Assessing Photosensitizer Targeting Using Meso-Tetra(Carboxyphenyl) Porphyrin
Abstract
:1. Introduction
2. Results and Discussion
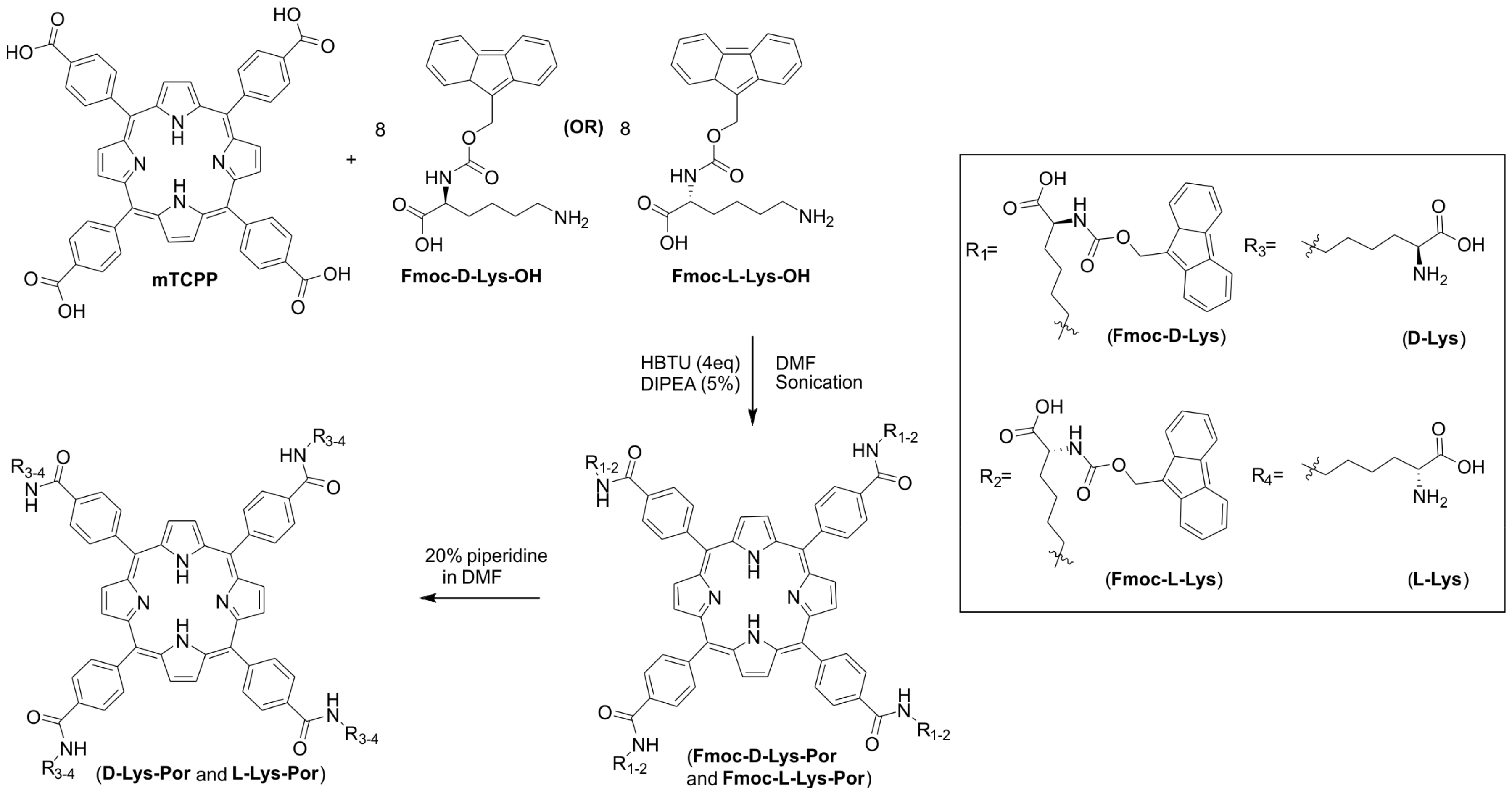
2.1. Synthesis and Formulation of Lysine-Conjugated Porphyrin
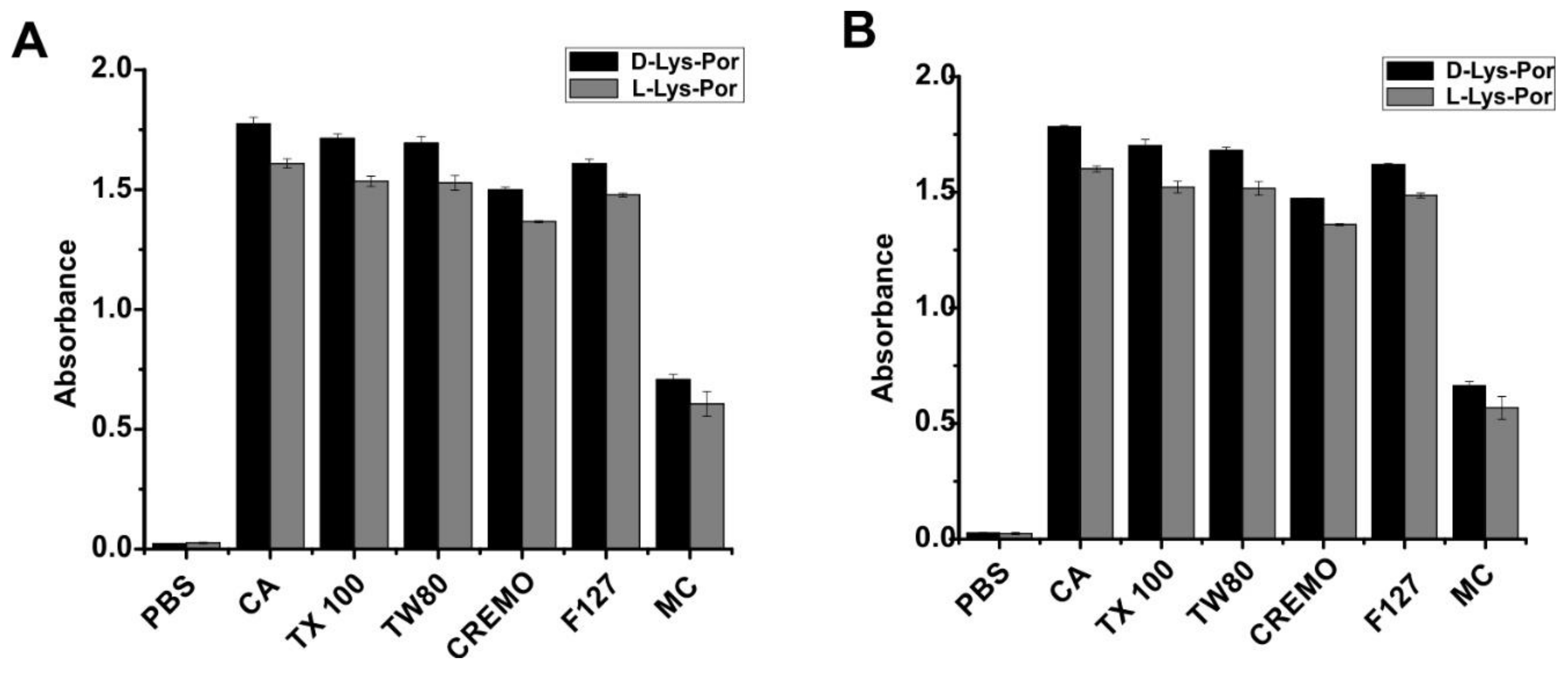
2.2. Photophysical Studies
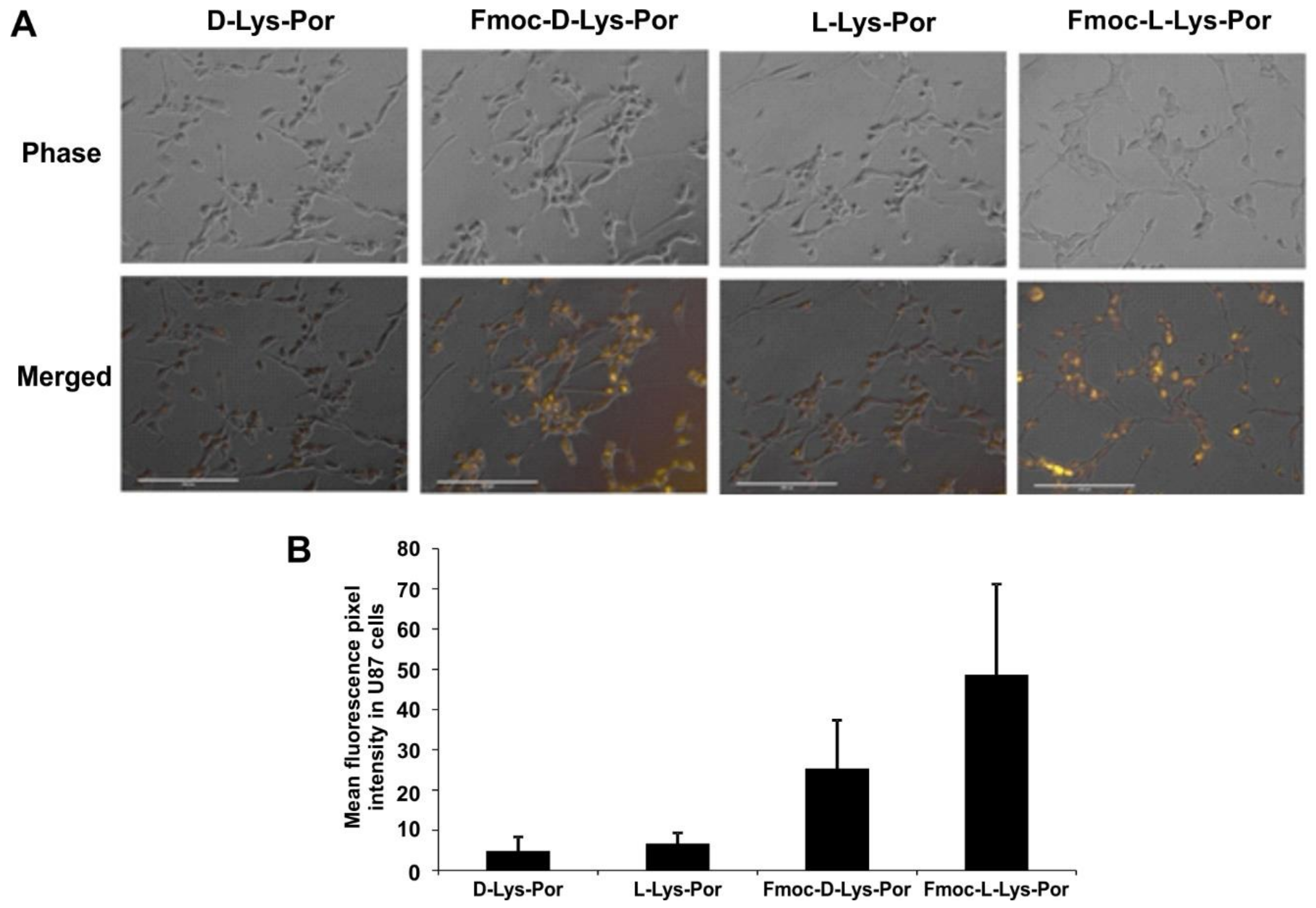
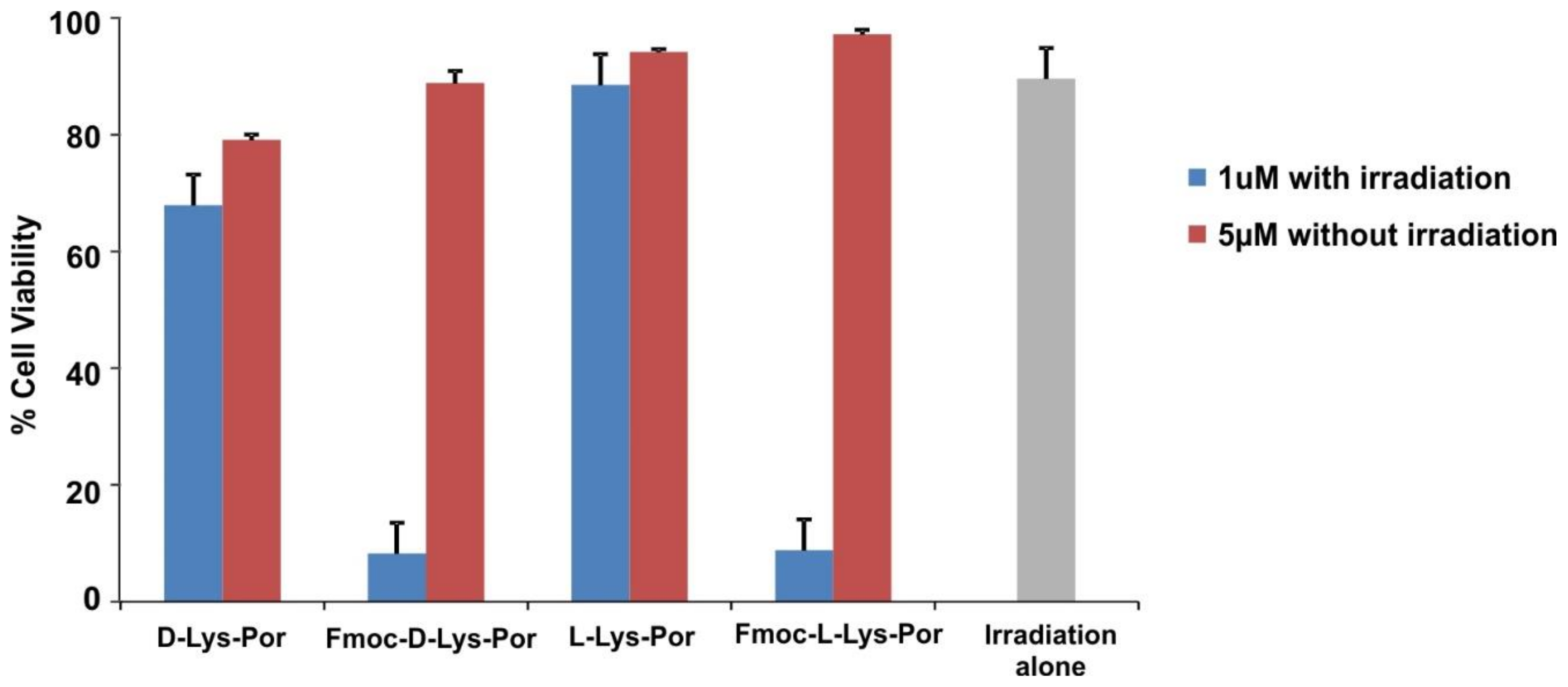
2.3. In Vitro Cell Studies
3. Conclusions
4. Materials and Methods
Materials
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Konan, Y.N.; Gurny, R.; Allémann, E. State of the art in the delivery of photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2002, 66, 89–106. [Google Scholar] [CrossRef]
- Lovell, J.F.; Liu, T.W.; Chen, J.; Zheng, G. Activatable photosensitizers for imaging and therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Bagnato, V.S.; Cuenca, R.; Downie, G.H.; Sibata, C.H. The future of photodynamic therapy in oncology. Future Oncol. 2006, 2, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, R. Photodynamic therapy in historical perspective. Rev. Contemp. Pharmacother. 1999, 10, 1–17. [Google Scholar]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978, 38, 2628–2635. [Google Scholar] [PubMed]
- Henderson, B.W.; Busch, T.M.; Vaughan, L.A.; Frawley, N.P.; Babich, D.; Sosa, T.A.; Zollo, J.D.; Dee, A.S.; Cooper, M.T.; Bellnier, D.A. Photofrin photodynamic therapy can significantly deplete or preserve oxygenation in human basal cell carcinomas during treatment, depending on fluence rate. Cancer Res. 2000, 60, 525–529. [Google Scholar] [PubMed]
- Engbrecht, B.W.; Menon, C.; Kachur, A.V.; Hahn, S.M.; Fraker, D.L. Photofrin-mediated photodynamic therapy induces vascular occlusion and apoptosis in a human sarcoma xenograft model. Cancer Res. 1999, 59, 4334–4342. [Google Scholar] [PubMed]
