Anti-Inflammatory and Antinociceptive Studies of Hydroalcoholic Extract from the Leaves of Phyllanthus brasiliensis (Aubl.) Poir. and Isolation of 5-O-β-d-Glucopyranosyljusticidin B and Six Other Lignans
Abstract
:1. Introduction
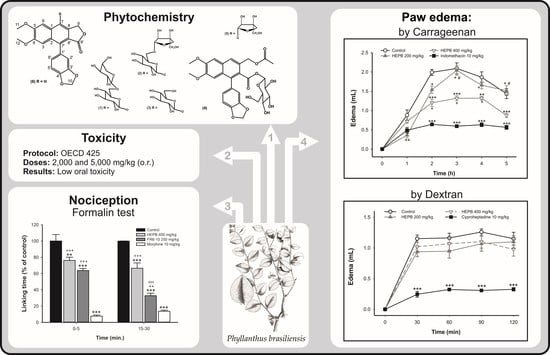
2. Results and Discussion
2.1. Pharmacological Assays
2.1.1. Carrageenan-Induced Rat Paw Edema
2.1.2. Dextran-Induced Rat Paw Edema
2.1.3. Formalin Test
2.1.4. Acute Oral Toxicity
2.2. General Chemistry
2.3. Isolation of Compounds
3. Materials and Methods
3.1. Pharmacological Assays
3.1.1. Drugs and Chemical Compounds
3.1.2. Animals
3.1.3. Carrageenan-Induced Rat Paw Edema
3.1.4. Dextran-Induced Rat Paw Edema
3.1.5. Formalin Test
3.1.6. Acute Oral Toxicity
3.1.7. Statistical Analysis
3.2. Collection, Identification and Preparation of Crude Extract
3.3. General Chemistry
3.4. Isolation of Compounds
3.4.1. HPLC Profile
3.4.2. Compound 3
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Silva, M.J.; Sales, M.F. Phyllanthus L. (Phyllanthaceae) em Pernambuco, Brasil. Acta Bot. Bras. 2007, 21, 79–98. [Google Scholar] [CrossRef]
- Calixto, J.B.; Santos, A.R.; Filho, V.C.; Yunes, R.A. A Review of the Plants of the Genus Phyllanthus: Their Chemistry, Pharmacology, and Therapeutic Potential. Med. Res. Rev. 1998, 18, 225–258. [Google Scholar] [CrossRef]
- Chouhan, H.S.; Singh, S.K. Phytochemical analysis, antioxidant and anti-inflammatory activities of Phyllanthus simplex. J. Ethnopharmacol. 2011, 137, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.K.; Fang, S.; Tzeng, Y. Anti-inflammatory activities of constituents isolated from Phyllanthus polyphyllus. J. Ethnopharmacol. 2006, 103, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Andel, T.V. The diverse uses of fish-poison plants in northwest Guyana. Econ. Bot. 2000, 54, 500–512. [Google Scholar] [CrossRef]
- Kupchan, S.M.; LaVoie, E.J.; Branfman, A.R.; Fei, B.Y.; Bright, W.M.; Bryan, R.F. Phyllanthocin, a novel bisabolane aglycone from the antileukemic glycoside, phyllanthoside. J. Am. Chem. Soc. 1977, 99, 3199–3201. [Google Scholar] [CrossRef] [PubMed]
- Breitbach, U.B.; Niehues, M.; Lopes, N.P.; Faria, J.E.Q.; Brandao, M.G.L. Amazonian Brazilian medicinal plants described by C.F.P. von Martius in the 19th century. J. Ethnopharmacol. 2013, 147, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Unander, D.W.; Webster, G.L.; Blumberg, B.S. Usage and bioassays in Phyllanthus (Euphorbiaceae): A compilation III. The subgenera Eriococcus, Conami, Gomphidium, Bo tryan thus, Xylophylla and Phyllanthodendron, and a complete list of the species cited in the three-part series. J. Ethnopharmacol. 1992, 36, 103–112. [Google Scholar] [CrossRef]
- Santos, A.R.; De Campos, R.O.; Miguel, O.G.; Filho, V.C.; Siani, A.C.; Yunes, R.A.; Calixto, J.B. Antinociceptive properties of extracts of new species of plants of the genus Phyllanthus (Euphorbiaceae). J. Ethnopharmacol. 2000, 72, 229–238. [Google Scholar] [CrossRef]
- Sharma, P.K.; Kumar, S.; Kumar, P.; Kaushik, P.; Kaushik, D.; Dhingra, Y.; Aneja, K.R. Synthesis and biological evaluation of some pyrazolylpyrazolines as anti-inflammatory-antimicrobial agents. Eur. J. Med. Chem. 2010, 45, 2650–2655. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.H. Pharmacological Approaches to Natural Product Screening and Evaluation. In New Natural Products and Plant Drugs with Pharmacological, Biological or Therapeutical Activity; Wagner, H.K., Wolff, P.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1977; ISBN 978-3-642-66682-7. [Google Scholar]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenan-induced edema in the hind paw of the rat as an assay for anti-inflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Hunskaar, S.; Fasmer, O.B.; Hole, K. Formalin test in mice, a useful technique for evaluating mild analgesics. J. Neurosci. Methods 1985, 14, 69–76. [Google Scholar] [CrossRef]
- Calixto, J.B. Twenty-five years of research on medicinal plants in Latin America: A personal view. J. Ethnopharmacol. 2005, 100, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Kassuya, C.A.; Leite, D.F.; de Melo, L.V.; Rehder, V.L.; Calixto, J.B. Anti-inflammatory Properties of extracts, Fractions and Lignans isolated from Phyllanthus amarus. Planta Med. 2005, 71, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Gorski, F.; Corrêa, C.R.; Filho, V.C.; Yunes, R.A.; Calixto, J.B. Potent antinociceptive activity of a hydroalcoholic extract of Phyllanthus corcovadensis. J. Pharm. Pharmacol. 1993, 45, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.P.; Nautiyal, D.P.; Rather, M.A.; Bachheti, R.K. A new aryl naphthalide lignan from Justicia prostrate Gamble. Asian J. Chem. 2011, 23, 2125–2127. [Google Scholar]
