.L.; Waldron, K.W. Texture of Chinese Water Chestnut: Involvement of Cell Wall Phenolics. J. Sci. Food Agric. 1995, 68, 337–346. [Google Scholar] [CrossRef]
- Fry, S. Cross-Linking of Matrix Polymers in the Growing Cell Walls of Angiosperms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1986, 37, 165–186. [Google Scholar] [CrossRef]
- Jung, H.J.G.; Fahey, G.C. Effect of Phenolic Compound Removal on in vitro Forage Gigestibility. J. Agric. Food Chem. 1981, 29, 817–820. [Google Scholar] [CrossRef]
- Fry, S.C. The Growing Plant Cell Wall: Chemical and Metabolic Analysis; Longman Group Limited: Harlow, UK, 1988. [Google Scholar]
- Ikegawa, T.; Mayama, S.; Nakayashiki, H.; Kato, H. Accumulation of Diferulic Acid During the Hypersensitive Response of Oat Leaves to Puccinia coronata sp. avenae and its Role in the Resistance of Oat Tissues to Cell Wall Degrading Enzymes. Physiol. Mol. Plant Pathol. 1996, 48, 245–256. [Google Scholar] [CrossRef]
- Crandall, P.G.; Wicker, L. Pectin Internal Gel Strength: Theory, Measurement, and Methodology. In ACS Symposium Series Chemistry and Function of Pectins; ACS Publications: Washington, DC, USA, 1986; pp. 88–102. [Google Scholar]
- Oakenfull, D.; Scott, A. Hydrophobic Interaction in the Gelation of High Methoxyl Pectins. J. Food Sci. 1984, 49, 1093–1098. [Google Scholar] [CrossRef]
- Löfgren, C.; Walkenström, P.; Hermansson, A.-M. Microstructure and Rheological Behavior of Pure and Mixed Pectin Gels. Biomacromolecules 2002, 3, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Naresh, L.; Dhuldhoya, N.; Merchant, S.; Merchant, U. An Overview on Pectins. Times Food Process. J. 2006, 44–51. [Google Scholar]
- Pilgrim, G.W.; Walter, R.H.; Oakenfull, D.G. Jams, Jellies, and Preserves. In The Chemistry and Technology of Pectin; Walter, R.H., Ed.; Academic Press: San Diego, CA, USA, 1991. [Google Scholar]
- Fraeye, I.; Doungla, E.; Duvetter, T.; Moldenaers, P.; Loey, A.V.; Hendrickx, M. Influence of Intrinsic and Extrinsic Factors on Rheology of Pectin–calcium Gels. Food Hydrocoll. 2009, 23, 2069–2077. [Google Scholar] [CrossRef]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Corrigendum to “Advances in biomedical applications of pectin gels”. Int. J. Biol. Macromol. 2013, 55, 681–689. [Google Scholar] [CrossRef]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.; Thom, D. Biological Interactions between Polysaccharides and Divalent Cations: The egg-Box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Morris, E.R.; Powell, D.A.; Gidley, M.J.; Rees, D.A. Conformations and Interactions of Pectins. I. Polymorphism between Gel and Solid States of Calcium Polygalacturonate. J. Mol. Biol. 1982, 155, 507–516. [Google Scholar] [CrossRef]
- Thibault, J.-F.; Guillon, F.; Rombouts, F.M. Gelation of Sugar Beet Pectins by Oxidative Coupling. In The Chemistry and Technology of Pectins; Walter, R., Ed.; Academic Press: San Diego, CA, USA, 1991; pp. 109–118. [Google Scholar]
- Figueroa-Espinoza, M.C.; Rouau, X. Oxidative Cross-Linking of Pentosans by a Fungal Laccase and Horseradish Peroxidase: Mechanism of Linkage between Feruloylated Arabinoxylans. Cereal Chem. J. 1998, 75, 259–265. [Google Scholar] [CrossRef]
- Oosterveld, A.; Beldman, G.; Voragen, A.G.J. Oxidative Cross-linking of Pectic Polysaccharides from Sugar Beet Pulp. Carbohydr. Res. 2000, 328, 199–207. [Google Scholar] [CrossRef]
- Saulnier, L.; Thibault, J.-F. Ferulic Acid and Diferulic Acids as Components of Sugar-beet Pectins and Maize Bran Heteroxylans. J. Sci. Food Agric. 1999, 79, 396–402. [Google Scholar] [CrossRef]
- Oosterveld, A.; Grabber, J.H.; Beldman, G.; Ralph, J.; Voragen, A.G.J. Formation of Ferulic Acid Dehydrodimers through Oxidative Cross-linking of Sugar Beet Pectin. Carbohydr. Res. 1997, 300, 179–181. [Google Scholar] [CrossRef]
- Roberfroid, M. Prebiotics: The Concept Revisited. J. Nutr. 2007, 137, 830S–837S. [Google Scholar] [CrossRef] [PubMed]
- Blaut, M. Relationship of Prebiotics and Food to Intestinal Microflora. Eur. J. Nutr. 2002, 41, 11–16. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, D.J.A.; Gassul, M.A.; Leeds, A.R.; Metz, G.; Dilawari, J.B.; Slavin, B.; Blendis, L.M. Effect of Dietary Fiber on Complication of Gastric Surgery. Gastroenterology 1977, 72, 215–227. [Google Scholar]
- Schwartz, S.E.; Levine, R.A.; Singh, A.; Schiedecker, J.R.; Track, N.S. Sustained Pectin Ingestion Delays Gastric Emptying. Gastroenterology 1982, 83, 812–817. [Google Scholar] [PubMed]
- Hotchkiss, A.T.; Olano-Martin, E.; Grace, W.E.; Gibson, G.R.; Rastall, R.A. Pectic Oligosaccharides as Prebiotics. J. Am. Chem. Soc. 2003, 5, 54–62. [Google Scholar]
- Gómez, B.C.A.; Gullón, B.; Remoroza, C.; Schols, H.A.; Parajó, J.C.; Alonso, J.L. Purification, Characterization, and Prebiotic Properties of Pectic Oligosaccharides from Orange Peel Wastes. J. Agric. Food Chem. 2014, 62, 9769–9782. [Google Scholar] [CrossRef] [PubMed]
