1. Introduction
Alzheimer diseases (AD) is the most common kind of neurodegeneration disease in the elderly, characterized by deterioration of cognitive functions, extracellular Aβ deposits and intracellular neurofibrillary tangles, other pathology features include the cholinergic neurons and synaptic degeneration/loss, cerebral amyloid angiopathy (CAA), neuroinflammation, and oxidative damage [
1,
2].
AD is so chronic and complex disease. To date, there is no current disease-modifying therapy available for the treatment of this disorder. In spite of extensive academic, pharmaceutical, and medicinal research, numerous drug candidates targeting Aβ or β-secretase have failed in clinical trials. This means drugs with single targets have less therapeutic effects or cause side effects when used to prevent or treat AD. Therefore, the new strategy towards development of safe agents with multiple targets, natural polyphenols are likely to comply with these requirements due to the advantages of multi-target effects and fewer side effects.
Flavonoids are the largest group of polyphenols available from dietary fruits and vegetables and have been found to play a neuroprotective role by inhibiting or modifying the self-assembly of the amyloid-β (Aβ) peptide into oligomers and fibrils, which are linked to the pathogenesis of Alzheimer’s disease [
3]. Scutellarin is a flavonoid found in the traditional Chinese herbal medicine Erigeron breviscapus (vant.), and has been widely used in clinical to treat cardiovascular diseases and cerebrovascular injury [
4]). Recently, scutellarin has been proven to: promote neuroprotective effects by inhibition of microglia inflammatory activation [
5], attenuate the neurotoxicity of Aβ and protects against Aβ-induced learning and memory deficits in rats [
6], exert potential neuroprotective effects on AD. However, up until now the long term multiple antiamyloidogenic and fibril-destabilizing effects have not been studied. In this research, we report that scutellarin efficiently inhibits oligomerization and fibril formation through binding to Aβ42. In the meantime, scutellarin mitigates amyloid pathology and related cognitive deficits after nine months of treatment in APP/PS1 transgenic mice. This research reveals scutellarin is a potential multi-targeting agent against AD.
2. Materials and Methods
2.1. Aβ Disaggregation Assay
2.1.1. Aβ Preparation
