The Multifunctional Role of Chitosan in Horticultural Crops; A Review
Abstract
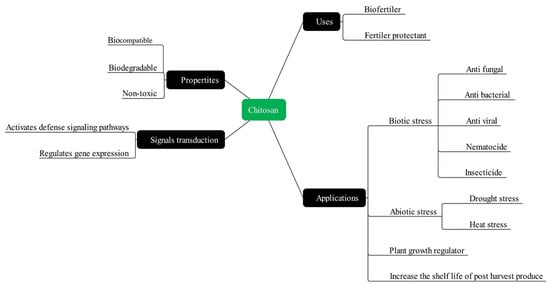
:1. Introduction
2. Sources of Chitosan
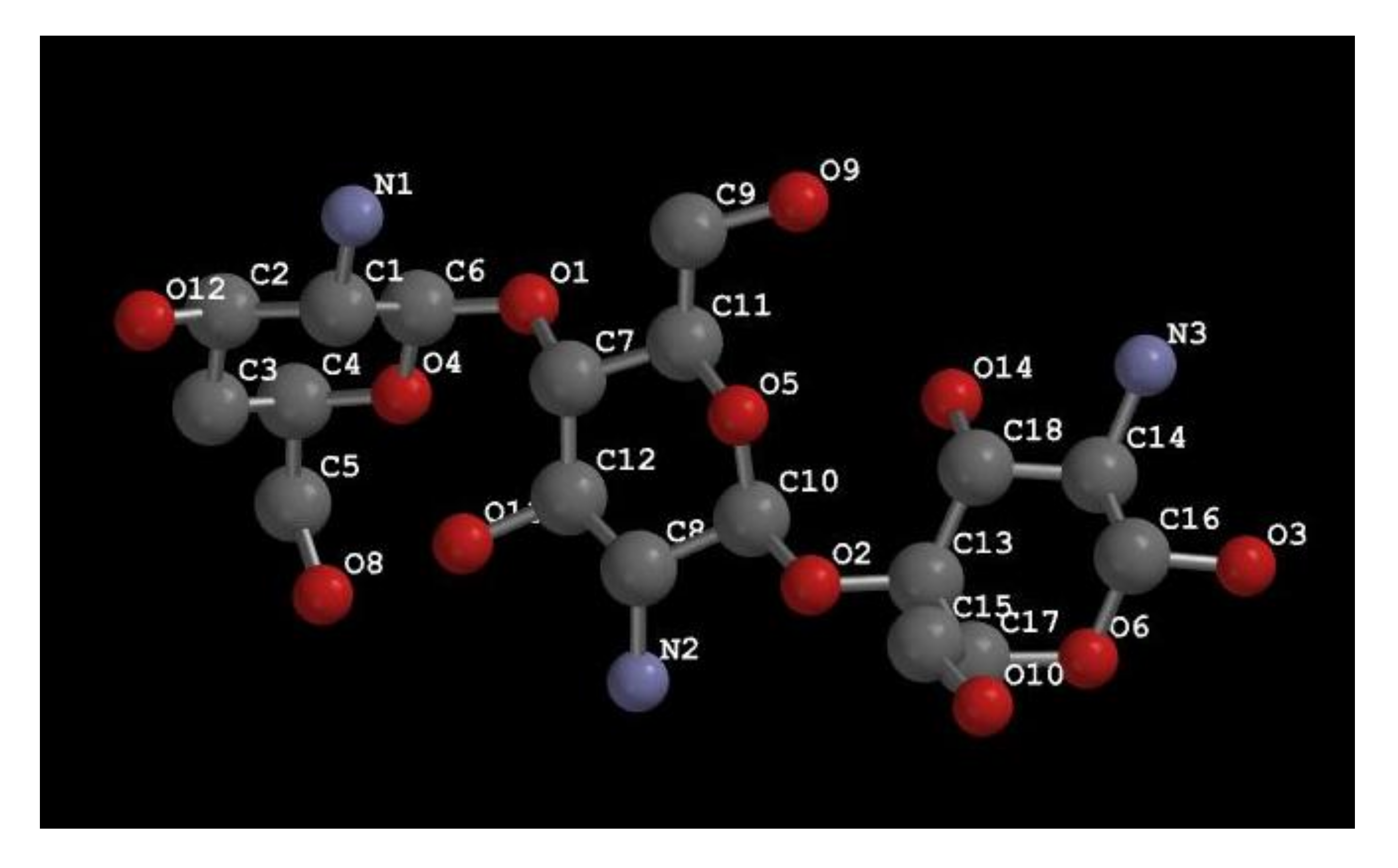
3. Structure and Characterization of Chitosan
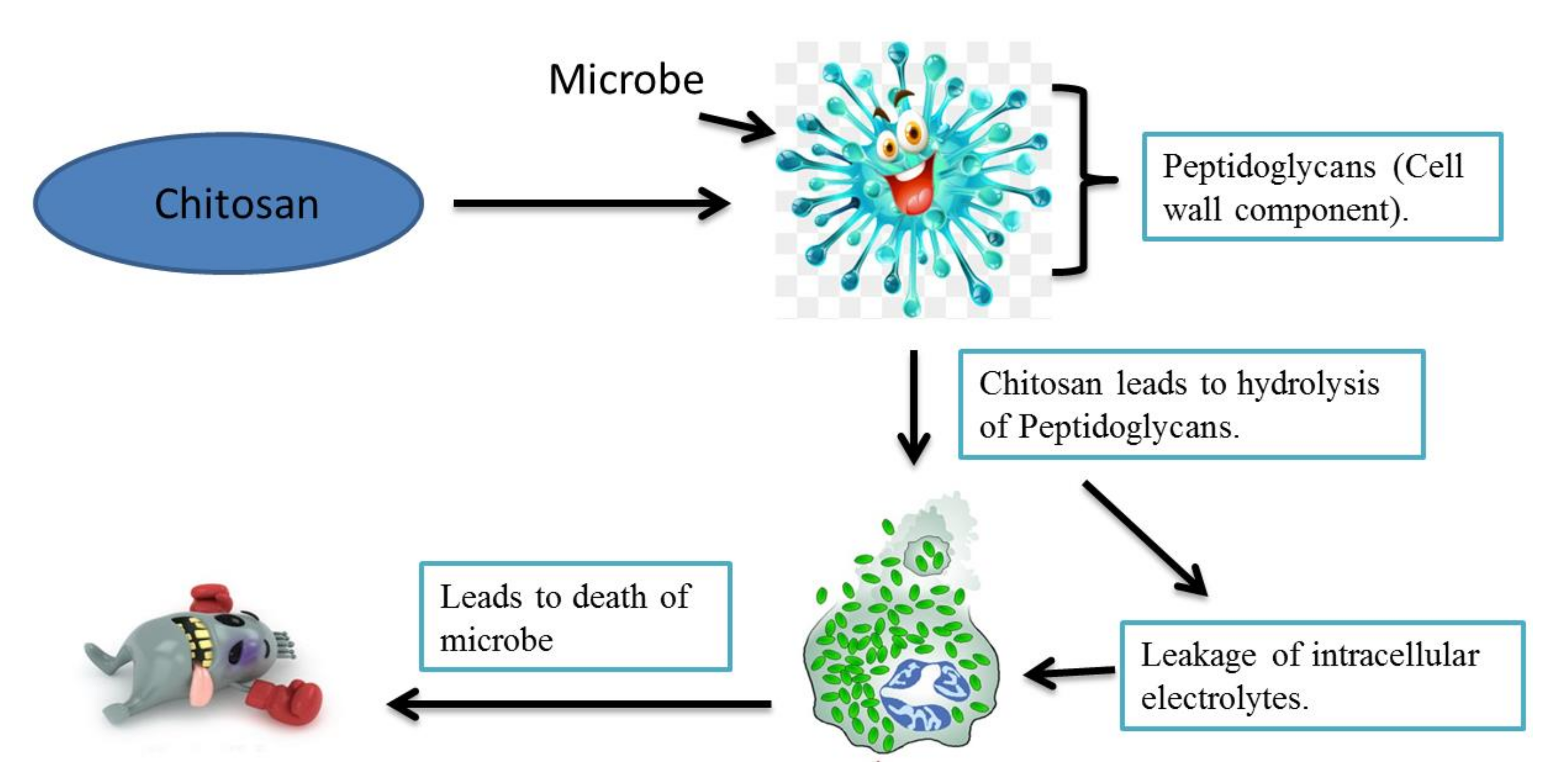
4. Antimicrobial Activity of Chitosan
4.1. Chitosan Effect on Fungi, Bacteria, and Nematodes
4.2. Effect of Chitosan on Viral Diseases
5. Chitosan as an Insecticide
6. Chitosan’s Effects on Abiotic Stresses
6.1. Effect on Drought Stress
6.2. Effect on Heat Stress
7. Chitosan Effects on Fruits and Vegetables
7.1. Chitosan Effects on Plant Growth, Yield Attributes, and Physiological Activities
7.2. Chitosan Effects on Post-Harvest Fruits and Vegetables
8. Chitosan Effects on Gene Expression
9. Chitosan as a Bio-Fertilizer and Fertilizer Protectant
10. Conclusions
Acknowledgments
Conflicts of Interest
References
- Dash, M.; Chiellini, F.; Ottenbrite, R.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Natural chelating polymers; alginic acid, chitin and chitosan. In Natural Chelating Polymers; Alginic Acid, Chitin and Chitosan; Pergamon Press: Oxford, UK, 1973. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Kaya, M.; Mujtaba, M.; Bulut, E.; Akyuz, B.; Zelencova, L.; Sofi, K. Fluctuation in physicochemical properties of chitins extracted from different body parts of honeybee. Carbohydr. Polym. 2015, 132, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Sofi, K.; Sargin, I.; Mujtaba, M. Changes in physicochemical properties of chitin at developmental stages (larvae, pupa and adult) of Vespa crabro (wasp). Carbohydr. Polym. 2016, 145, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Bitim, B.; Mujtaba, M.; Koyuncu, T. Surface morphology of chitin highly related with the isolated body part of butterfly (Argynnis pandora). Int. J. Biol. Macromol. 2015, 81, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 2008, 57, 397–430. [Google Scholar] [CrossRef]
- Shamov, M.; Bratskaya, S.Y.; Avramenko, V. Interaction of carboxylic acids with chitosan: Effect of pK and hydrocarbon chain length. J. Colloid Interface Sci. 2002, 249, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Cervera, M.F.; Heinämäki, J.; de la Paz, N.; López, O.; Maunu, S.L.; Virtanen, T.; Hatanpää, T.; Antikainen, O.; Nogueira, A.; Fundora, J. Effects of spray drying on physicochemical properties of chitosan acid salts. AAPS PharmSciTech 2011, 12, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Akyuz, L.; Sargin, I.; Mujtaba, M.; Salaberria, A.M.; Labidi, J.; Cakmak, Y.S.; Koc, B.; Baran, T.; Ceter, T. Incorporation of sporopollenin enhances acid–base durability, hydrophobicity, and mechanical, antifungal and antioxidant properties of chitosan films. J. Ind. Eng. Chem. 2017, 47, 236–245. [Google Scholar] [CrossRef]
- Akyuz, L.; Kaya, M.; Koc, B.; Mujtaba, M.; Ilk, S.; Labidi, J.; Salaberria, A.M.; Cakmak, Y.S.; Yildiz, A. Diatomite as a novel composite ingredient for chitosan film with enhanced physicochemical properties. Int. J. Biol. Macromol. 2017, 105, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Salaberria, A.M.; Andres, M.A.; Kaya, M.; Gunyakti, A.; Labidi, J. Utilization of flax (Linum usitatissimum) cellulose nanocrystals as reinforcing material for chitosan films. Int. J. Biol. Macromol. 2017, 104, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Allan, C.R.; Hadwiger, L.A. The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp. Mycol. 1979, 3, 285–287. [Google Scholar] [CrossRef]
- Barber, M.; Bertram, R.; Ride, J. Chitin oligosaccharides elicit lignification in wounded wheat leaves. Physiol. Mol. Plant Pathol. 1989, 34, 3–12. [Google Scholar] [CrossRef]
- Chaouat, C. Conception de Nouveaux Systèmes de Formulation d'actifs Dépigmentants, en vue de leur Utilisation par voie Cutanée; Université de Toulouse, Université Toulouse III-Paul Sabatier: Toulouse, France, 2013. [Google Scholar]
- Chirkov, S.N.; Surguchova, N.; Atabekov, J.G. Chitosan inhibits systemic infections caused by DNA-containing plant viruses. Arch. Phytopathol. Plant Prot. 1994, 29, 21–24. [Google Scholar] [CrossRef]
- Li, B.; Shi, Y.; Shan, C.; Zhou, Q.; Ibrahim, M.; Wang, Y.; Wu, G.; Li, H.; Xie, G.; Sun, G. Effect of chitosan solution on the inhibition of Acidovorax citrulli causing bacterial fruit blotch of watermelon. J. Sci. Food Agric. 2013, 93, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Agbodjato, N.A.; Noumavo, P.A.; Adjanohoun, A.; Agbessi, L.; Baba-Moussa, L. Synergistic effects of plant growth promoting rhizobacteria and chitosan on in vitro seeds germination, greenhouse growth, and nutrient uptake of maize (Zea mays L.). Biotechnol. Res. Int. 2016, 2016, 7830182. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Baran, T.; Erdoğan, S.; Menteş, A.; Özüsağlam, M.A.; Çakmak, Y.S. Physicochemical comparison of chitin and chitosan obtained from larvae and adult Colorado potato beetle (Leptinotarsa decemlineata). Mater. Sci. Eng. C 2014, 45, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Cakmak, Y.S.; Baran, T.; Asan-Ozusaglam, M.; Mentes, A.; Tozak, K.O. New chitin, chitosan, and O-carboxymethyl chitosan sources from resting eggs of Daphnia longispina (Crustacea); with physicochemical characterization, and antimicrobial and antioxidant activities. Biotechnol. Bioprocess Eng. 2014, 19, 58–69. [Google Scholar] [CrossRef]