- Gómez, B.C.A.; Gullón, B.; Yáñez, R.; Schols, H.; Alonso, J.L. Prebiotic Potential of Pectins and Pectic Oligosaccharides Derived from Lemon Peel Wastes and Sugar Beet Pulp: A comparative Evaluation. J. Funct. Foods 2016, 20, 108–121. [Google Scholar] [CrossRef]
- Chung, W.S.F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A.S.; Wells, J.M.; Flint, H.J.; Duncan, S.H. Prebiotic Potential of Pectin and Pectic Oligosaccharides to Promote Anti-Inflammatory Commensal Bacteria in the Human Colon. FEMS Microbiol. Ecol. 2017, 93, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Moriya, M.; Sakurai, M.H.; Kiyohara, H.; Tabuchi, Y.; Yamada, H. Corrigendum to “Stimulatory Effect of a Pectic Polysaccharide from a Medicinal Herb, the Roots of Bupleurum falcatum L., on G-CSF Secretion from Intestinal Epithelial Cells”. Int. Immunopharmacol. 2008, 8, 1713. [Google Scholar] [CrossRef]
- Gullón, B.; Gómez, B.; Martínez-Sabajanes, M.; Yáñez, R.; Parajó, J.; Alonso, J. Pectic Oligosaccharides: Manufacture and Functional properties. Trends. Food Sci. Technol. 2013, 30, 153–161. [Google Scholar] [CrossRef]
- Islamova, Z.I.; Ogai, D.K.; Abramenko, O.I.; Lim, A.L.; Abduazimov, B.B.; Malikova, M.K.; Rakhmanberdyeva, R.K.; Khushbaktova, Z.A.; Syrov, V.N. Comparative Assessment of the Prebiotic Activity of Some Pectin Polysaccharides. Pharm. Chem. J. 2017, 51, 288–291. [Google Scholar] [CrossRef]
- Sousa, R.; Florindo, M.; Mendes, M.; Araújo, D.; Goes, I.; Alves, P.; Pinto, Í. Hypoglycemic Effect of New Pectin Isolated from Passiflora glandulosa cav in Aalloxan-induced Diabetic Mice. World J. Pharm. Pharm. Sci. 2015, 4, 1571–1586. [Google Scholar]
- Makarova, E.; Górnaś, P.; Konrade, I.; Tirzite, D.; Cirule, H.; Gulbe, A.; Pugajeva, I.; Seglina, D.; Dambrova, M. Acute Anti-Hyperglycaemic Effects of an Unripe Apple Preparation containing Phlorizin in Healthy Volunteers: A Preliminary Study. J. Sci. Food Agric. 2015, 95, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Wicker, L.; Kim, Y.; Kim, M.J.; Thirkield, B.; Lin, Z.; Jung, J. Pectin as a Bioactive Polysaccharide–Extracting Tailored Function from Less. Food Hydrocoll. 2014, 42, 251–259. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, M.; Yang, Z.; Pan, S. Anti-Diabetic Effect of Citrus Pectin in Diabetic Rats and Potential Mechanism via PI3K/Akt Signaling Pathway. Int. J. Biol. Macromol. 2016, 89, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Maciel, V.; Yoshida, C.; Pereira, S.; Goycoolea, F.; Franco, T. Electrostatic Self-Assembled Chitosan-Pectin Nano- and Microparticles for Insulin Delivery. Molecules 2017, 22, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Rascón-Chu, A.; Díaz-Baca, J.A.; Carvajal-Millán, E.; Pérez-López, E.; Hotchkiss, A.T.; Gonzáles-Ríos, H.; Balaldrán-Quintana, R.; Campa-Mada, A. Electrosprayed Core-shell Composite Microbeads based on Pectin-Arabinoxylans for Insulin Carrying: Aggregation and Size Dispersion Control. Polymers 2018, 10, 108. [Google Scholar] [CrossRef]
- Gunness, P.; Gidley, M.J. Mechanisms Underlying the Cholesterol-Lowering Properties of Soluble Dietary Fibre Polysaccharides. Food Funct. 2010, 1, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Theuwissen, E.; Mensink, R.P. Water-soluble Dietary Fibers and Cardiovascular Disease. Physiol. Behav. 2008, 94, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Naqash, F.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gani, A. Emerging Concepts in the Nutraceutical and Functional Properties of Pectin—A Review. Carbohydr. Polym. 2017, 168, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Espinal-Ruiz, M.; Restrepo-Sánchez, L.-P.; Narváez-Cuenca, C.-E.; Mcclements, D.J. Impact of Pectin Properties on Lipid Digestion under Simulated Gastrointestinal Conditions: Comparison of Citrus and Banana Passion Fruit (Passifloratripartita var. mollissima) Pectins. Food Hydrocoll. 2016, 52, 329–342. [Google Scholar] [CrossRef]
- Andersen, O.; Aaseth, J. Molecular Mechanisms of in Vivo Metal Chelation: Implications for Clinical Treatment of Metal Intoxications. Environ. Health Perspect. 2002, 110, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Ahmady-Asbchin, S.; Andres, Y.; Gerente, C.; Cloirec, P.L. Natural Seaweed Waste as Sorbent for Heavy Metal Removal from Solution. Environ. Technol. 2009, 30, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Khotimchenko, Y.; Khozhaenko, E.; Kovalev, V.; Khotimchenko, M. Cerium Binding Activity of Pectins Isolated from the Seagrasses Zostera marina and Phyllospad ixiwatensis. Mar. Drugs 2012, 10, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Khozhaenko, E.; Kovalev, V.; Podkorytova, E.; Khotimchenko, M. Removal of the Metal Ions from Aqueous Solutions by Nanoscaled Low Molecular Pectin Isolated from Seagrass Phyllospadix watensiss. Sci. Total Environ. 2016, 565, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Kyomugasho, C.; Gwala, S.; Christiaens, S.; Kermani, Z.J.; Loey, A.M.V.; Grauwet, T.; Hendrickx, M.E. Pectin Nanostructure Influences Pectin-cation Interactions and in vitro-bioaccessibility of Ca2+, Zn2+, Fe2+ and Mg2+-ions in Model Systems. Food Hydrocoll. 2017, 62, 299–310. [Google Scholar] [CrossRef]