Synthetic Aβ42 peptide corresponding to the human sequence was purchased from Tocris Bioscience, which was dissolved in HFIP at a concentration of 1 mg/mL and was separated into aliquots in sterile Eppendorf Tube (100 μg/tube), followed by incubation at room temperature for 24 h in the fume hood to form clear peptide film, the resulting peptide films were dried under vacuum overnight and stored at −20 °C before assaying [
7].
2.1.2. Thioflavin T Fluorescence Assay
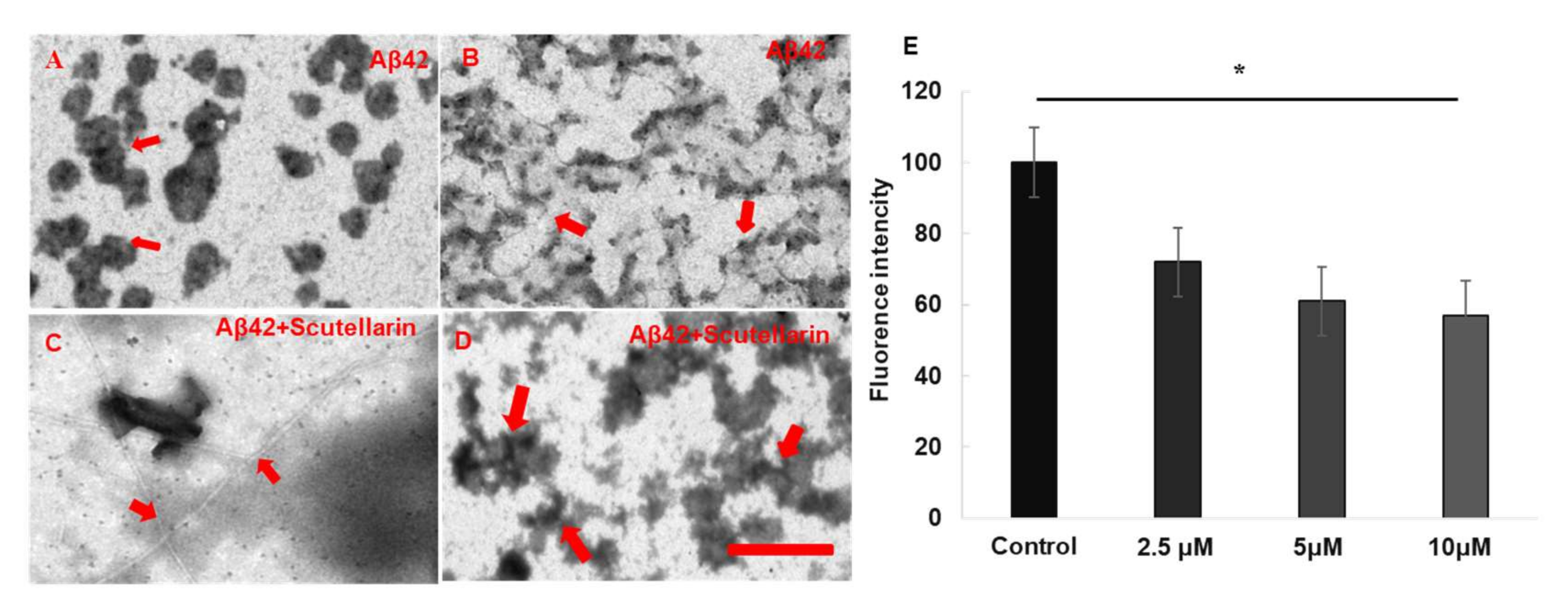
Thioflavin T (Th. T) dye fluorescence is used regularly to quantify the formation and inhibition of amyloid fibrils in the presence of anti-amyloidogenic compounds such as polyphenols [
8]. To investigate the disaggregation effect of scutellarin on preformed Aβ fibril, 100 μg Aβ42 oligomer was pre-incubated in DMEM at 37 °C for 10 days to form Aβ fibril. Following incubation, Aβ alone, or in the presence of the concentration gradient of scutellarin (purity > 98.5%, Yunnan Plant Pharmaceutical, Yunnan, China,
Figure 1) for an additional three days at 37 °C, 5 μM Thioflavin T solution was added to the samples. Emission spectra were recorded at 482 nm upon excitation at 450 nm (SpectraMax M2, Molecular Devices). Measurement was repeated three times.
2.1.3. Electron Microscopy (EM) Assay
In brief, copper grids were preplaced on the bottom of wells in a 24-well plate where 100 μg Aβ42 monomer were incubated with or without scutellarin at a concentration gradient for seven days at 37 °C. Following, a drop of the solution was placed on a 150 mesh Formvar coated grid for 30 s. After drying, the grid was stained with 2% (wt/vol) aqueous phosphotungstic acid for 20 min. The observations were performed on a Hitachi7650 TEM equipped with MegaView 3 Digital Camera (Hitachi, Tokyo, Japan).
2.2. SH-SY5Y Cell Culture and Viability Assay
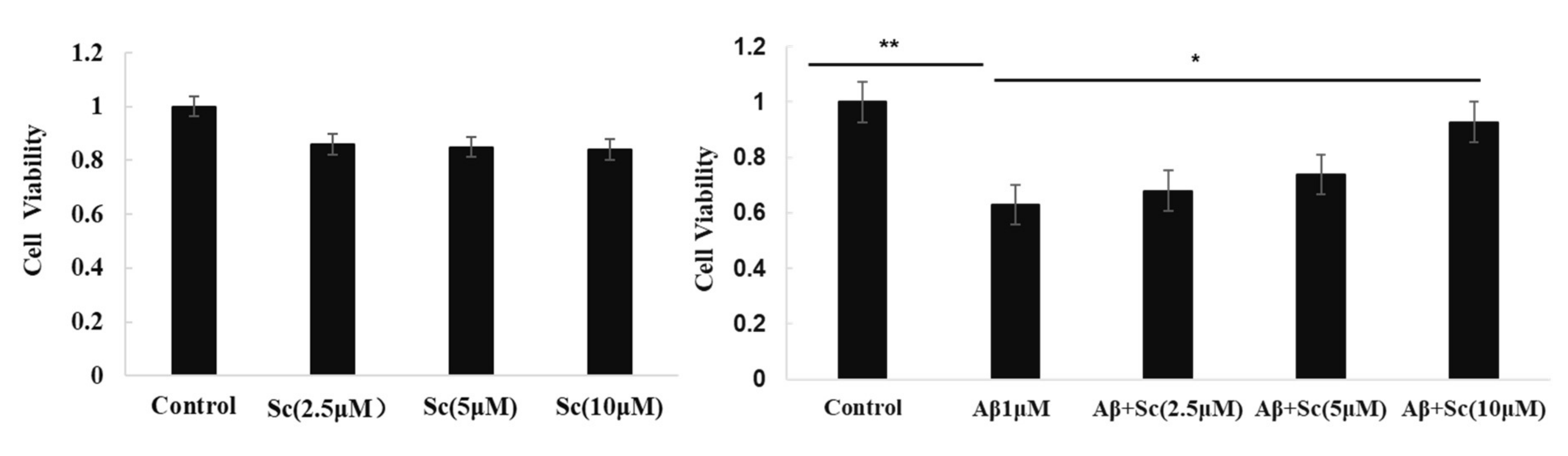
The SH-SY5Y cell line was obtained from BeNa Culture Collection (Shanghai, China). Cells were plated in 96-well plates containing complete medium and cultured for 24 h at 37 °C and then, the cells were treated with 1 μM Aβ42, Scutellarin (2.5, 5, or 10 μM) or 1 μM Aβ42 with Scutellarin (2.5, 5, or 10 μM) for another 24 h, followed by incubation with MTT (0.5 mg/mL) for 4 h and 10% SDS solution for another 15 min at 37 °C. The absorbance was measured at 560 nm with a microplate reader (Spectra Max M2, Molecular Devices, San Jose, CA, USA).