- Kumar, M.R.; Muzzarelli, R.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.P.; Martínez, M.V.; Hernández, J.L.; Yusty, M.L. High-performance liquid chromatographic determination of chitin in the snow crab, Chionoecetes opilio. J. Chromatogr. A 2006, 1116, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ganesh, E.A. Extraction of chitin from trash crabs (Podophthalmus vigil) by an eccentric method. Curr. Res. Biol. Sci. 2010, 2, 72–75. [Google Scholar]
- Sperstad, S.V.; Haug, T.; Paulsen, V.; Rode, T.M.; Strandskog, G.; Solem, S.T.; Styrvold, O.B.; Stensvåg, K. Characterization of crustins from the hemocytes of the spider crab, Hyas araneus, and the red king crab, Paralithodes camtschaticus. Dev. Comp. Immunol. 2009, 33, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Hajji, S.; Younes, I.; Ghorbel-Bellaaj, O.; Hajji, R.; Rinaudo, M.; Nasri, M.; Jellouli, K. Structural differences between chitin and chitosan extracted from three different marine sources. Int. J. Biol. Macromol. 2014, 65, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Seyyar, O.; Baran, T.; Erdoğan, S.; Kar, M. A physicochemical characterization of fully acetylated chitin structure isolated from two spider species: With new surface morphology. Int. J. Biol. Macromol. 2014, 65, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.J.; Knight, D.P.; Vollrath, F. Chitin in the silk gland ducts of the spider Nephila edulis and the silkworm Bombyx mori. PLoS ONE 2013, 8, e73225. [Google Scholar] [CrossRef] [PubMed]
- Synowiecki, J.; Al-Khateeb, N.A.A.Q. Mycelia of Mucor rouxii as a source of chitin and chitosan. Food Chem. 1997, 60, 605–610. [Google Scholar] [CrossRef]
- Mathur, N.K.; Narang, C.K. Chitin and chitosan, versatile polysaccharides from marine animals. J. Chem. Educ. 1990, 67, 938. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives, and applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Chobot, V.; Kremenak, J.; Opletal, L. Phytotherapeutic aspects of diseases of the circulatory system. 4. Chitin and chitosan. Ceska a Slovenska Farmacie: Casopis Ceske Farmaceuticke Spolecnosti a Slovenske farmaceuticke Spolecnosti 1995, 44, 190–195. [Google Scholar]
- Kaya, M.; Asan-Ozusaglam, M.; Erdogan, S. Comparison of antimicrobial activities of newly obtained low molecular weight scorpion chitosan and medium molecular weight commercial chitosan. J. Biosci. Bioeng. 2016, 121, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Abdou, E.S.; Nagy, K.S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Mahlous, M.; Tahtat, D.; Benamer, S.; Khodja, A.N. Gamma irradiation-aided chitin/chitosan extraction from prawn shells. Nucl. Instrum. Methods Phys. Res. Sec. B Beam Interact. Mater. At. 2007, 265, 414–417. [Google Scholar] [CrossRef]
- Zhang, M.; Haga, A.; Sekiguchi, H.; Hirano, S. Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. Int. J. Biol. Macromol. 2000, 27, 99–105. [Google Scholar] [CrossRef]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Jiang, C.; Yang, Q. Extraction and characterization of chitin from the beetle Holotrichia parallela motschulsky. Molecules 2012, 17, 4604–4611. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Baran, T. Description of a new surface morphology for chitin extracted from wings of cockroach (Periplaneta americana). Int. J. Biol. Macromol. 2015, 75, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Baran, T.; Mentes, A.; Asaroglu, M.; Sezen, G.; Tozak, K.O. Extraction and characterization of α-chitin and chitosan from six different aquatic invertebrates. Food Biophys. 2014, 9, 145–157. [Google Scholar] [CrossRef]
- Tanaka, K.; Katsura, N.; Saku, T.; Kasuga, S. Composite texture of chitin and keratin in an animal organ, Lingula seta. Polym. J. 1988, 20, 119–123. [Google Scholar] [CrossRef]
- Chaussard, G.; Domard, A. New aspects of the extraction of chitin from squid pens. Biomacromolecules 2004, 5, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Saito, T.; Isogai, A. Preparation of chitin nanofibers from squid pen β-chitin by simple mechanical treatment under acid conditions. Biomacromolecules 2008, 9, 1919–1923. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Abuzaytoun, R. Chitin, chitosan, and co-products: Chemistry, production, applications, and health effects. Adv. Food Nutr. Res. 2005, 49, 93–135. [Google Scholar] [PubMed]
- Jang, M.K.; Kong, B.G.; Jeong, Y.I.; Lee, C.H.; Nah, J.W. Physicochemical characterization of α-chitin, β-chitin, and γ-chitin separated from natural resources. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T.; Saman, I.; Asan Ozusaglam, M.; Cakmak, Y.S.; Menteş, A. Physicochemical characterization of chitin and chitosan obtained from resting eggs of Ceriodaphnia quadrangula (Branchiopoda: Cladocera: Daphniidae). J. Crustacean Biol. 2014, 34, 283–288. [Google Scholar] [CrossRef]
- Il’Ina, A.; Varlamov, V. Hydrolysis of chitosan in lactic acid. Appl. Biochem. Microbiol. 2004, 40, 300–303. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Ichikawa, S.; Hiruta, O.; Sato, S.; Mukataka, S. Factors affecting the composition of oligosaccharides produced in chitosan hydrolysis using immobilized chitosanases. Biotechnol. Prog. 2002, 18, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Shuai, X.; Unger, F.; Simon, M.; Bi, D.; Kissel, T. The depolymerization of chitosan: Effects on physicochemical and biological properties. Int. J. Pharm. 2004, 281, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Chen, J. Changes of polydispersity and limiting molecular weight of ultrasound-treated chitosan. Adv. Chitin Sci. 2000, 4, 361–366. [Google Scholar]