- Eliaz, I.; Hotchkiss, A.T.; Fishman, M.L.; Rode, D. The Effect of Modified Citrus Pectin on Urinary Excretion of Toxic Elements. Phytother. Res. 2006, 20, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Y.; Liang, L.; Fan, X.; Yu, Z.; Hotchkiss, A.T.; Wilk, B.J.; Eliaz, I. The Role of Modified Citrus Pectin as an Effective Chelator of Lead in Children Hospitalized with Toxic Lead Levels. Altern. Ther. Health Med. 2008, 14, 34–38. [Google Scholar] [PubMed]
- Khotimchenko, M.; Kolenchenko, E.; Khotimchenko, Y. Zinc-Binding Activity of Different Pectin Compounds in Aqueous Solutions. J. Colloid Interface Sci. 2008, 323, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Leclere, L.; Cutsem, P.V.; Michiels, C. Anti-Cancer activities of pH- or heat-Modified pectin. Front. Pharmacol. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guess, B.W.; Scholz, M.C.; Strum, S.B.; Lam, R.Y.; Johnson, H.J.; Jennrich, R.I. Modified Citrus Pectin (MCP) Increases the Prostate-Specific Antigen Doubling Time in Men with Prostate Cancer: A Phase II Pilot Study. Prostate Cancer Prostatic Dis. 2003, 6, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.L.; Dreaden, T.M.; Theobald, L.K.; Tran, N.M.; Beal, T.L.; Eid, M.; Gao, M.Y.; Shirley, R.B.; Stoffel, M.T.; Kumar, M.V.; et al. Pectin Induces Apoptosis in Human Prostate Cancer Cells: Correlation of Apoptotic Function with Pectin Structure. Glycobiology 2007, 17, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.; Djaldetti, M.; Salman, H.; Bessler, H. Effect of Citrus Pectin on Malignant Cell Proliferation. Biomed. Pharmacother. 2010, 64, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Niu, Y.; Wu, H.; Sun, Y.; Li, Q.; Kong, X.; Liu, L.; Mei, Q. Modified Apple Polysaccharides Could Induce Apoptosis in Colorectal Cancer Cells. J. Food Sci. 2010, 75, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wang, P.; Du, Z.; Wang, W.; Cong, Q.; Zheng, C.; Jin, C.; Ding, K.; Shao, C. Structural Elucidation of a Pectin from Flowers of Lonicera japonica and its Antipancreatic Cancer Activity. Int. J. Biol. Macromol. 2016, 88, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Sathisha, U.V.; Jayaram, S.; Nayaka, H.; Dharmesh, S.M. Inhibition of Galectin-3 Mediated Cellular Interactions by Pectic Polysaccharides from Dietary Sources. Glycoconj. J. 2007, 24, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-Y.; Huang, Z.-L.; Yang, G.-H.; Lu, W.-Q.; Yu, N.-R. Inhibitory Effect of Modified Citrus Pectin on Liver Metastases in a Mouse Colon Cancer Model. World J. Gastroenterol. 2008, 14, 7386–7391. [Google Scholar] [CrossRef] [PubMed]
- Glinsky, V.V.; Raz, A. Modified Citrus Pectin Anti-Metastatic Properties: One Bullet, Multiple Targets. Carbohydr. Res. 2009, 344, 1788–1791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lan, Y.; Zheng, Y.; Liu, F.; Zhao, D.; Mayo, K.H.; Zhou, Y.; Tai, G. Identification of the Bioactive Components from pH-modified Citrus Pectin and their Inhibitory Effects on Galectin-3 Function. Food Hydrocoll. 2016, 58, 113–119. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, F.; Liu, X.; Ange, K.S.; Zhang, A.; Li, Q.; Linhardt, R.J. Isolation of a Lectin binding Rhamnogalacturonan-I containing Pectic Polysaccharide from Pumpkin. Carbohydr. Polym. 2017, 163, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Rhamnogalacturonan I containing Jomogalacturonan Inhibits Colon Cancer Cell Proliferation by decreasing ICAM1 Expression. Carbohydr. Polym. 2015, 132, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Modified Sugar Beet Pectin Induces Apoptosis of Colon Cancer Cells Via an Interaction with the Neutral Sugar Side-chains. Carbohydr. Polym. 2016, 136, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Azémar, M.; Hildenbrand, B.; Haering, B.; Heim, M.E.; Unger, C. Clinical Benefit in Patients with Advanced Solid Tumors Treated with Modified Citrus Pectin: A Prospective Pilot Study. J. Clin. Oncol. 2007, 1, 73–80. [Google Scholar] [CrossRef]
- Katav, T.; Liu, L.; Traitel, T.; Goldbart, R.; Wolfson, M.; Kost, J. Modified pectin-Based Carrier for Gene Delivery: Cellular Barriers in Gene Delivery Course. J. Control. Release 2008, 130, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Munarin, F.; Guerreiro, S.G.; Grellier, M.A.; Tanzi, M.C.; Barbosa, M.A.; Petrini, P.; Granja, P.L. Pectin-Based Injectable Biomaterials for Bone Tissue Engineering. Biomacromolecules 2011, 12, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, P.; Gandhimathi, C.; Venugopal, J.R.; Becker, D.L.; Ramakrishna, S.; Srinivasan, D.K. Controlled Release of Drugs in Electrosprayed Nanoparticles for Bone Tissue Engineering. Adv. Drug Deliv. Rev. 2015, 94, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.; Nasi, W. Gels for Use in Wound Management. World Patent No. WO/2008/015475, 7 February 2008. [Google Scholar]