2.3. Animals
APP/PS1 transgenic mice and age-matching non-transgenic (WT) mice were purchased from Nanjing Biomedical Research Institute of Nanjing University and were bred in the Kunming Medical University animal house. Five or six mice were housed in each cage with free access to standard food and water and were maintained under standard laboratory conditions. The APP/PS1 transgenic mice were constructed on a C57BL/6 background and bear a chimeric mouse/human (Mo/Hu) APP695 with mutations linked to familial AD (KM 593/594 NL) and human PS1 carrying the exon-9-deleted variant associated with familial AD (PS1dE9) in one locus under control of a brain- and neuron-specific murine Thy-1 promoter element [
9]. Genotypes of the offspring were determined by PCR analysis of tail DNA.
2.4. Diet Treatment
A total of 24 transgenic mice and 12 wild type littermates (C57BL/6 mice, half male and half female) were used: the transgenic mice were randomly divided into Scutellarin Tg group (N = 12, half males and half females), control Tg group (N = 12, half males and half females) and wild-type mice group (N = 12, half males and half females). The scutellarin (Tg) group were treated with scutellarin mixed food (50 mg/kg) [
10]. Control (Tg) and wild-type mice group were fed with standard commercial food (Beijing KeaoXieli Feed Company, Beijing, China). Beginning at three months of age, the mice consumed both diets ad libitum for nine months. This study was carried out in strict compliance with the Guidelines for the Animal Care and Use of China. The protocols were approved by the Animal Ethics Committee of Kunming Medical University.
2.5. Behavioral Procedures
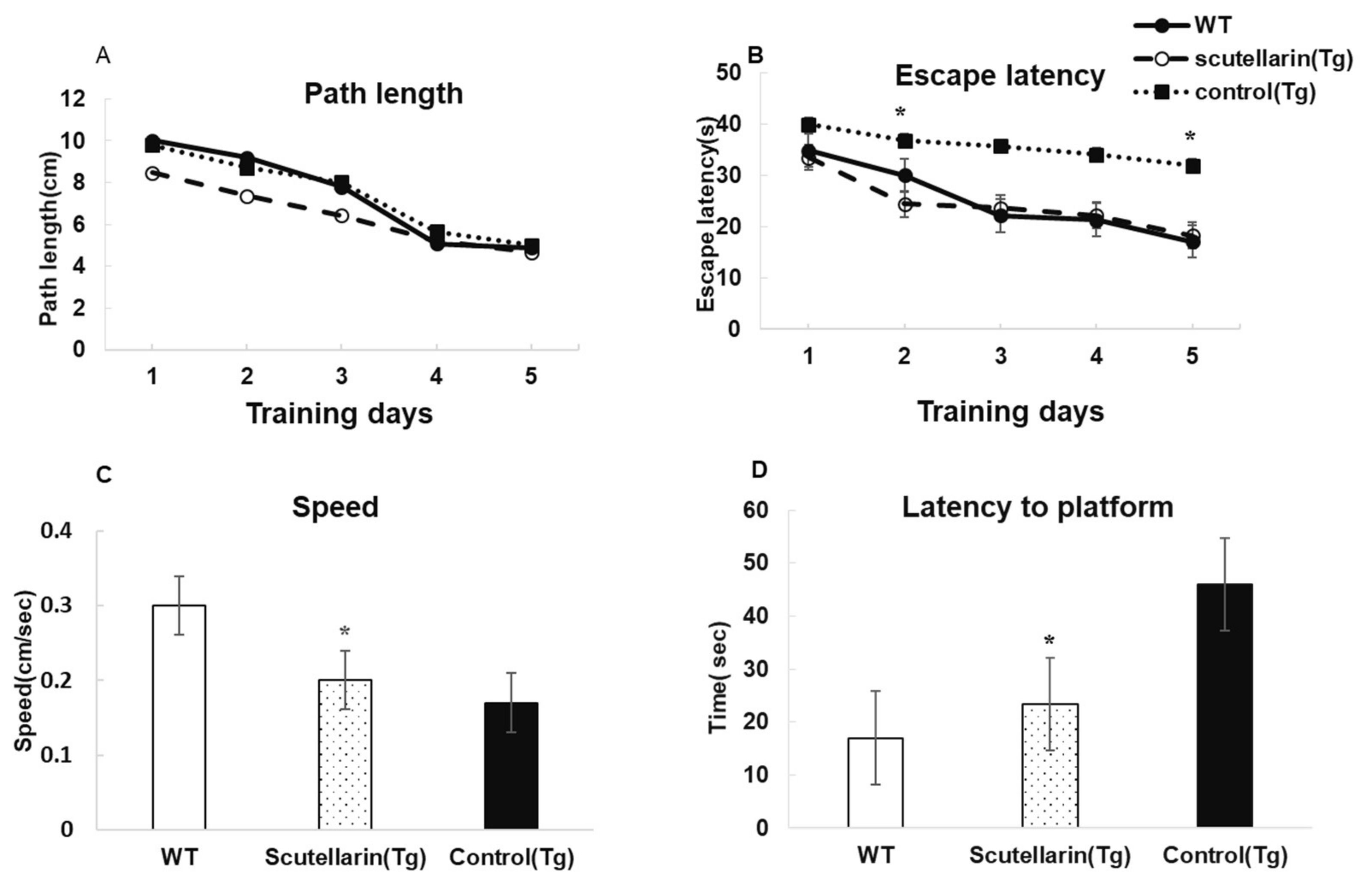