- Hawary, D.L.; Motaleb, M.A.; Farag, H.; Guirguis, O.W.; Elsabee, M.Z. Water-soluble derivatives of chitosan as a target delivery system of 99mTc to some organs in vivo for nuclear imaging and biodistribution. J. Radioanal. Nucl. Chem. 2011, 290, 557–567. [Google Scholar] [CrossRef]
- Khanjari, A.; Karabagias, I.; Kontominas, M. Combined effect of N, O-carboxymethyl chitosan and oregano essential oil to extend shelf life and control Listeria monocytogenes in raw chicken meat fillets. LWT-Food Sci. Technol. 2013, 53, 94–99. [Google Scholar] [CrossRef]
- Xiao, B.; Wan, Y.; Wang, X.; Zha, Q.; Liu, H.; Qiu, Z.; Zhang, S. Synthesis and characterization of N-(2-hydroxy)propyl-3-trimethyl ammonium chitosan chloride for potential application in gene delivery. Colloids Surf. B Biointerfaces 2012, 91, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Goy, R.C.; Britto, D.D.; Assis, O.B. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Liang, C.; Yuan, F.; Liu, F.; Wang, Y.; Gao, Y. Structure and antimicrobial mechanism of ɛ-polylysine–chitosan conjugates through Maillard reaction. Int. J. Biol. Macromol. 2014, 70, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.B.; Arul, J.; Ait-Barka, E.; Angers, P.; Richard, C.; Castaigne, F. EVect of Chitosan on Growth and Toxin Production by Alternaria alternata f. sp. lycopersici. Biocontrol Sci. Technol. 1998, 8, 33–43. [Google Scholar] [CrossRef]
- Goy, R.C.; Morais, S.T.; Assis, O.B. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Revista Brasileira de Farmacognosia 2016, 26, 122–127. [Google Scholar] [CrossRef]
- Jinasena, D.; Pathirathna, P.; Wickramarachchi, S.; Marasinghe, E. Use of chitosan to control anthracnose on “Embul” banana. In Proceedings of the 2011 International Conference on Asia Agriculture and Animal IPCBEE, Hong Kong, China, 2 July 2011; pp. 56–60. [Google Scholar]
- Cheah, L.; Page, B.; Shepherd, R. Chitosan coating for inhibition of sclerotinia rot of carrots. N. Z. J. Crop Hortic. Sci. 1997, 25, 89–92. [Google Scholar] [CrossRef]
- Ben-Shalom, N.; Fallik, E. Further suppression of Botrytis cinerea disease in cucumber seedlings by chitosan-copper complex as compared with chitosan alone. Phytoparasitica 2003, 31, 99–102. [Google Scholar] [CrossRef]
- Moret, A.; Muñoz, Z.; Garcés, S. Control of powdery mildew on cucumber cotyledons by chitosan. J. Plant Pathol. 2009, 375–380. [Google Scholar]
- Long, L.T.; Tan, L.V.; Boi, V.N.; Trung, T.S. Antifungal activity of water-soluble chitosan against Colletotrichum capsici in post-harvest chili pepper. J. Food Process. Preserv. 2017. [Google Scholar] [CrossRef]
- Mandal, S. Induction of phenolics, lignin and key defense enzymes in eggplant (Solanum melongena L.) roots in response to elicitors. Afr. J. Biotechnol. 2010, 9, 8038–8047. [Google Scholar]
- Jitareerat, P.; Paumchai, S.; Kanlayanarat, S.; Sangchote, S. Effect of chitosan on ripening, enzymatic activity, and disease development in mango (Mangifera indica) fruit. N. Z. J. Crop Hortic. Sci. 2007, 35, 211–218. [Google Scholar] [CrossRef]
- Zeng, K.; Deng, Y.; Ming, J.; Deng, L. Induction of disease resistance and ROS metabolism in navel oranges by chitosan. Sci. Hortic. 2010, 126, 223–228. [Google Scholar] [CrossRef]
- Meng, X.; Yang, L.; Kennedy, J.F.; Tian, S. Effects of chitosan and oligochitosan on growth of two fungal pathogens and physiological properties in pear fruit. Carbohydr. Polym. 2010, 81, 70–75. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Hernández-López, M.; Bosquez-Molina, E.; Wilson, C. Effects of chitosan and plant extracts on growth of Colletotrichum gloeosporioides, anthracnose levels and quality of papaya fruit. Crop Prot. 2003, 22, 1087–1092. [Google Scholar] [CrossRef]
- Hassni, M.; El Hadrami, A.; Daayf, F.; Barka, E.A.; El Hadrami, I. Chitosan, antifungal product against Fusarium oxysporum f. sp. albedinis and elicitor of defence reactions in date palm roots. Phytopathol. Mediterr. 2004, 43, 195–204. [Google Scholar]
- Ma, Z.; Yang, L.; Yan, H.; Kennedy, J.F.; Meng, X. Chitosan and oligochitosan enhance the resistance of peach fruit to brown rot. Carbohydr. Polym. 2013, 94, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Sathiyabama, M.; Akila, G.; Einstein Charles, R. Chitosan-induced defence responses in tomato plants against early blight disease caused by Alternaria solani (Ellis and Martin) Sorauer. Arch. Phytopathol. Plant Prot. 2014, 47, 1777–1787. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Charles, R.E. Fungal cell wall polymer based nanoparticles in protection of tomato plants from wilt disease caused by Fusarium oxysporum f. sp. lycopersici. Carbohydr. Polym. 2015, 133, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Escudero, N.; Lopez-Moya, F.; Ghahremani, Z.; Zavala-Gonzalez, E.A.; Alaguero-Cordovilla, A.; Ros-Ibañez, C.; Lacasa, A.; Sorribas, F.J.; Lopez-Llorca, L.V. Chitosan increases tomato root colonization by Pochonia chlamydosporia and their combination reduces root-knot nematode damage. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Algam, S.; Xie, G.; Li, B.; Yu, S.; Su, T.; Larsen, J. Effects of Paenibacillus strains and chitosan on plant growth promotion and control of Ralstonia wilt in tomato. J. Plant Pathol. 2010, 92, 593–600. [Google Scholar]
- Nicaise, V. Crop immunity against viruses: Outcomes and future challenges. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Faoro, F. Induced Systemic Resistance against Systemic Viruses: A Feasible Approach? Available online: https://air.unimi.it/retrieve/handle/2434/229167/299904/Faoro%20F%20_%20IOBC%20Bull.%2089.pdf (accessed on 8 April 2018).