- Mishra, R.K.; Datt, M.; Pal, K.; Banthia, A.K. Preparation and Characterization of Amidated Pectin based Hydrogels for Drug Delivery System. J. Mater. Sci. Mater. Med. 2008, 19, 2275–2280. [Google Scholar] [CrossRef] [PubMed]
- Sande, S.A. Pectin-based Oral Drug Delivery to the Colon. Expert Opin. Drug Deliv. 2005, 2, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Penhasi, A. Preparation and Characterization of in-situ Ionic Cross-linked Pectin Films: II. Biodegradation and Drug Diffusion. Carbohydr. Polym. 2017, 157, 651–659. [Google Scholar] [CrossRef] [PubMed]
- De Souza, J.C.A.R.R.D.; Feitosa, J.P.; Ricardo, N.M.; Trevisan, M.T.S.; Paula, H.C.B.D.; Ulrich, C.M.; Owen, R.W. Spray-Drying Encapsulation of Mangiferin using Natural Polymers. Food Hydrocoll. 2013, 33, 10–18. [Google Scholar] [CrossRef]
- Wanasawas, P.; Sinchaipanid, N.; Fell, J.T.; Mitrevej, A. Influence of Pectin and Calcium Pectinate Films on in vitro Drug Release from Coated Theophylline Pellets. J. Drug Deliv. Sci. Technol. 2013, 23, 465–470. [Google Scholar] [CrossRef]
- Jantrawut, P.; Akazawa, H.; Ruksiriwanich, W. Anti-cancer Activity of Rutin Encapsulated in Low Methoxyl Pectin Beads. Int. J. Pharm. Pharm. Sci. 2014, 6, 199–202. [Google Scholar]
- Sriamornsak, P.; Wattanakorn, N.; Takeuchi, H. Study on the Mucoadhesion Mechanism of Pectin by Atomic Force Microscopy and Mucin-particle Method. Carbohydr. Polym. 2010, 79, 54–59. [Google Scholar] [CrossRef]
- Fallon, M.; Reale, C.; Davies, A.; Lux, A.E.; Kumar, K.; Stachowiak, A.; Galvez, R. Efficacy and Safety of Fentanyl Pectin Nasal Sprays Compared with Immediate-release Morphine Sulfate Tablets in the Treatment of Breakthrough Cancer Pain: A Multicenter, Randomized, Controlled, Double-blind, Double-dummy Multiple-crossover Study. J. Support. Oncol. 2011, 9, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.; Sitte, T.; Elsner, F.; Reale, C.; Espinosa, J.; Brooks, D.; Fallon, M. Consistency of Efficacy, Patient Acceptability, and Nasal Tolerability of Fentanyl Pectin Nasal Spray Compared with Immediate-Release Morphine Sulfate in Breakthrough Cancer Pain. J. Pain Symp. Manag. 2011, 41, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Ueberall, M.; Lorenzl, S.; Lux, E.; Voltz, R.; Perelman, M. Efficacy, Safety, and Tolerability of Fentanyl Pectin Nasal Spray in Patients with Breakthrough Cancer Pain. J. Pain Res. 2016, 9, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A. The Use of Mucoadhesive Polymers in Ocular Drug Delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Yates, K.M. In Situ Gel Formation of Pectin. U.S. Patent 6,777,000, 17 August 2004. [Google Scholar]
- Giunchedi, P.; Conte, U.; Chetoni, P.; Saettone, M. Pectin Microspheres as Ophthalmic Carriers for Piroxicam: Evaluation in vitro and in vivo in Albino Rabbits. Eur. J. Pharm. Sci. 1999, 9, 1–7. [Google Scholar] [CrossRef]
- Liu, L.; Fishman, M.L.; Kost, J.; Hicks, K.B. Pectin-Based Systems for Colon-Specific Drug Delivery via Oral Route. Biomaterials 2003, 24, 3333–3343. [Google Scholar] [CrossRef]
- Maestrelli, F.; Cirri, M.; Corti, G.; Mennini, N.; Mura, P. Development of enteric-Coated Calcium Pectinate Microspheres Intended for Colonic Drug Delivery. Eur. J. Pharm. Biopharm. 2008, 69, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.W.; Colombo, G.; Sonvico, F. Pectin Matrix as Oral Drug Delivery Vehicle for Colon Cancer Treatment. AAPS PharmSciTech 2011, 12, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Dev, R.K.; Bali, V.; Pathak, K. Novel Microbially Triggered Colon Specific Delivery System of 5-Fluorouracil: Statistical Optimization, in vitro, in vivo, Cytotoxic and Stability Assessment. Int. J. Pharm. 2011, 411, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Arnold, R.D.; Wicker, L. Pectin and Charge Modified Pectin Hydrogel Beads as a Colon-Targeted Drug Delivery Carrier. Colloids Surf. B 2013, 104, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Y.; Hsu, D.K.; Liu, F.-T. Expression of Galectin-3 Modulates T-cell Growth and Apoptosis. Proc. Natl. Acad. Sci. USA 1996, 93, 6737–6742. [Google Scholar] [CrossRef] [PubMed]
- Lopes da Silva, J.A.; Rao, M.A. Pectins: Structure, Functionality, and Uses. In Food Polysaccharides and Their Applications, 2nd ed.; Stephen, A.M., Phillips, G.O., Williams, P.A., Eds.; CRC Press/Taylor and Francis: Boca Raton, FL, USA, 2006; pp. 254–397. [Google Scholar]
- Toft, K. Interactions between Pectins and Alginates. Prog. Food Nutr. Sci. 1982, 6, 89–96. [Google Scholar]