The effect of scutellarin treatment on spatial learning and memory was assessed by Morris water maze (MWM) testing at the age of 12 months, a circular plastic pool (height 40 cm, diameter 120 cm) was filled with water (plus white dye) maintained at 22 ± 1 °C. An escape platform (11 cm in diameter) 1 cm below the water surface was used. The acquisition task consisted of six consecutive days of testing with four trials per day. In each trial, the animal was put into the water at one of four starting positions of non-platform quadrants respectively, and was given 60 s to find the hidden platform. If a mouse failed to find the platform within 60 s, the training was terminated, a maximum score of 60 s was assigned, and the mouse was manually guided to the hidden platform. The escape latency, path length and swim speed were recorded semi-automatically by a video tracking system (Stoelting Co., Wood Dale, IL, USA) and analyzed by image analyzing software. On the sixth day, a probe trial was performed, and mice were placed and released opposite the site where the platform had been located, the time spent in each quadrant and target annulus crossings were recorded [
11].
2.6. AD Type Pathology and Bioanalysis
2.6.1. Tissue Processing
The mice were executed after behavioral test, the mice were deeply anesthetized, the blood was collected from the retro orbital sinus and was centrifuged immediately at 1300× g for 8 min, the supernatant from these samples was collected for future biochemical detection.
The brain was perfused intracardially with chilled 0.1 M phosphate buffered saline (PBS) through the heart using a syringe and was quickly removed and placed on an ice-cold glass dish and bisected sagittally. The left-hemisphere were routinely fixed in 4% paraformaldehyde (pH 7.4) for 24 h and incubated in 30% sucrose at 4 °C for 36 h, the coronal sections were cut on a frozen microtome in 35 μm thick sections for staining. The right hemisphere was rapidly frozen in liquid nitrogen and kept at −80 °C until analysis.