- Oseroff, A.R.; Blumenson, L.R.; Wilson, B.D.; Mang, T.S.; Bellnier, D.A.; Parsons, J.C.; Frawley, N.; Cooper, M.; Zeitouni, N.; Dougherty, T.J. A dose ranging study of photodynamic therapy with porfimer sodium (Photofrin®) for treatment of basal cell carcinoma. Lasers Surg. Med. 2006, 38, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and nonporphyrin photosensitizers in oncology: Preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lovell, J.F. Porphyrins as theranostic agents from prehistoric to modern times. Theranostics 2012, 2, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Song, W.; Rieffel, J.; Lovell, J.F. Emerging applications of porphyrins in photomedicine. Front. Phys. 2015, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and Photodynamic Therapy: Mechanisms, Monitoring, and Optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [PubMed]
- Chitgupi, U.; Qin, Y.; Lovell, J.F. Targeted nanomaterials for phototherapy. Nanotheranostics 2017, 1, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Sharma, S.K.; Zhiyentayev, T.; Huang, Y.-Y.; Hamblin, M.R. Photodynamic Therapy: Photosensitizer Targeting and Delivery. In Drug Delivery in Oncology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 1569–1603. [Google Scholar]
- Derycke, A.S.; De Witte, P.A. Transferrin-mediated targeting of hypericin embedded in sterically stabilized PEG-liposomes. Int. J. Oncol. 2002, 20, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, L.B.; Boyle, R.W. Photodynamic therapy: Novel third-generation photosensitizers one step closer? Br. J. Pharmacol. 2008, 154, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Vrouenraets, M.B.; Visser, G.W.; Loup, C.; Meunier, B.; Stigter, M.; Oppelaar, H.; Stewart, F.A.; Snow, G.B.; van Dongen, G.A. Targeting of a hydrophilic photosensitizer by use of internalizing monoclonal antibodies: A new possibility for use in photodynamic therapy. Int. J. Cancer 2000, 88, 108–114. [Google Scholar] [CrossRef]