- Braccini, I.; Perez, S. Molecular Basis of Ca2+-Induced Gelation in Alginates and Pectins: The Egg-Box Model Revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.A.; Cooley, H.J. Rates of Structure Development during Gelation and Softening of High-methoxyl Pectin-sodium Alginate-fructose Mixtures. Food Hydrocoll. 1995, 9, 229–235. [Google Scholar] [CrossRef]
- Walkenstrom, P.; Kidman, S.; Hermansson, A.-M.; Rasmussen, P.B.; Hoegh, L. Microstructure and Rheological Behavior of Alginate/pectin Mixed Gels. Food Hydrocoll. 2003, 17, 593–603. [Google Scholar] [CrossRef]
- Morris, E.R. Mixed Polymer Gels. In Food Gels; Harris, R., Ed.; Elsevier: New York, NY, USA, 1990; pp. 291–359. [Google Scholar]
- Draget, K.I.; Smidsrød, O.; Skjåk-Bræk, G. Alginates from Algae. In Polysaccharides and Polyamides in the Food Industry. Properties, Production and Patents; Steinbüchel, A., Rhee, S.K., Eds.; Wiley Verlag GmbH & Co.: Weinham, Germany, 2005; pp. 1–30. [Google Scholar]
- Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Nishinari, K.; Funami, T.; Williams, P.A. Binding Behavior of Calcium to Polyuronates: Comparison of Pectin with Alginate. Carbohydr. Polym. 2008, 72, 334–341. [Google Scholar] [CrossRef]
- Rezvanian, M.; Ahmad, N.; Amin, M.C.I.M.; Ng, S.F. Optimization, Characterization, and in vitro Assessment of Alginate-pectin Ionic Cross-linked Hydrogel Film for Wound Dressing Applications. Int. J. Biol. Macromol. 2017, 97, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.J.; Chilvers, G.R. Cold Setting Alginate-pectin Mixed Gels. J. Sci. Food Agric. 1984, 35, 1370–1376. [Google Scholar] [CrossRef]
- Matto, M.; Husain, Q. Entrapment of Porous and Stable Concanavalin A–peroxidase Complex into Hybrid Calcium Alginate-pectin Gel. J. Chem. Technol. Biotechnol. 2006, 81, 1316–1323. [Google Scholar] [CrossRef]
- Krause, A.C.; da Silva, M.A.; Kieckbusch, T.G. Natamycin Release from Alginate/pectin Films for Food Packaging Applications. J. Food. Eng. 2012, 110, 18–25. [Google Scholar]
- Islan, G.C.A.A.; Verti, I.P.D.; Marchetti, S.G.; Castro, G.R. Studies of Ciprofloxacin Encapsulation on Alginate/Pectin Matrixes and Its Relationship with Biodisponibility. Appl. Biochem. Biotechnol. 2012, 167, 1408–1420. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Shah, T.; Amin, A. Polysaccharides: A Targeting Strategy for Colonic Drug Delivery. Expert Opin. Drug Deliv. 2011, 8, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Salyers, A.A.; Vercellotti, J.R.; West, S.E.; Wilkins, T.D. Fermentation of Mucin and Plant Polysaccharides by Strains of Bacteroides from the Human Colon. Appl. Environ. Microbiol. 1977, 33, 319–322. [Google Scholar] [PubMed]
- Eliaz, I.; Weil, E.; Wilk, B. Integrative Medicine and the Role of Modified Citrus Pectin/Alginates in Heavy Metal Chelation and Detoxification—Five Case Reports. Complement. Med. Res. 2008, 4, 358–364. [Google Scholar]
- Hsu, F.-Y.; Yu, D.-S.; Huang, C.-C. Development of pH-Sensitive Pectinate/Alginate Microspheres for Colon Drug Delivery. J. Mater. Sci. Mater. Med. 2013, 24, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Belščak-Cvitanović, A.; Bušić, A.; Barišić, L.; Vrsaljko, D.; Karlović, S.; Špoljarić, I.; Vojvodić, A.; Mršić, G.; Komes, D. Emulsion Templated Microencapsulation of Dandelion (Taraxacum officinale L.) Polyphenols and β-Carotene by Ionotropic Gelation of Alginate and Pectin. Food Hydrocoll. 2016, 57, 139–152. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Development and Characterization of Composite Edible Films Based on Sodium Alginate and Pectin. J. Food. Eng. 2013, 115, 459–465. [Google Scholar] [CrossRef]
- Seixasa, F.L.; Turbiani, F.R.B.; Salomão, P.G.; Souza, R.P.; Gimenes, M.L. Biofilms Composed of Alginate and Pectin: Effect of Concentration of Cross Linker and Plasticizer Agents. Chem. Eng. Trans. 2013, 32, 1693–1698. [Google Scholar]
- Jindal, M.; Kumar, V.; Rana, V.; Tiwary, A. An Insight into the Properties of Aegle marmelos Pectin–chitosan Cross-Linked Films. Int. J. Biol. Macromol. 2013, 52, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kowalonek, J. Studies of Chitosan/Pectin Complexes Exposed to UV Radiation. Int. J. Biol. Macromol. 2017, 103, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Birch, N.P.; Schiffman, J.D. Characterization of Self-Assembled Polyelectrolyte Complex Nanoparticles Formed from Chitosan and Pectin. Langmuir 2014, 30, 3441–3447. [Google Scholar] [CrossRef] [PubMed]
- Rashidova, S.S.; Milusheva, R.Y.; Semenova, L.N.; Mukhamedjanova, M.Y.; Voropaeva, N.L.; Vasilyeva, S.; Faizieva, R.; Ruban, I.N. Characteristics of Interactions in the Pectin–chitosan System. Chromatograpia 2004, 59, 779–782. [Google Scholar] [CrossRef]