2.6.2. Histological Staining and Imagine Analysis
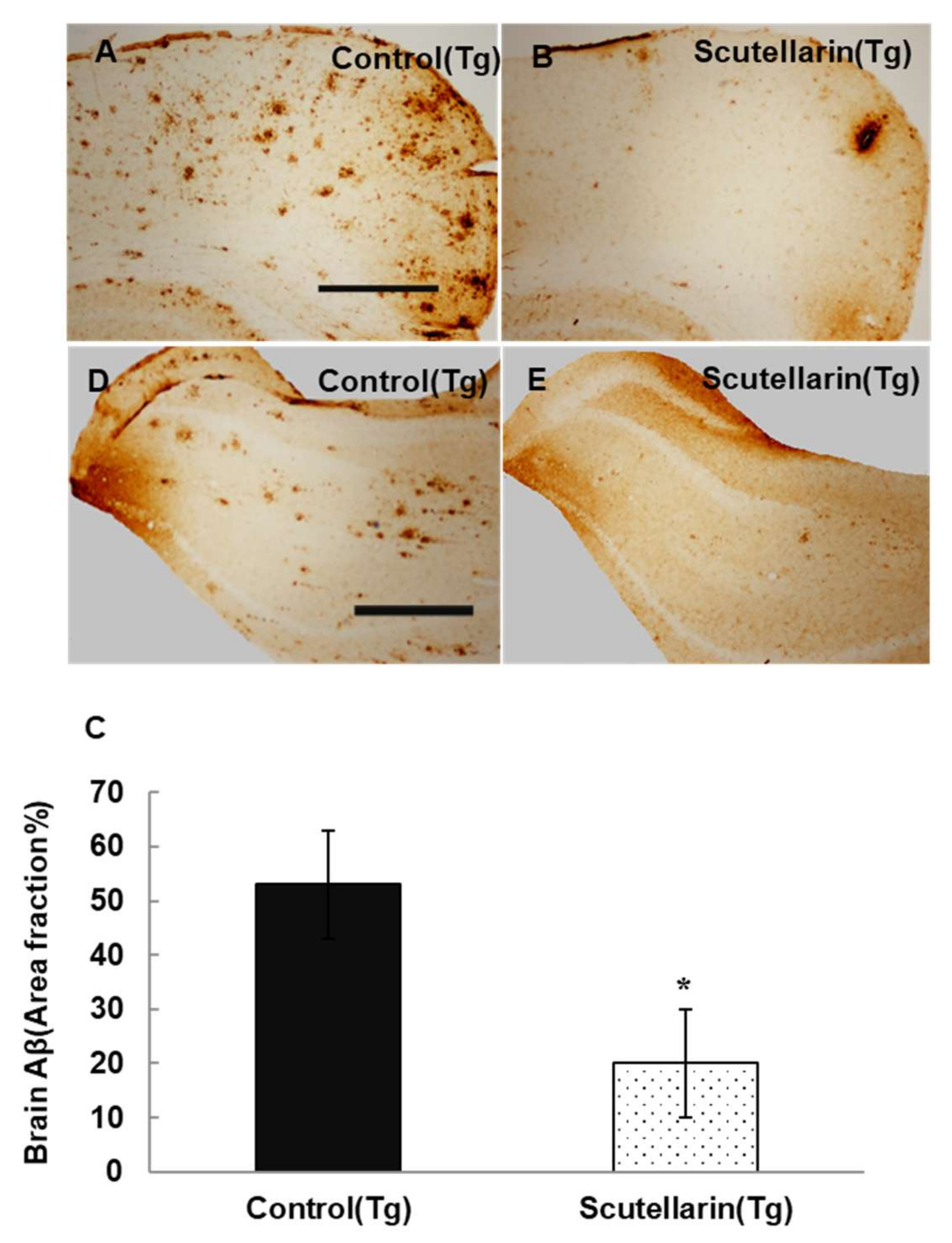
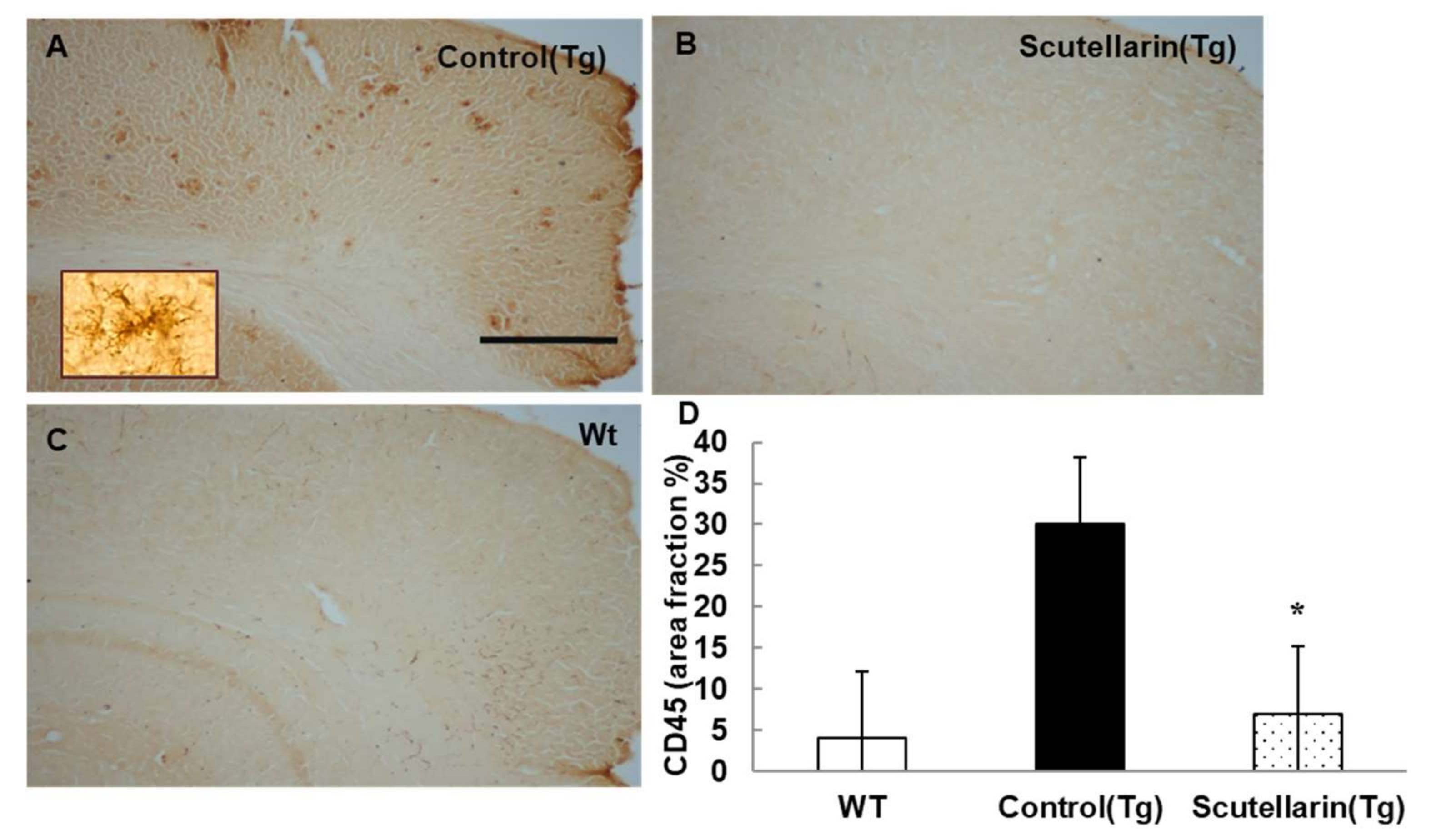
The basic immunohistochemical staining was carried out following IHC kit instruction (Slide Kit Chemical international, Inc., Millipore, Burlington, MA, USA). Five sections each 210 μm apart starting from septal hippocampus were randomly selected and stained with Aβ protein deposits (Biotin-conjugated mouse anti-Aβ antibody (6E10, Serotec, Oxfordshire, UK), activated microglia (rat monoclonal anti-CD45 Chemicon, Temecula, CA, USA), and astrocyte (rabbit poly-clonal anti-glial fibrillary acidic protein, Dako, Glostrup, Denmark)). Pretreatments were: incubation in a 3% H2O2 solution in distilled water for 20 min to block endogenous peroxidase; incubation in 70% formic acid for 15 min for antigen retrieval, sections were incubated with the primary antibodies overnight at +4 °C, followed by incubation with biotinylated secondary antibodies and visualization using the diaminobenzidine as chromogen. The reaction was stopped with phosphate buffer and sections were then mounted, cleared in xylene, and cover slipped with neutralized resin.
The region of neocortex and hippocampus manually was selected for analysis Aβ, microgliosis and astrocytosis burden. Stained specimens were analyzed with Olympus BX-51 microscope equipped with an Olympus DP-73 camera (Olympus, Tokyo, Japan). Images were collected at 4× magnification. Measurements were performed for a percentage of the area covered by the DAB staining.