- Tian, J.; Hao, X.; He, H. A new lignan and four new lignan glycosides from Mananthes patentiflora. Helv. Chim. Acta 2006, 89, 291–298. [Google Scholar] [CrossRef]
- Al-Abed, Y.; Sabri, S.; Zarga, M.A.; Shah, Z.; Atta-ur-Rahman, A. Chemical constituents of the flora of Jordan, part V-B. Three new arylnaphthalene lignan glucosides from Haplophyllum buxbaumii. J. Nat. Prod. 1990, 53, 1152–1161. [Google Scholar] [CrossRef]
- Pettit, G.R.; Schaufelberger, D.E. Isolation and structure of the cytostatic lignan glycoside phyllanthostatin A. J. Nat. Prod. 1988, 51, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, G.; Puricelli, L.; Piacente, S.; Caniato, R.; Filippini, R.; Cappelletti, E.M. Patavine, a new arylnaphthalene lignan glycoside from shoot cultures of Haplophyllum patavinum. Chem. Pharm. Bull. 2002, 50, 844–846. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.; Ruas, M.M.; Donate, P.M. Complete assignments of 1H and 13C NMR spectral data for arylnaphthalene lignan lactones. Magn. Reson. Chem. 2007, 45, 902–904. [Google Scholar] [CrossRef] [PubMed]
- Priyadharsini, R.P.; Parasuraman, S.; Raveendran, R.J. Evaluation of the antihypertensive activity and alpha adrenergic receptor interaction of cleistanthins A and B. J. Basic Clin. Pharm. 2014, 5, 109–114. [Google Scholar] [PubMed]
- Vijayakumar, B.; Parasuraman, S.; Raveendran, R.; Velmurugan, D. Identification of natural inhibitors against angiotensin I converting enzyme for cardiac safety using induced fit docking and MM-GBSA studies. Pharmacogn. Mag. 2014, 10, 639–644. [Google Scholar]
- Parasuraman, S.; Raveendran, R. Diuretic effects of cleistanthin A and cleistanthin B from the leaves of Cleistanthus collinus in wistar rats. J. Young Pharm. 2012, 4, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.P.; Pande, G.; Shanmugam, G. Cleistanthin B causes G1 arrest and inducesapoptosis in mammaliancells. Apoptosis 1998, 3, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, Y.; Zhelezova, I.; Atanasova, T.; Zaharieva, M.M.; Sasheva, P.; Ionkova, I.; Konstantinov, S. Cytotoxic effect of the biotechnologically-derived justicidin B on human lymphoma cells. Biotechnol. Lett. 2014, 36, 2177–2183. [Google Scholar] [CrossRef] [PubMed]
- Susplugas, S.; Hung, N.V.; Bignon, J.; Thoison, O.; Kruczynski, A.; Sevenet, T.; Gueritte, F. Cytotoxic arylnaphthalene lignans from a Vietnamese acanthaceae, Justicia patentiflora. J. Nat. Prod. 2005, 68, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Prieto, J.M.; Recio, M.C.; Giner, R.M.; Manez, S.; Massmanjan, A.; Waterman, P.G.; Rios, J.L. Topical Anti-Inflammatory Lignans from Haplophyllum hispanicum. Z. Naturforsch. C 1996, 51, 618–622. [Google Scholar] [PubMed]
- Clancy, R.M.; Amin, A.R.; Abramson, S.B. The role of nitric oxide in inflammation and immunity. Arthritis Rheumatol. 1998, 41, 1141–1151. [Google Scholar] [CrossRef]
- Hasko, G.; Szabo, C. IL-12 as a therapeutic target for pharmacological modulation in immune-mediated and inflammatory diseases: Regulation of T helper 1/T helper 2 responses. Br. J. Pharmacol. 1999, 127, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Momekov, G.; Konstantinov, S.; Dineva, I.; Ionkova, I. Effect of justicidin B—A potent cytotoxic and pro-apoptotic arylnaphtalene lignan on human breast cancer-derived cell lines. Neoplasma 2011, 58, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The Nuclear Factor NF-κB Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. An Hydroalcoholic Chamomile Extract Modulates Inflammatory and Immune Response in HT29 Cells and Isolated Rat Colon. Phytother. Res. 2016, 30, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Therien, M.; Fitzsimmons, B.J.; Scheigetz, J.; Macdonald, D.; Choo, L.Y.; Guay, J.; Falgueyret, J.P.; Riendeau, D. Justicidin E: A new leukotriene biosynthesis inhibitor. Bioorg. Med. Chem. Lett. 1993, 3, 2063–2066. [Google Scholar] [CrossRef]
- Radmark, O.; Werz, O.; Steinhilber, D.; Samuelsson, B. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim. Biophys. Acta 2015, 1851, 331–339. [Google Scholar] [CrossRef] [PubMed]
- OECD Organization of Economic Cooperation and Development. The Revised Up-and-Down Procedure: A Test Method for Determining the Acute Oral Toxicity of Chemicals; NIH Publication: Bethesda, MD, USA, 2001. Available online: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd_gl423.pdf (accessed on 12 December 2017).