- Chirkov, S.; Il’ina, A.; Surgucheva, N.; Letunova, E.; Varitsev, Y.A.; Tatarinova, N.Y.; Varlamov, V. Effect of chitosan on systemic viral infection and some defense responses in potato plants. Russ. J. Plant Physiol. 2001, 48, 774–779. [Google Scholar] [CrossRef]
- Bondok, A. Response of Tomato Plants to Salicyli c Acid and Chitosan under Infection with Tomato mosaic virus. Am.-Eur. J. Agric. Environ. Sci. 2015, 15, 1520–1529. [Google Scholar]
- Mishra, S.; Jagadeesh, K.S.; Krishnaraj, P.U.; Prem, S. Biocontrol of tomato leaf curl virus (ToLCV) in tomato with chitosan supplemented formulations of Pseudomonas sp. under field conditions. Aust. J. Crop Sci. 2014, 8, 347–355. [Google Scholar]
- Firmansyah, D. Use of Chitosan and Plant Growth Promoting Rhizobacteria to Control Squash Mosaic Virus on Cucumber Plants. Asian J. Plant Pathol. 2017, 11, 148–155. [Google Scholar]
- Nagorskaya, V.; Reunov, A.; Lapshina, L.; Davydova, V.; Yermak, I. Effect of chitosan on tobacco mosaic virus (TMV) accumulation, hydrolase activity, and morphological abnormalities of the viral particles in leaves of N. tabacum L. cv. Samsun. Virol. Sin. 2014, 29, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Meng, Q.; Zeng, H.; Wang, W.; Yin, H. Chitosan oligosaccharide induces resistance to Tobacco mosaic virus in Arabidopsis via the salicylic acid-mediated signalling pathway. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.; Rogge, T.M.; Stevens, C.V.; Höfte, M.; Steurbaut, W.; Smagghe, G. Insecticidal and fungicidal activity of new synthesized chitosan derivatives. Pest Manag. Sci. 2005, 61, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Sahab, A.; Waly, A.; Sabbour, M.; Nawar, L.S. Synthesis, antifungal and insecticidal potential of Chitosan (CS)-g-poly (acrylic acid)(PAA) nanoparticles against some seed borne fungi and insects of soybean. Int. J. Chem. Tech. Res. 2015, 8, 589–598. [Google Scholar]
- Li, Y.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Li, K.; Li, P. Preparation, Characterization, and Insecticidal Activity of Avermectin-Grafted-Carboxymethyl Chitosan. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Helmi, A.; Mohamed, H.I. Biochemical and Ultrastructural Changes of Some Tomato Cultivars after Infestation with Aphis gossypii Glover (Hemiptera: Aphididae) at Qalyubiyah, Egypt. Gesunde Pflanzen 2016, 68, 41–50. [Google Scholar] [CrossRef]
- Sobhy, I.S.; Mandour, N.S.; Sarhan, A.A. Tomato treatment with chemical inducers reduces the performance of Spodoptera littoralis (Lepidoptera: Noctuidae). Appl. Entomol. Zool. 2015, 50, 175–182. [Google Scholar] [CrossRef]
- Razmjou, J.; Mohammadi, M.; Hassanpour, M. Effect of vermicompost and cucumber cultivar on population growth attributes of the melon aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2011, 104, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Carletto, J.; Lombaert, E.; Chavigny, P.; Brévault, T.; Lapchin, L.; Vanlerberghe-masutti, F. Ecological specialization of the aphid Aphis gossypii Glover on cultivated host plants. Mol. Ecol. 2009, 18, 2198–2212. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Wang, S.; Li, X.; Zhang, F. Optimization and application of microencapsulated artificial diet for Orius sauteri (Hemiptera: Anthocoridae). Acta Entomol. Sin. 2010, 53, 891–900. [Google Scholar]
- Salehi-Lisar, S.Y.; Bakhshayeshan-Agdam, H. Drought Stress in Plants: Causes, Consequences, and Tolerance. In Drought Stress Tolerance in Plants; Springer: Berlin, Germany, 2016; Volume 1, pp. 1–16. [Google Scholar]
- Yang, F.; Hu, J.; Li, J.; Wu, X.; Qian, Y. Chitosan enhances leaf membrane stability and antioxidant enzyme activities in apple seedlings under drought stress. Plant Growth Regul. 2009, 58, 131–136. [Google Scholar] [CrossRef]
- Jiao, Z.; Li, Y.; Li, J.; Xu, X.; Li, H.; Lu, D.; Wang, J. Effects of exogenous chitosan on physiological characteristics of potato seedlings under drought stress and rehydration. Potato Res. 2012, 55, 293–301. [Google Scholar] [CrossRef]
- Gu, L. Effects of exogenous Chitosan on physiological characteristics of phalaenopsis seedlings under draught stress. Southwest China J. Agric. Sci. 2011, 24, 90–93. [Google Scholar]
- Pongprayoon, W.; Roytrakul, S.; Pichayangkura, R.; Chadchawan, S. The role of hydrogen peroxide in chitosan-induced resistance to osmotic stress in rice (Oryza sativa L.). Plant Growth Regul. 2013, 70, 159–173. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhang, X.; Merewitz, E.; Peng, Y.; Ma, X.; Huang, L.; Yan, Y. Metabolic pathways regulated by chitosan contributing to drought resistance in white clover. J. Proteome Res. 2017, 16, 3039–3052. [Google Scholar] [CrossRef] [PubMed]
- Górnik, K.; Grzesik, M.; Romanowska-Duda, B. The effect of chitosan on rooting of grapevine cuttings and on subsequent plant growth under drought and temperature stress. J. Fruit Ornam. Plant Res. 2008, 16, 333–343. [Google Scholar]
- Iriti, M.; Faoro, F. Abscisic acid is involved in chitosan-induced resistance to tobacco necrosis virus (TNV). Plant Physiol. Biochem. 2008, 46, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.-H.; Lee, S.C. Function of ABA in stomatal defense against biotic and drought stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef] [PubMed]
- McKersie, B.D.; Lesheim, Y. Stress and Stress Coping in Cultivated Plants; Springer: Berlin, Germany, 2013. [Google Scholar]
- Ibrahim, E.A.; Ramadan, W.A. Effect of zinc foliar spray alone and combined with humic acid or/and chitosan on growth, nutrient elements content and yield of dry bean (Phaseolus vulgaris L.) plants sown at different dates. Sci. Hortic. 2015, 184, 101–105. [Google Scholar] [CrossRef]
- Ng, L.M.; Melcher, K.; Teh, B.T.; Xu, H.E. Abscisic acid perception and signaling: Structural mechanisms and applications. Acta Pharmacol. Sin. 2014, 35, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wollenweber, B.; Jiang, D.; Liu, F.; Zhao, J. Water deficits and heat shock effects on photosynthesis of a transgenic Arabidopsis thaliana constitutively expressing ABP9, a bZIP transcription factor. J. Exp. Bot. 2008, 59, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-S.; Kim, Y.-M.; Hwang, O.-J.; Han, Y.-J.; Kim, S.Y.; Kim, J.-I. Overexpression of ArabidopsisABF3 gene confers enhanced tolerance to drought and heat stress in creeping bentgrass. Plant Biotechnol. Rep. 2013, 7, 165–173. [Google Scholar] [CrossRef]
- Zagzog, O.A.; Gad, M.M.; Hafez, N.K. Effect of Nano-chitosan on Vegetative Growth, Fruiting and Resistance of Malformation of Mango. Trends Hortic. Res. 2017, 6, 673–681. [Google Scholar]
- Reglinski, T.; Elmer, P.; Taylor, J.; Wood, P.; Hoyte, S. Inhibition of Botrytis cinerea growth and suppression of botrytis bunch rot in grapes using chitosan. Plant Pathol. 2010, 59, 882–890. [Google Scholar] [CrossRef]