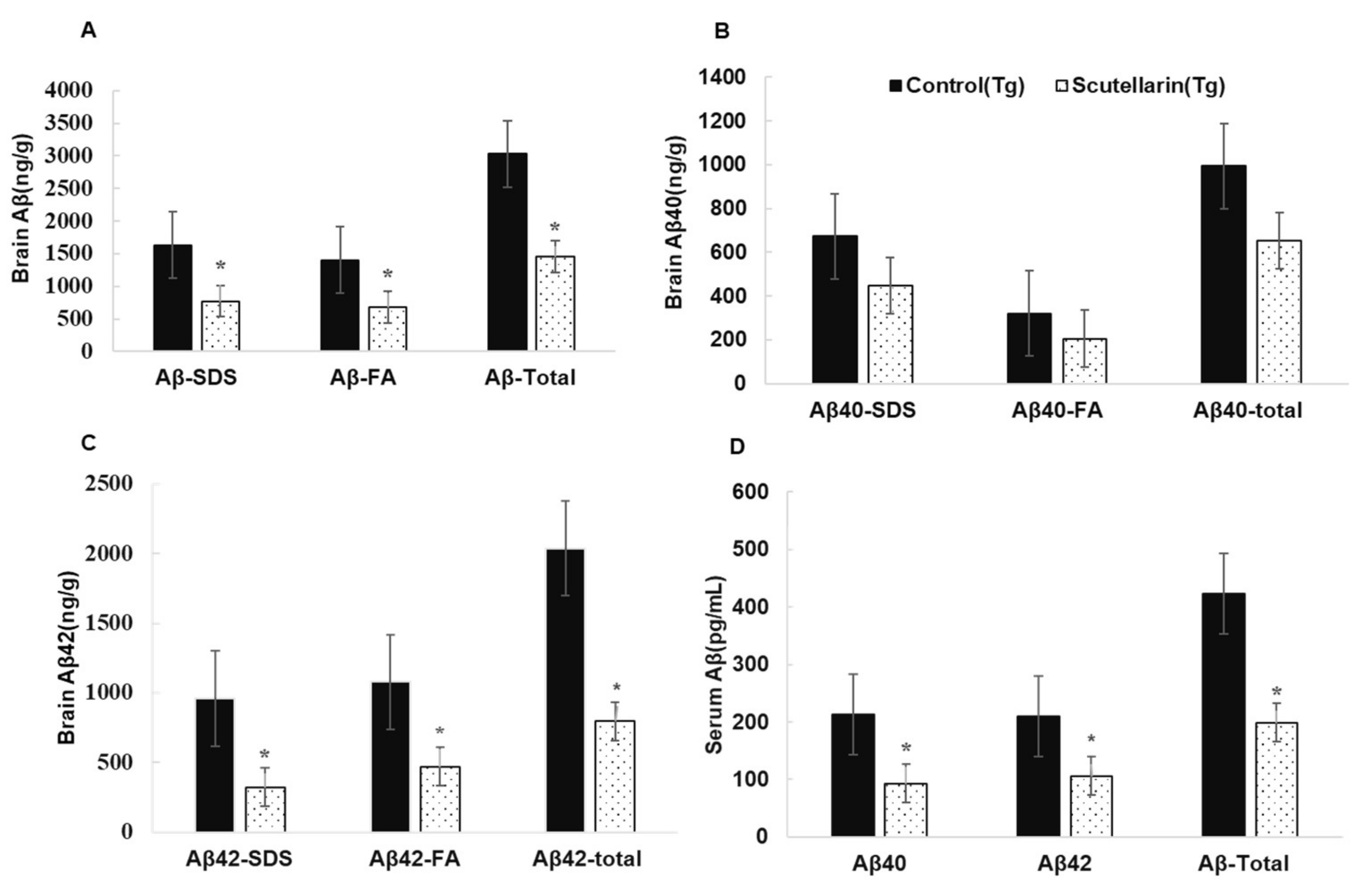
2.6.3. ELISA Assay for Soluble and Insoluble Aβ
The levels of soluble and insoluble Aβ in the brain of APP/PS1 mice were quantified according to the procedures previously described [
12]. Frozen brain was homogenized and sonicated in water containing 2% sodium dodecyl sulphate (SDS) and protease inhibitors (Roche, Basel, Switzerland). Homogenates were centrifuged at 100,000×
g for 1 h at 4 °C, the resultant supernatant was collected, representing the TBS-soluble fraction (Aβ-TBS). The resultant pellet was suspended and sonicated in water containing 2% sodium dodecyl sulfate (SDS) and centrifuged at 100,000×
g for 1 h at 4 °C, the resultant supernatant was collected, representing the SDS-soluble fraction (Aβ-SDS). The resultant pellet was extracted with 70% formic acid (FA) and the supernatant was collected, representing the SDS-insoluble fraction (Aβ-FA). Before ELISA assay, formic acid extracts were neutralized by 1:20 dilution into 1 M Trisphosphate buffer, pH 11, and then diluted in sample buffer. Then, ELISA kits were used to determine Aβ40 and Aβ42 levels in the brain and serum samples (Millipore, Burlington, MA, USA). The concentration of Aβ was expressed as picograms per milliliter (pg/mL). The concentration values of the samples fell within the linear section of the standard curve.
2.6.4. Quantification of Inflammatory Cytokines in the Mouse Plasma by ELISA
Plasma cytokine levels were measured using commercial assay kits according to the manufacturer’s directions (Thermo Scientific, Waltham, MA, USA). The concentration of inflammatory cytokines was presented as pg/mL.