- Ghaffari, A.; Navaee, K.; Oskoui, M.; Bayati, K.; Rafiee-Tehrani, M. Preparation and Characterization of Free Mixed-Film of Pectin/Chitosan/Eudragit® RS Intended for Sigmoidal Drug Delivery. Eur. J. Pharm. Biopharm. 2007, 67, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Recillas, M.; Silva, L.L.; Peniche, P.; Goycoolea, F.M.; Rinaudo, M.; Roman, J.S.; Argüelles, W.M. Thermo-and pH-responsive Polyelectrolyte Complex Membranes from Chitosan-g-N-isopropylacrylamide and Pectin. Carbohydr. Polym. 2011, 86, 1336–1343. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked Ionic Polysaccharides for Stimuli-Sensitive Drug Delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Pal, S. Modified Biopolymer-Dextrin Based Crosslinked Hydrogels: Application in Controlled Drug Delivery. RSC Adv. 2015, 5, 25014–25050. [Google Scholar] [CrossRef]
- Bernabé, P.; Peniche, C.; Argüelles-Monal, W. Swelling Behavior of Chitosan/Pectin Polyelectrolyte Complex Membranes. Effect of Thermal Cross-Linking. Polym. Bull. 2005, 55, 367–375. [Google Scholar] [CrossRef]
- Hiorth, M.; Kjøniksen, A.-L.; Knudsen, K.D.; Sande, S.A.; Nyström, B. Structural and Dynamical Properties of Aqueous Mixtures of Pectin and Chitosan. Eur. Polym. J. 2005, 41, 1718–1728. [Google Scholar] [CrossRef]
- Chen, P.-H.; Kuo, T.-Y.; Kuo, J.-Y.; Tseng, Y.-P.; Wang, D.-M.; Lai, J.-Y.; Hsieh, H.-J. Novel Chitosan–pectin Composite Membranes with Enhanced Strength, Hydrophilicity and Controllable Disintegration. Carbohydr. Polym. 2010, 82, 1236–1242. [Google Scholar] [CrossRef]
- Nordby, M.H.; Kjøniksen, A.L.; Nyström, B.; Roots, J. Thermoreversible Gelation of Aqueous Mixtures of Pectin and Chitosan. Rheology. Biomacromolecules 2003, 4, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, L.; Bianco-Peled, H. Pectin-chitosan Physical Hydrogels as Potential Drug Delivery Vehicles. Int. J. Biol. Macromol. 2017, 101, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Marudova, M.; Macdougall, A.J.; Ring, S.G. Pectin-chitosan Interactions and Gel Formation. Carbohydr. Res. 2004, 339, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Maciel, V.B.V.; Yoshida, C.M.; Franco, T.T. Chitosan/Pectin Polyelectrolyte Complex as a pH Indicator. Carbohydr. Polym. 2015, 132, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, P.; Ferreira, P.; Sousa, H.D.; Batista, P.; Rodrigues, M.; Correia, I.; Gil, M. Preparation and Chemical and Biological Characterization of a Pectin/Chitosan Polyelectrolyte Complex Scaffold for Possible Bone Tissue Engineering Applications. Int. J. Biol. Macromol. 2011, 48, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Birch, N.P.; Barney, L.E.; Pandres, E.; Peyton, S.R.; Schiffman, J.D. Thermal-Responsive Behavior of a Cell Compatible Chitosan/Pectin Hydrogel. Biomacromolecules 2015, 16, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.A.; Kӧk, M.S.; Harding, S.E.; Adams, G. Polysaccharide Drug Delivery Systems Based on Pectin and Chitosan. Biotechnol. Genet. Eng. Rev. 2010, 27, 257–284. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Mishra, A.; Raval, P.; Patel, H.; Gupta, A.; Shah, D. Chitosan-pectin Polyelectrolyte Complex as a Carrier for Colon Targeted Drug Delivery. J. Young Pharm. 2013, 5, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, H.; Zhu, X.; Hou, Y.; Liu, W.; Yang, G.; Jiang, A. Pectin-chitosan Complex: Preparation and Application in Colon-specific Capsule. Int. J. Agric. Biol. Eng. 2015, 8, 151–160. [Google Scholar]
- Rampino, A.; Borgogna, M.; Bellich, B.; Blasi, P.; Virgilio, F.; Cesàro, A. Chitosan-pectin Hybrid Nanoparticles Prepared by Coating and Blending Techniques. Eur. J. Pharm. Sci. 2016, 84, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Dubin, P.; Kayitmazer, A.; Turksen, S. Polyelectrolyte-protein Complexes. Curr. Opin. Colloid Interface Sci. 2005, 10, 52–78. [Google Scholar] [CrossRef]
- Alonso-Sande, M.C.A.D.; Cuña, M.; Remuñán-López, C.; Teijeiro-Osorio, D.; Alonso-Lebrero, J.L.; Alonso, M.J. Formation of New Glucomannan-Chitosan Nanoparticles and Study of Their Ability to Associate and Deliver Proteins. Macromolecules 2006, 39, 4152–4158. [Google Scholar] [CrossRef]
- Tolstoguzov, V. Some Thermodynamic Considerations in Food Formulation. Food Hydrocoll. 2003, 17, 1–23. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Pilosof, A.M.R. Protein-polysaccharide Interactions at Fluid Interfaces. Food Hydrocoll. 2011, 25, 1925–1937. [Google Scholar] [CrossRef]
- Burgess, D. Practical Analysis of Complex Coacervate Systems. J. Colloid Interface Sci. 1990, 140, 227–238. [Google Scholar] [CrossRef]
- Syrbe, A.; Bauer, W.J.; Klostermeyer, H. Polymer Science Concepts in Dairy Systems: An Overview of Milk Protein and Food Hydrocolloid Interaction. Int. Dairy J. 1998, 8, 179–193. [Google Scholar] [CrossRef]
- Li, X.; Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Yao, X.; Zhang, Y.; Zhao, M.; Zhang, K.; Jiang, F. Complexation of Bovine Serum Albumin and Sugar Beet Pectin: Structural Transitions and Phase Diagram. Langmuir 2012, 28, 10164–10176. [Google Scholar] [CrossRef] [PubMed]