- Schneider, R.; Schmitt, F.; Frochot, C.; Fort, Y.; Lourette, N.; Guillemin, F.; Müller, J.-F.; Barberi-Heyob, M. Design, synthesis, and biological evaluation of folic acid targeted tetraphenylporphyrin as novel photosensitizers for selective photodynamic therapy. Biorg. Med. Chem. 2005, 13, 2799–2808. [Google Scholar] [CrossRef] [PubMed]
- Gravier, J.; Schneider, R.; Frochot, C.; Bastogne, T.; Schmitt, F.; Didelon, J.; Guillemin, F.; Barberi-Heyob, M. Improvement of meta-tetra (hydroxyphenyl) chlorin-like photosensitizer selectivity with folate-based targeted delivery. Synthesis and in vivo delivery studies. J. Med. Chem. 2008, 51, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Z.; Blessington, D.; Li, H.; Busch, T.M.; Madrak, V.; Miles, J.; Chance, B.; Glickson, J.D.; Zheng, G. Pyropheophorbide 2-Deoxyglucosamide: A New Photosensitizer Targeting Glucose Transporters. Bioconjug. Chem. 2003, 14, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Spring, B.Q.; Abu-Yousif, A.O.; Palanisami, A.; Rizvi, I.; Zheng, X.; Mai, Z.; Anbil, S.; Sears, R.B.; Mensah, L.B.; Goldschmidt, R.; et al. Selective treatment and monitoring of disseminated cancer micrometastases in vivo using dual-function, activatable immunoconjugates. Proc. Natl. Acad. Sci. USA 2014, 111, E933–E942. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Tirand, L.; Frochot, C.; Vanderesse, R.; Thomas, N.; Gravier, J.; Guillemin, F.; Barberi-Heyob, M. Recent improvements in the use of synthetic peptides for a selective photodynamic therapy. Anti-Cancer Agents Med. Chem. 2006, 6, 469–488. [Google Scholar] [CrossRef]
- Zheng, X.; Morgan, J.; Pandey, S.K.; Chen, Y.; Tracy, E.; Baumann, H.; Missert, J.R.; Batt, C.; Jackson, J.; Bellnier, D.A.; et al. Conjugation of 2-(1′-Hexyloxyethyl)-2-devinylpyropheophorbide-a (HPPH) to Carbohydrates Changes its Subcellular Distribution and Enhances Photodynamic Activity in Vivo. J. Med. Chem. 2009, 52, 4306–4318. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Graham, A.; Shibata, M.; Missert, J.R.; Oseroff, A.R.; Dougherty, T.J.; Pandey, R.K. Synthesis of β-Galactose-Conjugated Chlorins Derived by Enyne Metathesis as Galectin-Specific Photosensitizers for Photodynamic Therapy. J. Org. Chem. 2001, 66, 8709–8716. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hui, L.; Foster, D.A.; Drain, C.M. Efficient Synthesis and Photodynamic Activity of Porphyrin-Saccharide Conjugates: Targeting and Incapacitating Cancer Cells. Biochemistry 2004, 43, 10918–10929. [Google Scholar] [CrossRef] [PubMed]
- Frochot, C.; Stasio, B.D.; Vanderesse, R.; Belgy, M.-J.; Dodeller, M.; Guillemin, F.; Viriot, M.-L.; Barberi-Heyob, M. Interest of RGD-containing linear or cyclic peptide targeted tetraphenylchlorin as novel photosensitizers for selective photodynamic activity. Bioorg. Chem. 2007, 35, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Veracoechea, F.J.; Frenkel, D. Designing super selectivity in multivalent nano-particle binding. Proc. Natl. Acad. Sci. USA 2011, 108, 10963–10968. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.; Kim, S.-J.; Kim, Y. Porphyrinic metal-organic frameworks from custom-designed porphyrins. CrystEngComm 2016, 18, 345–368. [Google Scholar] [CrossRef]
- Zhou, C.; Afonso, D.; Valetti, S.; Feiler, A.; Cardile, V.; Graziano, A.C.E.; Conoci, S.; Sortino, S. Targeted Photodynamic Therapy with a Folate/Sensitizer Assembly Produced from Mesoporous Silica. Chem. Eur. J. 2017, 23, 7672–7676. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Jiang, D.; Kamkaew, A.; Valdovinos, H.F.; Im, H.-J.; Feng, L.; England, C.G.; Goel, S.; Barnhart, T.E.; Liu, Z.; et al. Renal-Clearable PEGylated Porphyrin Nanoparticles for Image-Guided Photodynamic Cancer Therapy. Adv. Funct. Mater. 2017, 27, 1702928. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wei, H.; He, Q.; Ferreira, C.A.; Kutyreff, C.J.; Ni, D.; Rosenkrans, Z.T.; Cheng, L.; Yu, F.; Engle, J.W.; et al. Efficient Uptake of 177Lu-Porphyrin-PEG Nanocomplexes by Tumor Mitochondria for Multimodal-Imaging-Guided Combination Therapy. Angew. Chem. Int. Ed. 2018, 57, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Hernandez, R.; Geng, J.; Sun, H.; Song, W.; Chen, F.; Graves, S.A.; Nickles, R.J.; Cheng, C.; Cai, W.; et al. A porphyrin-PEG polymer with rapid renal clearance. Biomaterials 2016, 76, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Song, W.; Chen, G.; Reynard, J.M.; Ohulchanskyy, T.Y.; Prasad, P.N.; Bright, F.V.; Lovell, J.F. Pd-Porphyrin-Cross-Linked Implantable Hydrogels with Oxygen-Responsive Phosphorescence. Adv. Healthc. Mater. 2014, 3, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chauhan, S.; Geng, J.; Qin, Y.; Watson, D.F.; Lovell, J.F. Implantable Tin Porphyrin-PEG Hydrogels with pH-Responsive Fluorescence. Biomacromolecules 2017, 18, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Lovell, J.F.; Roxin, A.; Ng, K.K.; Qi, Q.; McMullen, J.D.; DaCosta, R.S.; Zheng, G. Porphyrin-Cross-Linked Hydrogel for Fluorescence-Guided Monitoring and Surgical Resection. Biomacromolecules 2011, 12, 3115–3118. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Chen, L.-F.; Hu, P.; Chen, Z.-N. Tetranuclear gadolinium (III) porphyrin complex as a theranostic agent for multimodal imaging and photodynamic therapy. Inorg. Chem. 2014, 53, 4184–4191. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.; Ristic, Z.; Klauser, S.; Verrey, F. Activation of system l heterodimeric amino acid exchangers by intracellular substrates. EMBO J. 2002, 21, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, O.; Kanai, Y.; Chairoungdua, A.; Kim, D.K.; Segawa, H.; Nii, T.; Cha, S.H.; Matsuo, H.; Fukushima, J.-I.; Fukasawa, Y. Human l-type amino acid transporter 1 (LAT1): Characterization of function and expression in tumor cell lines. Biochim. Biophys. Acta 2001, 1514, 291–302. [Google Scholar] [CrossRef]
- Kanai, Y.; Segawa, H.; Miyamoto, K.-I.; Uchino, H.; Takeda, E.; Endou, H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J. Biol. Chem. 1998, 273, 23629–23632. [Google Scholar] [CrossRef] [PubMed]