2.7. Statistical Analysis
Results for experimental groups are presented as the mean ± SEM. In cases of equal variance, statistical differences were determined using one-way analysis of variance (ANOVA) followed by post-hoc (Tukey’s) tests for comparisons between groups. If homogeneity of variances was rejected, the ANOVA followed with the Dunnett’s test. The threshold for statistical significance was set to p < 0.05. All statistical analyses were performed using the SPSS 20.0 software (IBM, Chicago, IL, USA)
4. Discussion
Aβ is believed to play a critical role in the AD pathology process. Aβ peptides subsequently aggregate from monomers to oligomers, protofibrils, and fibrils, inducing cognitive deficits and a series of the deleterious cascades that exacerbate neuronal injury [
14], which consequently means they have been considered as a primary toxic species in AD [
15]. Therefore, developing multi-target drugs to antagonize the aggregation of various amyloid proteins and interfere with the pathological process becomes an attractive therapeutic strategy.
Here, we used a series of techniques to investigate anti-amyloidogenic effects of scutellarin on AD pathology. In vitro, we determined the effects of scutellarin on the destabilization of Aβ fibrils by thioflavin T fluorescence spectroscopy and electron microscopy. Our results present that scutellarin dependently destabilized preformed Aβ; altered the Aβ aggregation pathway to yield non-toxic, unstructured Aβ aggregates; and displayed anti-aggregation effects on Aβ. In the cell culture experiment, cell growth was remarkably inhibited by Aβ oligomers treatment. However, scutellarin reduced the Aβ-induced cytotoxicity and exerted a neuroprotective effect in the SH-SY5Y cell culture model.
Through an in vivo study, the neuroprotective effects of a scutellarin diet have been tested in APP/PS1 transgenic mouse model. Our data showed that scutellarin significantly improved behavioral impairment, decreased Aβ levels in the brain and the serum, exhibited strong cleaning capacity in the Tg mice brain and the periphery, and did not cause side effects.
Brain inflammation is one of the hallmarks of AD and originates in the central nervous system. Microglia and astrocytes are arguably the major sources of cytokines in AD. Aβ complexes interact with microglial and astrocytic expressed pattern recognition receptors that initiate innate immunity [
16]. This process leads to the production of toxic and inflammatory mediators such as hydrogen peroxide, nitric oxide, and cytokines—including interleukin (IL)-1β, IL-6, TNF-a, and IFN-γ etc.—that can recruit further microglia and astrocytes to the inflammatory site [
17]. The passage of cytokines through the blood–brain barrier allows for the ability to peripherally measure them. In the present study, we found scutellarin reduced neuroinflammation including Aβ plaque-associated microgliosis and activated astrocytes, cytokines levels of TNF-α and IL-6, and exerted anti-inflammatory properties through downregulation of pro-inflammatory cytokine expression, thereby contributing to improved cognition and decreased Aβ concentrations.
Obviously, these beneficial effects can be attributed to its structure of phenol, which enables it to penetrate the blood–brain barrier and helps to re-establish the redox regulation of proteins, transcription factors, and signaling cascade; successfully protects neuronal cells from oxidative damage; and potentially alleviates neuroinflammation of AD [
18,
19]. In addition, the interaction of scutellarin with Aβ induces conformational and structural changes in Aβ oligomers, disrupts hydrophobic and π–π interactions of Aβ peptides and prevents Aβ aggregation and toxicity. In turn, the Aβ oligomer–scutellarin interaction results in delayed fibril formation, reduces amyloid plaque load, and attenuates behavior deficits in APP/PS1 mice. A published paper has discussed the molecular mechanism of scutellarin prevention of AD pathology [
20]. Yuan et al. suggest that scutellarin regulates the activation of microglia via the Notch pathway and concurrently induces morphological and functional changes in activated microglia [
21]. Xu et al. propose that scutellarin can effectively upregulate the synthesis and release of NGF, glial cell line-derived neurotrophic factor (GDNF), and brain-derived neurotrophic factor (BDNF) [
22]. Chai et al. put forward that scutellarin can induce the expression of neurotrophin messenger RNAs and proteins through cyclic adenosine monophosphate response, element-binding protein (P-CREB), and p-Akt signaling and inhibit NO production in early stages of neuronal damage [
23]. These conclusions indicate that scutellarin can slow down the progression of AD-like neuropathology via anti-inflammation, anti-oxidation, anti-amyloidogenic properties, consistent with ferulic acid and fisetin which have shown effects on improving behavioral impairment and Alzheimer-like pathology in transgenic mice [
24,
25]. Although scutellarin exerts multiple beneficial functions in mouse models of AD, the key question remains unanswered: can scutellarin’s beneficial actions on animal models be translated to the human condition? In addition, due to its poor solubility and weak oral absorption, the clinical use of scutellarin is limited [
26]. In further research, we need to identify bioactive metabolites and dissect their targets in AD modification.