- Meng, X.; Li, B.; Liu, J.; Tian, S. Physiological responses and quality attributes of table grape fruit to chitosan preharvest spray and post-harvest coating during storage. Food Chem. 2008, 106, 501–508. [Google Scholar] [CrossRef]
- Ferri, M.; Dipalo, S.C.; Bagni, N.; Tassoni, A. Chitosan elicits mono-glucosylated stilbene production and release in fed-batch bioreactor cultures of grape cells. Food Chem. 2011, 124, 1473–1479. [Google Scholar] [CrossRef]
- Scortichini, M. Field efficacy of chitosan to control Pseudomonas syringae pv. actinidiae, the causal agent of kiwifruit bacterial canker. Eur. J. Plant Pathol. 2014, 140, 887–892. [Google Scholar] [CrossRef]
- Gayed, A.A.N.A.; Shaarawi, S.A.M.A.; Elkhishen, M.A.; Elsherbini, N.R.M. Pre-harvest application of calcium chloride and chitosan on fruit quality and storability of ‘Early Swelling’peach during cold storage. Ciência Agrotecnol. 2017, 41, 220–231. [Google Scholar] [CrossRef]
- Giacalone, G.; Chiabrando, V. Effect of preharvest and post-harvest application of chitosan coating on storage quality of nectarines. Acta Hortic. 2013, 1084, 675–680. [Google Scholar]
- Jail, N.G.D.; Luiz, C.; Neto, R.; Di Piero, R.M. High-density chitosan reduces the severity of bacterial spot and activates the defense mechanisms of tomato plants. Trop. Plant Pathol. 2014, 39, 434–441. [Google Scholar] [CrossRef]
- Reddy, M.B.; Angers, P.; Castaigne, F.; Arul, J. Chitosan effects on blackmold rot and pathogenic factors produced by Alternaria alternata in post-harvest tomatoes. J. Am. Soc. Hortic. Sci. 2000, 125, 742–747. [Google Scholar]
- Tsugita, T.; Takahashi, K.; Muraoka, T.; Fukui, H. The application of chitin/chitosan for agriculture. In Proceedings of the Special Session of the 7th Symposium on Chitin and Chitosan; Japanese Society for Chitin and Chitosan: Fukui, Japan, 1993; pp. 21–22. (In Japanese). [Google Scholar]
- Hirano, S.; Kitaura, S.; Sasaki, N.; Sakaguchi, H.; Sugiyama, M.; Hashimoto, K.; Tanatani, A. Chitin biodegradation and wound healing in tree bark tissues. J. Polym. Environ. 1996, 4, 261–265. [Google Scholar] [CrossRef]
- Kim, H.-J.; Chen, F.; Wang, X.; Rajapakse, N.C. Effect of chitosan on the biological properties of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2005, 53, 3696–3701. [Google Scholar] [CrossRef] [PubMed]
- Barka, E.A.; Eullaffroy, P.; Clément, C.; Vernet, G. Chitosan improves development, and protects Vitis vinifera L. against Botrytis cinerea. Plant Cell Rep. 2004, 22, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Wanichpongpan, P.; Suriyachan, K.; Chandrkrachang, S. Effects of Chitosan on the growth of Gerbera flower plant (Gerbera jamesonii). In Proceedings of the Eighth International Chitin and Chitosan Conference and Fourth Asia Pacific Chitin and Chitosan Symposium, Yamaguchi, Japan, 21–23 September 2000; Uragami, T., Kurita, K., Fukamizo, T., Eds.; pp. 198–201. [Google Scholar]
- Chandrkrachang, S. The application of chitin and chitosan in agriculture in Thailand. Adv. Chitin Sci. 2002, 5, 458–462. [Google Scholar]
- Xue, G.-X.; Gao, H.-Y.; Li, P.-M.; Zou, Q. Effects of chitosan treatment on physiological and biochemical characteristics in cucumber seedlings under low temperature. J. Plant Physiol. Mol. Biol. 2004, 30, 441–448. [Google Scholar]
- Chookhongkha, N.; Sopondilok, T.; Photchanachai, S. Effect of chitosan and chitosan nanoparticles on fungal growth and chilli seed quality. In Proceedings of the International Conference on Post-harvest Pest and Disease Management in Exporting Horticultural Crops-PPDM2012 973, Bangkok, Thailand, 21 February 2012; pp. 231–237. [Google Scholar]
- Ziani, K.; Ursúa, B.; Maté, J.I. Application of bioactive coatings based on chitosan for artichoke seed protection. Crop Prot. 2010, 29, 853–859. [Google Scholar] [CrossRef]
- Chookhongkha, N.; Miyagawa, S.; Jirakiattikul, Y.; Photchanachai, S. Chili growth and seed productivity as affected by chitosan. In Proceedings of the International Conference on Agriculture Technology and Food Sciences (ICATFS’2012), Manila, Philippines, 17–18 November 2012; pp. 17–18. [Google Scholar]
- Shehata, S.; Fawzy, Z.; El-Ramady, H. Response of cucumber plants to foliar application of chitosan and yeast under greenhouse conditions. Aust. J. Basic Appl. Sci. 2012, 6, 63–71. [Google Scholar]
- Van, S.N.; Minh, H.D.; Anh, D.N. Study on chitosan nanoparticles on biophysical characteristics and growth of Robusta coffee in green house. Biocatal. Agric. Biotechnol. 2013, 2, 289–294. [Google Scholar]
- Wang, Y.; Li, B.; Chen, X.; Shi, Y.; Zhou, Q.; Qiu, H.; Ibrahim, M.; Xie, G.; Sun, G. Effect of chitosan on seed germation, seedling growth and the clubroot control in Chinese cabbage. J. Food Agric. Environ. 2012, 10, 673–675. [Google Scholar]
- Kananont, N.; Pichyangkura, R.; Chanprame, S.; Chadchawan, S.; Limpanavech, P. Chitosan specificity for the in vitro seed germination of two Dendrobium orchids (Asparagales: Orchidaceae). Sci. Hortic. 2010, 124, 239–247. [Google Scholar] [CrossRef]
- Mondal, M.; Malek, M.; Puteh, A.; Ismail, M.; Ashrafuzzaman, M.; Naher, L. Effect of foliar application of chitosan on growth and yield in okra. Aust. J. Crop Sci. 2012, 6, 918–921. [Google Scholar]
- Amini, J. Induced resistance in potato plants against verticillium wilt invoked by chitosan and Acibenzolar-S-methyl. Aust. J. Crop Sci. 2015, 9, 570–576. [Google Scholar]
- Farouk, S.; Mosa, A.; Taha, A.; Ibrahim, H.M.; El-Gahmery, A. Protective effect of humic acid and chitosan on radish (Raphanus sativus, L. var. sativus) plants subjected to cadmium stress. J. Stress Physiol. Biochem. 2011, 7, 99–116. [Google Scholar]
- Saavedra, G.M.; Figueroa, N.E.; Poblete, L.A.; Cherian, S.; Figueroa, C.R. Effects of preharvest applications of methyl jasmonate and chitosan on post-harvest decay, quality and chemical attributes of Fragaria chiloensis fruit. Food Chem. 2016, 190, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Srisornkompon, P.; Pichyangkura, R.; Chadchawan, S. Chitosan Increased Phenolic Compound Contents in Tea (Camellia sinensis) Leaves by Pre-and Post-Treatments. J. Chitin Chitosan Sci. 2014, 2, 93–98. [Google Scholar] [CrossRef]
- González Gómez, H.; Ramírez Godina, F.; Ortega Ortiz, H.; Benavides Mendoza, A.; Robledo Torres, V.; Cabrera De la Fuente, M. Use of chitosan-PVA hydrogels with copper nanoparticles to improve the growth of grafted watermelon. Molecules 2017, 22, 1031. [Google Scholar]
- Abd-Alla, M.; Wafaa, M. New safe methods for controlling anthracnose disease of mango (Mangifera indica L.) fruits caused by Colletotrichum gloeosporioides (Penz.). J. Am. Sci. 2010, 8, 361–367. [Google Scholar]
- Abbasi, N.A.; Iqbal, Z.; Maqbool, M.; Hafiz, I.A. Post-harvest quality of mango (Mangifera indica L.) fruit as affected by chitosan coating. Pak. J. Bot 2009, 41, 343–357. [Google Scholar]