- Shikano, N.; Kanai, Y.; Kawai, K.; Ishikawa, N.; Endou, H. Characterization of 3-[125I] iodo-α-methyl-l-tyrosine transport via human l-type amino acid transporter 1. Nucl. Med. Biol. 2003, 30, 31–37. [Google Scholar] [CrossRef]
- Lahoutte, T.; Caveliers, V.; Camargo, S.M.; Franca, R.; Ramadan, T.; Veljkovic, E.; Mertens, J.; Bossuyt, A.; Verrey, F. SPECT and PET amino acid tracer influx via system l (h4F2hc-hLAT1) and its transstimulation. J. Nucl. Med. 2004, 45, 1591–1596. [Google Scholar] [PubMed]
- Uchino, H.; Kanai, Y.; Kim, D.K.; Wempe, M.F.; Chairoungdua, A.; Morimoto, E.; Anders, M.; Endou, H. Transport of amino acid-related compounds mediated by l-type amino acid transporter 1 (LAT1): Insights into the mechanisms of substrate recognition. Mol. Pharmacol. 2002, 61, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.C.; Bode, B.P. Amino acid transporters ASCT2 and LAT1 in cancer: Partners in crime? Semin. Cancer Biol. 2005, 15, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.H.; Su, H.; Hildebrandt, I.J.; Phelps, M.E.; Czernin, J.; Weber, W.A. Changes in tumor metabolism as readout for Mammalian target of rapamycin kinase inhibition by rapamycin in glioblastoma. Clin. Cancer Res. 2008, 14, 3416–3426. [Google Scholar] [CrossRef] [PubMed]
- Sasajima, T.; Miyagawa, T.; Oku, T.; Gelovani, J.G.; Finn, R.; Blasberg, R. Proliferation-dependent changes in amino acid transport and glucose metabolism in glioma cell lines. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Nodwell, M.B.; Yang, H.; Čolović, M.; Yuan, Z.; Merkens, H.; Martin, R.E.; Bénard, F.; Schaffer, P.; Britton, R. 18F-Fluorination of Unactivated C–H Bonds in Branched Aliphatic Amino Acids: Direct Synthesis of Oncological Positron Emission Tomography Imaging Agents. J. Am. Chem. Soc. 2017, 139, 3595–3598. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Nakanishi, K.; Berova, N. Porphyrins and metalloporphyrins: Versatile circular dichroic reporter groups for structural studies. Chirality 2000, 12, 237–255. [Google Scholar] [CrossRef]
