Light Emission from the Fe2+-EGTA-H2O2 System: Possible Application for the Determination of Antioxidant Activity of Plant Phenolics
Abstract
:1. Introduction
2. Results
2.1. Ultra Weak Photon Emission from Fe2+-EGTA-H2O2 System
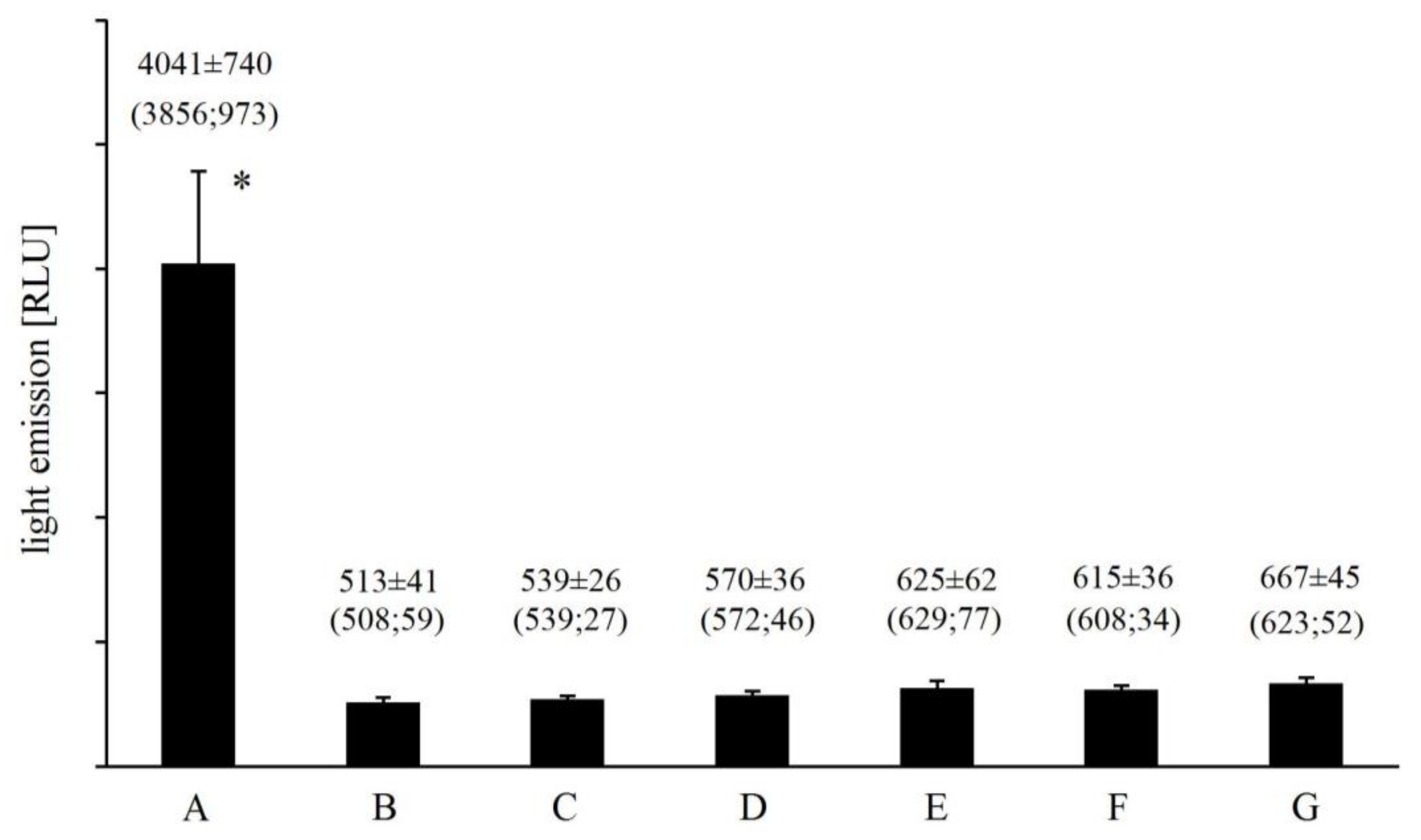
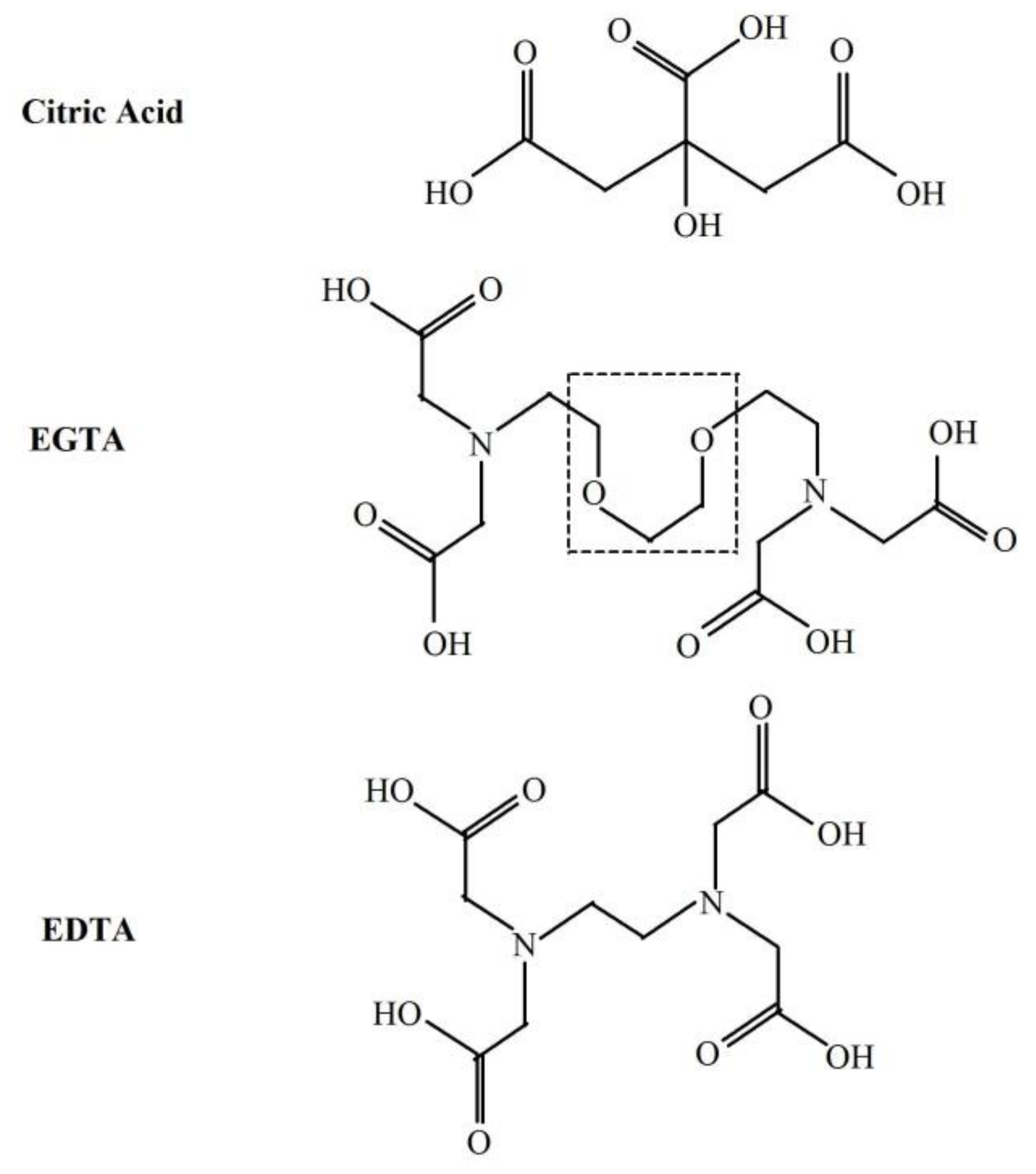
2.2. Effect of Iron and EGTA Replacement by other Divalent Cations and Metal Chelators on Light Emission from Fe2+-EGTA-H2O2 System
2.3. Effect of Reactive Oxygen Species Scavengers on Light Emission from Fe2+-EGTA-H2O2 System
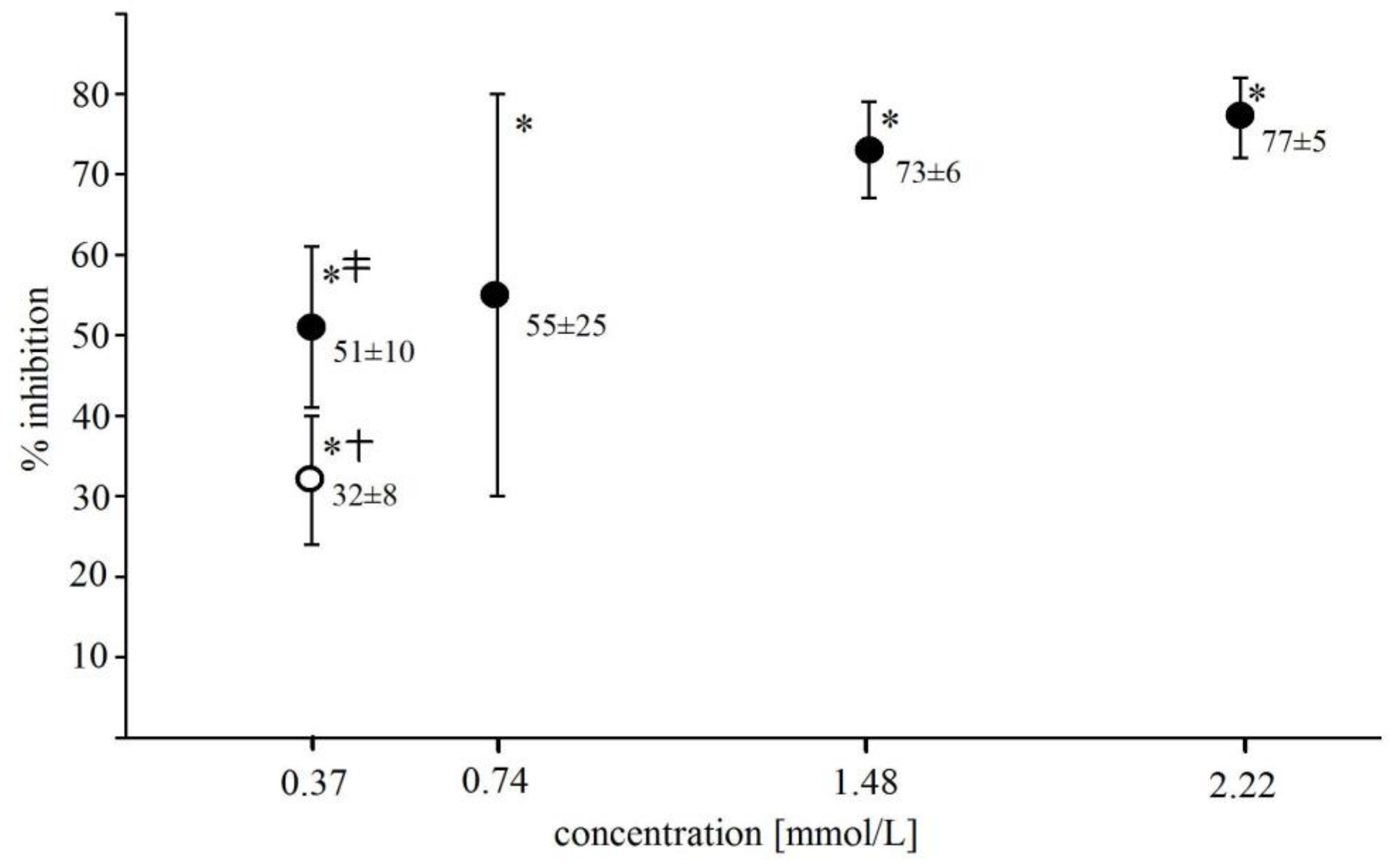
2.4. Effect of Selected Phenolics and Ascorbic Acid on Light Emission from Fe2+-EGTA-H2O2 System
3. Discussion
3.1. Light Emission from Fe2+-EGTA-H2O2 System
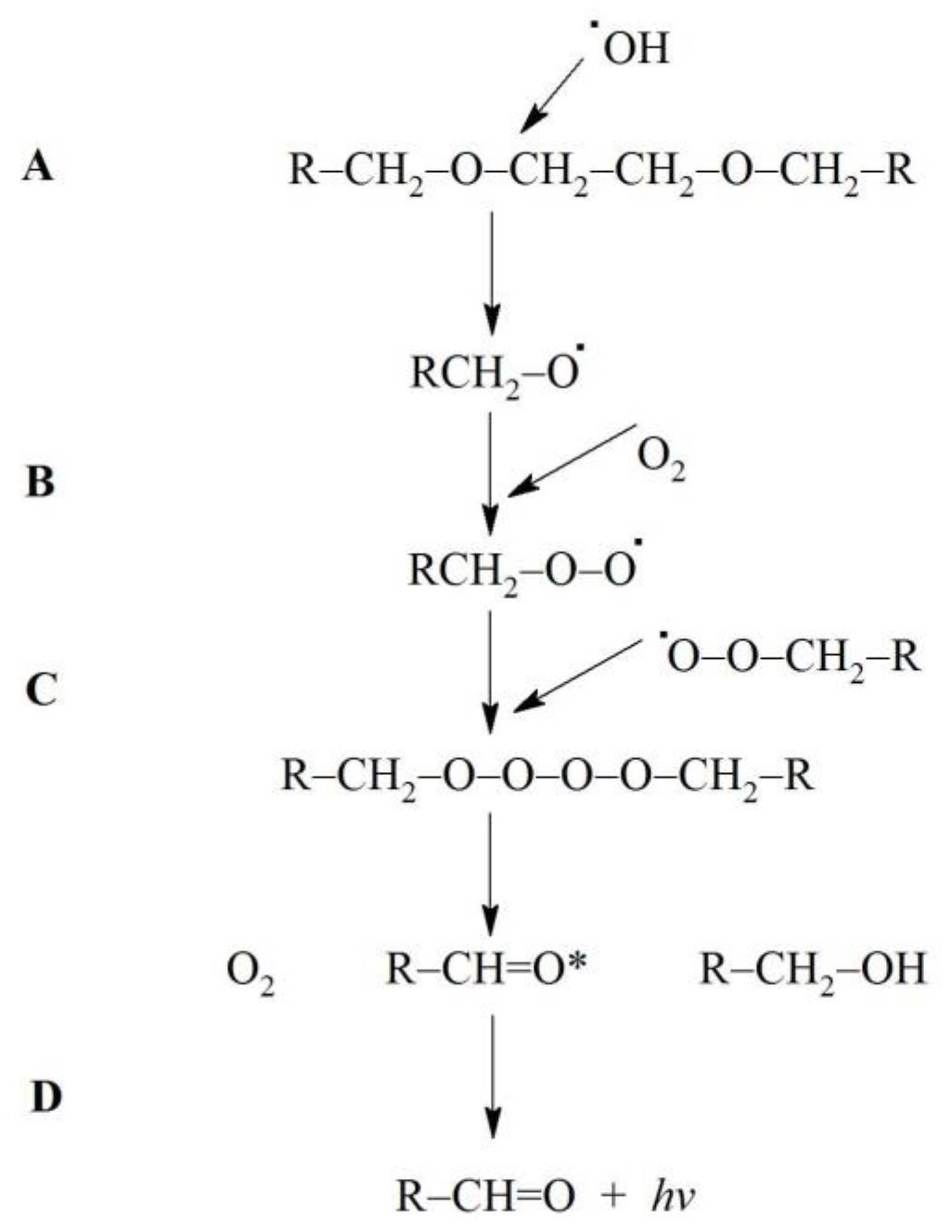
3.2. Plausible Mechanism of Light Generation from Fe2+-EGTA-H2O2 System
3.3. Inhibitory Effect of Phenolic Acids on Light Emission from Fe2+-EGTA-H2O2 System
3.4. Strengths and Weaknesses of the Study
4. Materials and Methods
4.1. Reagents
4.2. Light Emission from Fe2+-EGTA-H2O2 System
4.3. Effect of Iron and EGTA Replacement by other Divalent Cations and Metal Chelators on Light Emission from Fe2+-EGTA-H2O2 System
4.4. Determining the Effect of Reactive Oxygen Species Scavengers and Selected Phenolic Acids on Light Emission from Fe2+-EGTA-H2O2 System