- Zhu, X.; Wang, Q.; Cao, J.; Jiang, W. Effects of chitosan coating on post-harvest quality of mango (Mangifera indica L. cv. Tainong) fruits. J. Food Process. Preserv. 2008, 32, 770–784. [Google Scholar] [CrossRef]
- Abdel Fattah, A.; Ashoush, I.; Alnashi, B. Effect of Chitosan Edible Coating on Quality Attributes of Pomegranate Arils During Cold Storage. J. Food Dairy Sci. Mansoura Univ. 2016, 7, 435–442. [Google Scholar]
- Ghasemnezhad, M.; Zareh, S.; Rassa, M.; Sajedi, R.H. Effect of chitosan coating on maintenance of aril quality, microbial population and PPO activity of pomegranate (Punica granatum L. cv. Tarom) at cold storage temperature. J. Sci. Food Agric. 2013, 93, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Petriccione, M.; De Sanctis, F.; Pasquariello, M.S.; Mastrobuoni, F.; Rega, P.; Scortichini, M.; Mencarelli, F. The effect of chitosan coating on the quality and nutraceutical traits of sweet cherry during post-harvest life. Food Bioprocess Technol. 2015, 8, 394–408. [Google Scholar] [CrossRef]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of chitosan coating on the post-harvest quality and antioxidant enzyme system response of strawberry fruit during cold storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Plainsirichai, M.; Leelaphatthanapanich, S.; Wongsachai, N. Effect of chitosan on the quality of rose apples (Syzygium agueum Alston) cv. Tabtim Chan stored at an ambient temperature. APCBEE Procedia 2014, 8, 317–322. [Google Scholar] [CrossRef]
- Ghasemnezhad, M.; Shiri, M. Effect of chitosan coatings on some quality indices of apricot (Prunus armeniaca L.) during cold storage. Casp. J. Environ. Sci. 2010, 8, 25–33. [Google Scholar]
- Suseno, N.; Savitri, E.; Sapei, L.; Padmawijaya, K.S. Improving shelf-life of cavendish banana using chitosan edible coating. Procedia Chem. 2014, 9, 113–120. [Google Scholar] [CrossRef]
- El Guilli, M.; Hamza, A.; Clément, C.; Ibriz, M.; Ait Barka, E. Effectiveness of post-harvest treatment with chitosan to control citrus green mold. Agriculture 2016, 6, 12. [Google Scholar] [CrossRef]
- Drevinskas, T.; Naujokaitytė, G.; Maruška, A.; Kaya, M.; Sargin, I.; Daubaras, R.; Česonienė, L. Effect of molecular weight of chitosan on the shelf life and other quality parameters of three different cultivars of Actinidia kolomikta (kiwifruit). Carbohydr. Polym. 2017, 173, 269–275. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Casariego, A.; Diaz, R.; Roblejo, L. Effect of edible chitosan/zeolite coating on tomatoes quality during refrigerated storage. Emirates J. Food Agric. 2014, 26, 238. [Google Scholar] [CrossRef]
- Wójcik, W.; Zlotek, U. Use of chitosan film coatings in the storage of carrots (Daucus carota). Prog. Chem. Appl. Chitin Deriv. 2008, 13, 141–148. [Google Scholar]
- Moreira, M.D.R.; Ponce, A.; Ansorena, R.; Roura, S.I. Effectiveness of edible coatings combined with mild heat shocks on microbial spoilage and sensory quality of fresh cut broccoli (Brassica oleracea L.). J. Food Sci. 2011, 76, M367–M374. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.L.; Cabral, M.F.; Germano, T.A.; de Carvalho, W.M.; Brasil, I.M.; Gallão, M.I.; Moura, C.F.H.; Lopes, M.M.A.; de Miranda, M.R.A. Chitosan coating with trans-cinnamaldehyde improves structural integrity and antioxidant metabolism of fresh-cut melon. Post-harvest Biol. Technol. 2016, 113, 29–39. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Yang, H. Post-harvest chitosan-g-salicylic acid application alleviates chilling injury and preserves cucumber fruit quality during cold storage. Food Chem. 2015, 174, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Hadwiger, L.; Kendra, D.; Fristensky, B.; Wagoner, W. Chitosan both activates genes in plants and inhibits RNA synthesis in fungi. In Chitin in Nature and Technology; Springer: Belin, Germany, 1986; pp. 209–214. [Google Scholar]
- Limpanavech, P.; Chaiyasuta, S.; Vongpromek, R.; Pichyangkura, R.; Khunwasi, C.; Chadchawan, S.; Lotrakul, P.; Bunjongrat, R.; Chaidee, A.; Bangyeekhun, T. Chitosan effects on floral production, gene expression, and anatomical changes in the Dendrobium orchid. Sci. Hortic. 2008, 116, 65–72. [Google Scholar] [CrossRef]
- Hadwiger, L.A. Multiple effects of chitosan on plant systems: Solid science or hype. Plant Sci. 2013, 208, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.; Van Strien, E. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Ramkissoon, A.; Francis, J.; Bowrin, V.; Ramjegathesh, R.; Ramsubhag, A.; Jayaraman, J. Bio-efficacy of a chitosan based elicitor on Alternaria solani and Xanthomonas vesicatoria infections in tomato under tropical conditions. Ann. Appl. Biol. 2016, 169, 274–283. [Google Scholar] [CrossRef]
- Boon-Ek, Y.; Jitareerat, P.; Wongs-Aree, C.; Buanong, M.; Obsuwan, K. Expression of Plant Defense Genes in Pepper Seedlings Treated with Chitosan Solution. Southeast Asia Symp. Qual. Manag. Post-harvest Syst. 2013, 1088, 461–464. [Google Scholar] [CrossRef]
- Landi, L.; Feliziani, E.; Romanazzi, G. Expression of defense genes in strawberry fruits treated with different resistance inducers. J. Agric. Food Chem. 2014, 62, 3047–3056. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Gao, H. Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system, and post-harvest fruit quality of strawberries (Fragaria x aranassa Duch.). LWT-Food Sci. Technol. 2013, 52, 71–79. [Google Scholar] [CrossRef]
- Zhang, D.; Quantick, P.C. Antifungal effects of chitosan coating on fresh strawberries and raspberries during storage. J. Hortic. Sci. Biotechnol. 1998, 73, 763–767. [Google Scholar] [CrossRef]
- Feliziani, E.; Smilanick, J.; Margosan, D.; Mansour, M.; Romanazzi, G.; Gu, S.; Gohil, H.; Ames, Z.R. Preharvest fungicide, potassium sorbate, or chitosan use on quality and storage decay of table grapes. Plant Dis. 2013, 97, 307–314. [Google Scholar] [CrossRef]
- Berg, J.A.; Appiano, M.; Bijsterbosch, G.; Visser, R.G.; Schouten, H.J.; Bai, Y. Functional characterization of cucumber (Cucumis sativus L.) Clade V MLO genes. BMC Plant Biol. 2017, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Xia, W.; Shan, C.; Zhou, T.; Cai, W.; Zhang, W. Quaternized chitosan oligomers as novel elicitors inducing protection against B. cinerea in Arabidopsis. Int. J. Biol. Macromol. 2015, 72, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Teniente, L.; Durán-Flores, F.D.D.; Chapa-Oliver, A.M.; Torres-Pacheco, I.; Cruz-Hernández, A.; González-Chavira, M.M.; Ocampo-Velázquez, R.V.; Guevara-González, R.G. Oxidative and molecular responses in Capsicum annuum L. after hydrogen peroxide, salicylic acid and chitosan foliar applications. Int. J. Mol. Sci. 2013, 14, 10178–10196. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, S.; Meng, C.; Jin, Q.; Fan, H.; Lin, Y.; Cai, Y. Cloning and expression analysis of transcription factor gene DoWRKY1 in Dendrobium officinale. China J. Chin. Mater. Med. 2015, 40, 2807–2813. [Google Scholar]