- Mcclements, D.J. Non-Covalent Interactions between Proteins and Polysaccharides. Biotechnol. Adv. 2006, 24, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.G.; Decker, E.A.; Mcclements, D.J. Formation of Biopolymer Particles by Thermal Treatment of β-Lactoglobulin–pectin Complexes. Food Hydrocoll. 2009, 23, 1312–1321. [Google Scholar] [CrossRef]
- Jones, O.G.; Lesmes, U.; Dubin, P.; Mcclements, D.J. Effect of Polysaccharide Charge on Formation and Properties of Biopolymer Nanoparticles created by Heat Treatment of β-Lactoglobulin-pectin Complexes. Food Hydrocoll. 2010, 24, 374–383. [Google Scholar] [CrossRef]
- Baracat, M.M.; Nakagawa, A.M.; Casagrande, R.; Georgetti, S.R.; Verri, W.A.; Freitas, O.D. Preparation and Characterization of Microcapsules Based on Biodegradable Polymers: Pectin/Casein Complex for Controlled Drug Release Systems. AAPS PharmSciTech 2012, 13, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.; Rolin, C.; Ipsen, R. Stabilization of Acidified Skimmed Milk with HM Pectin. Food Hydrocoll. 2010, 24, 291–299. [Google Scholar] [CrossRef]
- Nasseri, A.T.; Thibault, J.-F.; Ralet, M.C. Citrus Pectin: Structure and Application in Acid Dairy Drinks. Tree Sci. Biotechnol. 2008, 2, 60–70. [Google Scholar]
- Piculell, L.; Bergfeld, K.; Nilsson, S. Factors Determining Phase Behavior of Multi-Component Polymer Systems; Nottingham University Press: Nottingham, UK, 1995; pp. 13–35. [Google Scholar]
- Gilsenan, P.; Richardson, R.; Morris, E. Associative and Segregative Interactions between Gelatin and Low-Methoxy Pectin: Part 1. Associative Interactions in the Absence of Ca2+. Food Hydrocoll. 2003, 17, 723–737. [Google Scholar] [CrossRef]
- Lundin, L.; Norton, I.T.; Foster, T.J.; Williams, M.A.K.; Hermansson, A.-M.; Bergström, E. Phase Separation in Mixed Biopolymer Systems. In Gums and Stabilisers for the Food Industry; The Royal Society of Chemistry: Cambridge, UK, 2000; pp. 168–180. [Google Scholar]
- Wu, B.; McClements, D.J. Functional Hydrogel Microspheres: Parameters affecting Electrostatic Assembly of Biopolymer Particles Fabricated from Gelatin and Pectin. Food Res. Int. 2015, 72, 231–240. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, D.; Xu, X.; Guo, S.; Jin, Z. Rheological, Textural and Microstructure Analysis of the High-Methoxy Pectin/Gelatin Mixed Systems. J. Texture Stud. 2007, 38, 577–600. [Google Scholar] [CrossRef]
- Farrés, I.F.; Moakes, R.J.; Norton, I.T. Designing Biopolymer Fluid Gels: A Microstructural Approach. Food Hydrocoll. 2014, 42, 362–372. [Google Scholar] [CrossRef]
- Shewan, H.M.; Stokes, J.R. Review of Techniques to Manufacture Micro-hydrogel Particles for the Food Industry and their Applications. J. Food Eng. 2013, 119, 781–792. [Google Scholar] [CrossRef]
- Gupta, B.; Tummalapalli, M.; Deopura, B.; Alam, M. Preparation and Characterization of in-Situ Cross linked Pectin–gelatin Hydrogels. Carbohydr. Polym. 2014, 106, 312–318. [Google Scholar] [CrossRef] [PubMed]
- DeMars, L.L.; Ziegler, G.R. Texture and Structure of Gelatin/Pectin-Based Gummy Confections. Food Hydrocoll. 2001, 15, 643–653. [Google Scholar] [CrossRef]
- Wu, B.; Degner, B.; McClements, D.J. Soft Matter Strategies for Controlling Food Texture: Formation of Hydrogel Particles by Biopolymer Complex Coacervation. J. Phys. Condens. Matter. 2014, 26, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Lim, B.; Chow, K.; Chong, S.; Chang, Y. Using Fish Gelatin and Pectin to make a Low-Fat Spread. Food Hydrocoll. 2008, 22, 1637–1640. [Google Scholar] [CrossRef]
- Saravanan, M.K.; Panduranga, R. Pectin-gelatin and Alginate-gelatin Complex Coacervation for Controlled Drug Delivery: Influence of Anionic Polysaccharides and Drugs being encapsulated on Physicochemical Properties of Microcapsules. Carbohydr. Polym. 2010, 80, 808–816. [Google Scholar] [CrossRef]
- Silva, D.F.; Favaro-Trindade, C.S.; Rocha, G.A.; Thomazini, M. Microencapsulation of Lycopene by Gelatin–pectin Complex Coacervation. J. Food Process. Preserv. 2012, 36, 185–190. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Min, Y.K.; Lee, B.T. Nanoparticle Biphasic Calcium Phosphate loading on Gelatin-Pectin Scaffold for Improved Bone Regeneration. Tissue Eng. Part A 2015, 21, 1376–1387. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Majeed, A.B.A.; Banthia, A.K. Development and Characterization of Pectin/gelatin Hydrogel Membranes for Wound Dressing. Int. J. Plast. Technol. 2011, 15, 82–95. [Google Scholar] [CrossRef]
- Tummalapalli, M.; Berthet, M.; Verrier, B.; Deopura, B.L.; Alam, M.S.; Gupta, B. Drug Loaded Composite Oxidized Pectin and Gelatin Networks for Accelerated Wound Healing. Int. J. Pharm. 2016, 505, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Tummalapalli, M.; Berthet, M.; Verrier, B.; Deopura, B.L.; Alam, M.S.; Gupta, B. Composite Wound Dressings of Pectin and Gelatin with Aloe Vera and Curcumin as Bioactive Agents. Int. J. Biol. Macromol. 2016, 82, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Autio, K.; Kuuva, T.; Roininen, K.; Lahteenmaki, L. Rheological Properties, Microstructure and Sensory Perception of High-amylose Starch-pectin Mixed Gels. J. Texture Stud. 2002, 33, 473–486. [Google Scholar] [CrossRef]