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| UPE | ultra-weak photon emission |
| •OH | hydroxyl radical |
| ROS | reactive oxygen species |
| EDTA | ethylenediaminetetraacetic acid, disodium salt |
| EGTA | ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid |
| RLU | relative light units |
| SOD | superoxide dismutase |
| DMSO | dimethyl sulfoxide |
| O2− | superoxide radical |
| O2(1Δg) | singlet oxygen |
| 3(R=C)* | triplet excited carbonyl groups |
Appendix A. Chemical Compounds Studied in This Article
References
- Cifra, M.; Pospíšil, P. Ultra-weak photon emission from biological samples: Definition, mechanisms, properties, detection and applications. J. Photochem. Photobiol. B 2014, 139, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, E.; Varsavsky, A.I.; Boveris, A.; Chance, B. Oxygen- or organic hydroperoxide-induced chemiluminescence of brain and liver homogenates. Biochem. J. 1981, 198, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Pospíšil, P. Linoleic acid-induced ultra-weak photon emission from Chlamydomonas reinhardtii as a tool for monitoring of lipid peroxidation in the cell membranes. PLoS ONE 2011, 6, e22345. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Kaneda, T. Extra-week chemiluminescence of organ homogenate and blood in tocopherol-deficient rats. J. Nutr. Sci. Vitaminol. 