- Liu, Y.; Wisniewski, M.; Kennedy, J.F.; Jiang, Y.; Tang, J.; Liu, J. Chitosan and oligochitosan enhance ginger (Zingiber officinale Roscoe) resistance to rhizome rot caused by Fusarium oxysporum in storage. Carbohydr. Polym. 2016, 151, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Berumen-Varela, G.; Coronado-Partida, D.; Ochoa-Jiménez, V.; Chacón-López, A.; Gutiérrez-Martínez, P. Effect of chitosan on the induction of disease resistance against Colletotrichum sp. in mango (Mangifera indica L) cv. Tommy Atkins 2015, 66, 16–21. [Google Scholar]
- Lu, L.; Liu, Y.; Yang, J.; Azat, R.; Yu, T.; Zheng, X. Quaternary chitosan oligomers enhance resistance and biocontrol efficacy of Rhodosporidium paludigenum to green mold in satsuma orange. Carbohydr. Polym. 2014, 113, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Coqueiro, D.S.O.; de Souza, A.A.; Takita, M.A.; Rodrigues, C.M.; Kishi, L.T.; Machado, M.A. Transcriptional profile of sweet orange in response to chitosan and salicylic acid. BMC Genom. 2015, 16, 288. [Google Scholar] [CrossRef] [PubMed]
- Petriccione, M.; Mastrobuoni, F.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of chitosan treatment on strawberry allergen-related gene expression during ripening stages. J. Food Sci. Technol. 2017, 54, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, C.; Hoffmann, T.; Escobar, N.M.; Ludemann, F.; Botella, M.A.; Valpuesta, V.; Schwab, W. The strawberry fruit Fra a allergen functions in flavonoid biosynthesis. Mol. Plant 2010, 3, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Kamalipourazad, M.; Sharifi, M.; Maivan, H.Z.; Behmanesh, M.; Chashmi, N.A. Induction of aromatic amino acids and phenylpropanoid compounds in Scrophularia striata Boiss. cell culture in response to chitosan-induced oxidative stress. Plant Physiol. Biochem. 2016, 107, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, H.; Hu, Y.; Liu, Y. Chitosan controls post-harvest decay on cherry tomato fruit possibly via the mitogen-activated protein kinase signaling pathway. J. Agric. Food Chem. 2015, 63, 7399–7404. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.A.; Hubbard, J.C.; Liu, L.; Davis, R.M.; Subbarao, K.V. Effects of chitin and chitosan on the incidence and severity of Fusarium yellows of celery. Plant Dis. 1998, 82, 322–328. [Google Scholar] [CrossRef]
- Murphy, J.G.; Rafferty, S.M.; Cassells, A.C. Stimulation of wild strawberry (Fragaria vesca) arbuscular mycorrhizas by addition of shellfish waste to the growth substrate: Interaction between mycorrhization, substrate amendment and susceptibility to red core (Phytophthora fragariae). Appl. Soil Ecol. 2000, 15, 153–158. [Google Scholar] [CrossRef]
- Inc, N.T. Efficacit´e du Lysozyme dans le Contrˆolede la Croissance des Agents Pathog`enes des Plants de Serres; Neova Technologies Inc.: Abbotsford, BC, Canada, 2010. [Google Scholar]
- O’Herlihy, E.A.; Duffy, E.M.; Cassells, A.C. The effects of arbuscular mycorrhizal fungi and chitosan sprays on yield and late blight resistance in potato crops from microplants. Folia Geobot. 2003, 38, 201–207. [Google Scholar] [CrossRef]
- Ohta, K.; Atarashi, H.; Shimatani, Y.; Matsumoto, S.; Asao, T.; Hosoki, T. Effects of chitosan with or without nitrogen treatments on seedling growth in Eustoma grandiflorum (Raf.) Shinn. Cv. Kairyou Wakamurasaki. J. Jpn. Soc. Hortic. Sci. 2000, 69, 63–65. [Google Scholar] [CrossRef]
- Spiegel, Y.; Kafkafi, U.; Pressman, E. Evaluation of a protein-chitin derivative of crustacean shells as a slow-release nitrogen fertilizer on Chinese cabbage. J. Hortic. Sci. 1988, 63, 621–627. [Google Scholar] [CrossRef]
- Chen, Y.-E.; Yuan, S.; Liu, H.-M.; Chen, Z.-Y.; Zhang, Y.-H.; Zhang, H.-Y. A combination of chitosan and chemical fertilizers improves growth and disease resistance in Begonia× hiemalis Fotsch. Hortic. Environ. Biotechnol. 2016, 57, 1–10. [Google Scholar] [CrossRef]
- Manjula, K.; Podile, A. Chitin-supplemented formulations improve biocontrol and plant growth promoting efficiency of Bacillus subtilis AF 1. Can. J. Microbiol. 2001, 47, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Bakiyalakshmi, S.V.; Valli, V.; Swarnila, R.D.L. Isolation and Application of Chitin and Chitosan from crab shell. Int. J. Curr. Microbiol. Appl. Sci. 2016, 91–99. [Google Scholar]
- Silva, W.O.; Stamford, N.P.; Silva, E.V.; Santos, C.E.; Freitas, A.D.S.; Silva, M.V. The impact of biofertilizers with diazotrophic bacteria and fungi chitosan on melon characteristics and nutrient uptake as an alternative for conventional fertilizers. Sci. Hortic. 2016, 209, 236–240. [Google Scholar] [CrossRef]
- Salachna, P.; Wilas, J.; Zawadzińska, A. The effect of chitosan coating of bulbs on the growth and flowering of Ornithogalum saundersiae. In Proceedings of the XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014), Brisbane, Australia, 17–22 August 2014; Volume 1104, pp. 115–118. [Google Scholar]
- Salachna, P.; Grzeszczuk, M.; Soból, M. Effects of Chitooligosaccharide Coating Combined with Selected Ionic Polymers on the Stimulation of Ornithogalum saundersiae Growth. Molecules 2017, 22, 1903. [Google Scholar] [CrossRef] [PubMed]
- Trenkel, M.E. Controlled-Release and Stabilized Fertilizers in Agriculture; International Fertilizer Industry Association Paris: Paris, France, 1997; Volume 11. [Google Scholar]
- Savci, S. Investigation of effect of chemical fertilizers on environment. Apcbee Procedia 2012, 1, 287–292. [Google Scholar] [CrossRef]
- Tamme, T.; Reinik, M.; Roasto, M.; Juhkam, K.; Tenno, T.; Kiis, A. Nitrates and nitrites in vegetables and vegetable-based products and their intakes by the Estonian population. Food Addit. Contam. 2006, 23, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Zebarth, B.; Drury, C.; Tremblay, N.; Cambouris, A. Opportunities for improved fertilizer nitrogen management in production of arable crops in eastern Canada: A review. Can. J. Soil Sci. 2009, 89, 113–132. [Google Scholar] [CrossRef]
- Wu, L.; Liu, M. Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water-retention. Carbohydr. Polym. 2008, 72, 240–247. [Google Scholar] [CrossRef]
- Corradini, E.; De Moura, M.; Mattoso, L. A preliminary study of the incorparation of NPK fertilizer into chitosan nanoparticles. Express Polym. Lett. 2010, 4, 509–515. [Google Scholar] [CrossRef]
- Hussain, M.R.; Devi, R.R.; Maji, T.K. Controlled release of urea from chitosan microspheres prepared by emulsification and cross-linking method. Iranian Polym. J. 2012, 21, 473–479. [Google Scholar] [CrossRef]
- Hähndel, R.; Aktiengesellschaft, B.; Reply to the request on controlled-release fertilizers. Personal communication, 1997.