Sample Availability: Samples of the compounds are not available from the authors. |






| Moiety | Position | 3 | |||
|---|---|---|---|---|---|
| δC | δH (J in Hz) | HMBC (H to C) | COSY (H to H) | ||
| Aglycone moiety | 1 | 140.4 a | |||
| 2 | 120.8 | ||||
| 3 | 140.5 a | ||||
| 4 | 116.9 | 8.45 s | 2, 5, 10 | 12 | |
| 5 | 145.4 | ||||
| 6 | 144.4 | ||||
| 7 | 154.6 | ||||
| 8 | 104.0 | 6.98 s | 1, 7 | OCH3-7 | |
| 9 | 129.8 b | ||||
| 10 | 131.4 b | ||||
| 11 | 172.3 | ||||
| 12 | 70.1 | 5.43 s | 3, 4, 11 | 4 | |
| 1′ | 130.3 | ||||
| 2′ | 111.5 | 6.78 d (1.5) | 3′, 4′, 6′ | 6′ | |
| 3′ | 149.0 | ||||
| 4′ | 149.0 | ||||
| 5′ | 109.0 | 6.95 d (7.8) | 1′, 3′, 4′ | ||
| 6′ | 124.6 | 6.75 dd (1.5, 7.8) | 2′ | 2′ | |
| 7′ | 102.6 | 6.03 d (1.2) 6.04 d (1.2) | 3′, 4′ | ||
| OCH3-6 | 62.0 | 3.97 s | 6 | ||
| OCH3-7 | 56.1 | 3.74 s | 7 | 8 | |
| Sugar moiety | 1″ | 105.7 | 5.16 d (7.8) | 5 | |
| 2″ | 75.7 | 3.65 m | |||
| 3″ | 77.9 | 3.49 m | |||
| 4″ | 71.3 | 3.46 m | |||
| 5″ | 78.3 | 3.20 m | |||
| 6″ | 62.2 | 3.69 m | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, L.D.C.; Negrão-Neto, R.; Pamplona, S.; Fernandes, L.; Barros, M.; Fontes-Júnior, E.; Maia, C.; E Silva, C.Y.Y.; Silva, M.N.d. Anti-Inflammatory and Antinociceptive Studies of Hydroalcoholic Extract from the Leaves of Phyllanthus brasiliensis (Aubl.) Poir. and Isolation of 5-O-β-d-Glucopyranosyljusticidin B and Six Other Lignans. Molecules 2018, 23, 941. https://doi.org/10.3390/molecules23040941
Borges LDC, Negrão-Neto R, Pamplona S, Fernandes L, Barros M, Fontes-Júnior E, Maia C, E Silva CYY, Silva MNd. Anti-Inflammatory and Antinociceptive Studies of Hydroalcoholic Extract from the Leaves of Phyllanthus brasiliensis (Aubl.) Poir. and Isolation of 5-O-β-d-Glucopyranosyljusticidin B and Six Other Lignans. Molecules. 2018; 23(4):941. https://doi.org/10.3390/molecules23040941
Chicago/Turabian StyleBorges, Luziane Da C., Raimundo Negrão-Neto, Sônia Pamplona, Luanna Fernandes, Mayra Barros, Enéas Fontes-Júnior, Cristiane Maia, Consuelo Y. Yoshioka E Silva, and Milton Nascimento da Silva. 2018. "Anti-Inflammatory and Antinociceptive Studies of Hydroalcoholic Extract from the Leaves of Phyllanthus brasiliensis (Aubl.) Poir. and Isolation of 5-O-β-d-Glucopyranosyljusticidin B and Six Other Lignans" Molecules 23, no. 4: 941. https://doi.org/10.3390/molecules23040941