1981, 27, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Kaneda, T.; Takyu, C.; Inaba, H. Characteristics of tissue ultraweak chemiluminescence in rats fed with autoxidized linseed oil. J. Nutr. Sci. Vitaminol. 1983, 29, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, E.; Boveris, A.; Chance, B. Chemiluminescence of lipid vesicles supplemented with cytochrome c and hydroperoxide. Biochem. J. 1980, 188, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Chen, H.; Zhou, Y.; Ogawa, N.; Lin, J.M. Self-catalytic degradation of ortho-chlorophenol with Fenton’s reagent studied by chemiluminescence. J. Environ. Sci. 2012, 24, 550–557. [Google Scholar] [CrossRef]
- Ivanova, I.P.; Trofimova, S.V.; Piskarev, I.M.; Aristova, N.A.; Burhina, O.E.; Soshnikova, O.O. Mechanism of chemiluminescence in Fenton reaction. J. Biophys. Chem. 2012, 3, 88–100. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Lu, C.; Lin, J.M. Improved chemiluminescence in fenton-like reaction via dodecylbenzene-sulfonate-intercalated layered double hydroxides. J. Phys. Chem. C 2012, 116, 14711–14716. [Google Scholar] [CrossRef]
- Friedman, A.; Arosio, P.; Finazzi, D.; Koziorowski, D.; Galazka-Friedman, J. Ferritin as an important player in neurodegeneration. Parkinsonism Relat. Disord. 2011, 17, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Iron and carcinogenesis: From Fenton reaction to target genes. Redox Rep. 2002, 7, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, P.; Prasad, A.; Rác, M. Role of reactive oxygen species in ultra-weak photon emission in biological systems. J. Photochem. Photobiol. B 2014, 139, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Pospišil, P. Two-dimensional imaging of spontaneous ultra-weak photon emission from the human skin: Role of reactive oxygen species. J. Biophotonics 2011, 4, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M. Highly sensitive imaging for ultra-weak photon emission from living organisms. J. Photochem. Photobiol. B 2014, 139, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Balcerczyk, A.; Sowa, K.; Bartosz, G. Metal chelators react also with reactive oxygen and nitrogen species. Biochem. Biophys. Res. Commun. 2007, 352, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, J.M.; Maidt, L.; Poyer, L. Superoxide dismutase and Fenton chemistry. Reaction of ferric-EDTA complex and ferric-bipyridyl complex with hydrogen peroxide without the apparent formation of iron(II). Biochem. J. 1990, 269, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Martinez, G.R.; Medeiros, M.H.; Di Mascio, P. Singlet molecular oxygen generated by biological