- Ribó, J.M.; Crusats, J.; Sagués, F.; Claret, J.; Rubires, R. Chiral sign induction by vortices during the formation of mesophases in stirred solutions. Science 2001, 292, 2063–2066. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Tokarz, D.; Cisek, R.; Ng, K.K.; Wang, F.; Chen, J.; Barzda, V.; Zheng, G. Organized Aggregation of Porphyrins in Lipid Bilayers for Third Harmonic Generation Microscopy. Angew. Chem. Int. Ed. 2015, 54, 13928–13932. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Dougherty, T.J. How does photodynamic therapy work? Photochem. Photobiol. 1992, 55, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Flors, C.; Fryer, M.J.; Waring, J.; Reeder, B.; Bechtold, U.; Mullineaux, P.M.; Nonell, S.; Wilson, M.T.; Baker, N.R. Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, Singlet Oxygen Sensor Green®. J. Exp. Bot. 2006, 57, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Kersemans, V.; Cornelissen, B.; Kersemans, K.; Bauwens, M.; Dierckx, R.A.; De Spiegeleer, B.; Mertens, J.; Slegers, G. 123/125I-labelled 2-iodo-l-phenylalanine and 2-iodo-d-phenylalanine: Comparative uptake in various tumour types and biodistribution in mice. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 919–927. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chitgupi, U.; Lovell, J.F.; Rajendiran, V. Assessing Photosensitizer Targeting Using Meso-Tetra(Carboxyphenyl) Porphyrin. Molecules 2018, 23, 892. https://doi.org/10.3390/molecules23040892
Chitgupi U, Lovell JF, Rajendiran V. Assessing Photosensitizer Targeting Using Meso-Tetra(Carboxyphenyl) Porphyrin. Molecules. 2018; 23(4):892. https://doi.org/10.3390/molecules23040892
Chicago/Turabian StyleChitgupi, Upendra, Jonathan F. Lovell, and Venugopal Rajendiran. 2018. "Assessing Photosensitizer Targeting Using Meso-Tetra(Carboxyphenyl) Porphyrin" Molecules 23, no. 4: 892. https://doi.org/10.3390/molecules23040892