- Zhang, J.; Tan, W.; Zhang, Z.; Song, Y.; Li, Q.; Dong, F.; Guo, Z. Synthesis, characterization, and the antifungal activity of chitosan derivatives containing urea groups. Int. J. Biol. Macromol. 2018, 109, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
| Aquatic | Terrestrial | Microorganisms |
|---|---|---|
| Crustaceans Crab Chionoecetes opilio [25] Podophthalmus vigil [26] Paralithodes amtschaticus [27] Carcinus mediterraneus [28] | Arthropods Spiders Geolycosa vultuosa [29] Hogna radiate [29] Nephila edulis [30] | Fungi (cell walls) Ascomydes Mucom rouxii [31] Blastomycota Blastocladiaceae [32] Chytridiomycota Chytridiaceae [33] Protista Brown algae [34] Planta Green algae [34] |
| Scorpionxs Mesobuthus gibbosus [35] | ||
| Water lobster Crayfish [36] | ||
| Prawn Aristens antennatus [37] | Beetles Bombyx mori [38] Holotrichia parallela [39] Leptinotarsa decemlineata [38] Cockroaches [40] | |
| Krill Daphnia longispina [22] Anax imperator [41] Hydrophilus piceus [41] Notonecta glauca [41] Agabus bipustulatus [41] Asellus aquaticus [41] | ||
| Brachiopods Lingula seta [42] | ||
| Mollusca Squid pens Loligo sp [43] Todarodes pacificus [44] | ||
| Coelenterata |
| Crop | Concentration | Pathogen/Pest | Defense Mechanism | Mode of Application | References |
|---|---|---|---|---|---|
| Banana | 1.0% (w/v) | Anthracnose | Arresting fungal activity | In-vivo | [59] |
| Carrots | 2 or 4% (w/v) | Sclerotinia sclerotiorum | Antifungal activity | In Vitro | [60] |
| Cucumber | 0.2 g L−1 | Botrytis cinerea | Antifungal | Foliar spray | [61] |
| Cucumber | 2% (w/v) | Sphaerotheca fuliginea | Antifungal | Petri dish treatment | [62] |
| Chilli pepper | 0.32% (w/v) | Colletotrichum capsici | Hijacked fungal activity | In vivo | [63] |
| Eggplant | 20 mL | Ralstonia solanacearum | Reduce fungal caused wilt | Cotton leaf disk elicitation method | [64] |
| Mango | 1% (w/v) | Colletotrichum gloeosporioides | Fungus inhibition | Post-harvest coating | [65] |
| Orange | 2% (w/v) | Penicillium italicum and Penicillium digitatum | Fungicidal effect | Post-harvest coating | [66] |
| Pear | 25 g/L | A. kikuchiana and P. piricola | Antifungal activity | Post-harvest treatment | [67] |
| Papaya | 1.5% (w/v) | C. gloeosporioides | Fungicidal effect | In situ | [68] |
| Palm | 1 mg mL−1 | Fusarium oxysporum | Inhibition of root fungal activity | Soil inoculation | [69] |
| Peach | 0.5 g L−1 | Monilinia fructicola | Antioxidant and antifungal | Dipping in solution | [70] |
| Tomato | 1 mg/mL | Alternaria solani | Antibacterial defense | Foliar application | [71] |
| Tomato | 0.1% (w/v) | Fusarium oxysporum f. sp. Lycopersici | Antifungal | Foliar application | [72] |
| Tomato | 0.1 mg mL−1 | Pochonia chlamydosporia | Nematocidal effect | Fertigation | [73] |
| Tomato | 10 mg L−1 | R. solanacearum | Antibacterial | Seed treatment | [74] |
| Crop | Functions | Reference |
|---|---|---|
| Artichoke | Improved seed germination and plant growth | [122] |
| Chili | Leaf area, canopy diameter and plant height | [123] |
| Cucumber | Triggered vegetative growth and quality of cucumber fruits | [124] |
| Coffee | Plant height and leaf area | [125] |
| Chinese cabbage | Uniform seed germination and enhanced seedling growth | [126] |
| Dendrobium formosum orchid | Enhanced seed germination | [127] |
| Eggplant | Improved antioxidant activity and total phenolic content | [64] |
| Grapevine | Increased number of internode and improved rooting | [97] |
| Okra | Plant height, leaf number and fruit yield | [128] |
| Potato | Increased the fresh weight of tuber and overall yield | [129] |
| Peach | Induced antioxidant activity and defense-related enzymes | [70] |
| Radish | Enhanced the nutrient uptake efficiency and mimic cadmium stress | [130] |
| Strawberry | Produced fruits with an increased shelf life | [131] |
| Tomato | Improved fruit and productivity | [71,72] |
| Tea | Enhanced the phenolic content up to 9% | [132] |
| Watermelon | Increased in weight of fresh and dry seedlings, Stimulated the growth of the primary stems, the root system, and an increase in stomatal width. | [19,133] |
| Crop | Genes | Expression | Functions | Reference |
|---|---|---|---|---|
| Arabidopsis | PAD3 | ↑ | Induced resistance against Botrytis cinerea | [162] |
| Capsicum annuum | cat1, pal, pr1 | ↑ | Maintain plant fitness and keep the defense system alert for the upcoming stress. | [163] |
| Dendrobium officinale | DoWRKY1 | ↑ | Proper regulation of plant metabolic and physiological properties. | [164] |
| Ginger | GLU, PAL | ↑ | Triggered tolerance against rhizome rot caused by Fusarium oxysporum | [165] |
| Mango | POD | ↑ | Altered the severity of anthracnose disease by inducing enzymatic activity. | [166] |
| Peach | POD, GLU | ↑ | Enhanced resistance against brown rot | [70] |
| Satsuma Orange | CHI, PAL | ↑ | Inhibiting the fungal decay process in orange fruit caused Penicillium digitatum. | [167] |
| Sweet orange | Cellulose synthase | ↓ | Reduce cell wall growth and increase resistance against biotic stress. | [168] |
| Strawberry | Fra a1, Fra a3, Fra a4 | ↑ | Fruit development, flavonoid biosynthesis and fruit ripening | [169,170] |
| Scrophularia striata Boiss | PAL | ↑ | Improved antioxidant activities and increased the production of phenylpropanoid | [171] |
| Tomato | MPK3, MPK6, PR1a1 | ↑ | Increase resistance against grey mold caused decay via the activation of MAPK signaling pathway. | [172] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharif, R.; Mujtaba, M.; Ur Rahman, M.; Shalmani, A.; Ahmad, H.; Anwar, T.; Tianchan, D.; Wang, X. The Multifunctional Role of Chitosan in Horticultural Crops; A Review. Molecules 2018, 23, 872. https://doi.org/10.3390/molecules23040872
Sharif R, Mujtaba M, Ur Rahman M, Shalmani A, Ahmad H, Anwar T, Tianchan D, Wang X. The Multifunctional Role of Chitosan in Horticultural Crops; A Review. Molecules. 2018; 23(4):872. https://doi.org/10.3390/molecules23040872
Chicago/Turabian StyleSharif, Rahat, Muhammad Mujtaba, Mati Ur Rahman, Abdullah Shalmani, Husain Ahmad, Toheed Anwar, Deng Tianchan, and Xiping Wang. 2018. "The Multifunctional Role of Chitosan in Horticultural Crops; A Review" Molecules 23, no. 4: 872. https://doi.org/10.3390/molecules23040872