- Khondkar, D.; Tester, R.F.; Hudson, N.; Karkalas, J.; Morrow, J. Rheological Behavior of Uncross-Linked and Cross-Linked Gelatinized Waxy Maize Starch with Pectin Gels. Food Hydrocoll. 2007, 21, 1296–1301. [Google Scholar] [CrossRef]
- Carbinatto, F.M.; Castro, A.D.D.; Cury, B.S.; Magalhães, A.; Evangelista, R.C. Physical Properties of Pectin–high Amylose Starch Mixtures Cross-Linked with Sodium Trimetaphosphate. Int. J. Pharm. 2012, 423, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Carbinatto, F.M.; Castro, A.D.D.; Evangelista, R.C.; Cury, B.S. Insights into the Swelling Process and Drug Release Mechanisms from Cross-Linked Pectin/High Amylose Starch Matrices. Asian J. Pharm. 2014, 9, 27–34. [Google Scholar] [CrossRef]
- Fishman, M.; Coffin, D.; Konstance, R.; Onwulata, C. Extrusion of Pectin/Starch Blends Plasticized with Glycerol. Carbohydr. Polym. 2000, 41, 317–325. [Google Scholar] [CrossRef]
- Dafe, A.; Etemadi, H.; Dilmaghani, A.; Mahdavinia, G.R. Investigation of Pectin/Starch Hydrogel as a Carrier for Oral Delivery of Probiotic Bacteria. Int. J. Biol. Macromol. 2017, 97, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Starch-Pectin Matrices for Encapsulation of Ascorbic Acid. Master’s Thesis, University of Nebraska-Lincoln, Lincoln, NE, USA, 2014; 41p. [Google Scholar]
- Desai, K.G. Properties of Tableted High-Amylose Corn Starch-Pectin Blend Microparticles Intended for Controlled Delivery of Diclofenac Sodium. J. Biomater. Appl. 2007, 21, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Soares, G.A.; de Castro, A.D.; Cury, B.S.F.; Evangelista, R.C. Blends of Cross-linked High Amylose Starch/pectin loaded with Diclofenac. Carbohydr. Polym. 2013, 91, 135–142. [Google Scholar] [CrossRef] [PubMed]





| Biomedical/Pharmaceutical Applications | Pectin Source Obtention | Degree of Esterification (%) | Reference |
|---|---|---|---|
| Prebiotic effect | Orange peel wastes | NA | [138]. |
| Sugar beet pulp/lemon peel | NA | [139]. | |
| Sugar beet pulp | NA | [140]. | |
| Roots of Bupleurum falcatum | NS | [141,142]. | |
| Apple pomace, tangerine peel and wild plant | NA | [143]. | |
| Glucose levels | Passiflora glandulosa | 35 | [144]. |
| Unripe apple | NS | [145]. | |
| Citrus pectin | NS | [146,147]. | |
| Insuline delivery | Pectin/Chitosan | NS | [148]. |
| Pectin/Arabinoxylans | NS | [149]. | |
| Cholesterol leves | Soluble dietary fiber | NA | [150,151,152]. |
| Banana passion fruit | 52 | [153]. | |
| Metal removal | Seagrass, citrus pectin | 60 | [154,155,156]. |
| Seagrass P. iwatensis | 2.54–6.91 | [157]. | |
| Commercial citrus/sugar beet | 16,35 | [158]. | |
| Citrus pectin | 3.8 | [159]. | |
| Citrus pectin | NS | [160]. | |
| Citrus pectin | 1 | [161]. | |
| Cancer prevention | Modified pectin | NS | [162]. |
| Prostate cancer | Modified citrus pectin | NS | [163]. |
| Citrus pectin (P-9135) | 6.7 | [164]. | |
| Colon cancer | Citrus pectin/Modified apple | 30,60,90 | [165,166]. |
| Pancreatic cancer | Flowers of L. japonica | NS | [167]. |
| Breast cancer | Pectic polysaccharides | NS | [168]. |
| Metastasis | Modified citrus pectin | NS | [169,170]. |
| Modified citrus pectin | 6.7 | [171]. | |
| Pumpkin | 5.4 | [172]. | |
| Antiproliferative effect | Citrus pectin | NS | [173]. |
| Modified sugar beet | 18,55,57,62 | [174]. | |
| Modified citrus pectin | NS | [175]. | |
| Gen delivery | Amidated citrus pectin | 26 | [176]. |
| Tissue engineering | Modified pectin with oligopeptides | LM | [177,178]. |
| Wound healing | Amidated pectin | LM | [179,180]. |
| Drug encapsulation | Citrus, pumpkin | 48,44;8.1;30 | [181,182,183,184,185]. |
| Unipectine OF300C | |||
| Nasal drug delivery | Commercial pectin | 29,38,70 | [186]. |
| Fentanyl Pectin | NA | [187,188,189]. | |
| Ocular drug delivery | Mucoadhesive polymers | NA | [190,191]. |
| Cesapectin® | 32 | [192]. | |
| Colon drug delivery | Citrus pectin | 36,25; 10,35 | [181,193,194,195,196,197]. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. https://doi.org/10.3390/molecules23040942
Lara-Espinoza C, Carvajal-Millán E, Balandrán-Quintana R, López-Franco Y, Rascón-Chu A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules. 2018; 23(4):942. https://doi.org/10.3390/molecules23040942
Chicago/Turabian StyleLara-Espinoza, Claudia, Elizabeth Carvajal-Millán, René Balandrán-Quintana, Yolanda López-Franco, and Agustín Rascón-Chu. 2018. "Pectin and Pectin-Based Composite Materials: Beyond Food Texture" Molecules 23, no. 4: 942. https://doi.org/10.3390/molecules23040942