hydroperoxides. J. Photochem. Photobiol. B 2014, 139, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Wijk, E.P.; Wijk, R.V. Multi-site recording and spectral analysis of spontaneous photon emission from human body. Forsch. Komplementarmed. Klass. Naturheilkd. 2005, 12, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Rác, M.; Sedlářová, M.; Pospíšil, P. The formation of electronically excited species in the human multiple myeloma cell suspension. Sci. Rep. 2015, 5, 8882. [Google Scholar] [CrossRef] [PubMed]
- Antiñolo, M.; Ocaña, A.J.; Aranguren, J.P.; Lane, S.I.; Albaladejo, J.; Jiménez, E. Atmospheric degradation of 2-chloroethyl vinyl ether, allyl ether and allyl ethyl ether: Kinetics with OH radicals and UV photochemistry. Chemosphere 2017, 181, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann-Ingrand, S.; Favreliere, S.; Fauconneau, B.; Mauco, G.; Tallineau, C. Plasmalogen degradation by oxidative stress: Production and disappearance of specific fatty aldehydes and fatty alpha-hydroxyaldehydes. Free Radic. Biol. Med. 2001, 31, 1263–1271. [Google Scholar] [CrossRef]
- Castro, G.; Casado, J.; Rodríguez, I.; Ramil, M.; Ferradás, A.; Cela, R. Time-of-flight mass spectrometry assessment of fluconazole and climbazole UV and UV/H2O2 degradability: Kinetics study and transformation products elucidation. Water Res. 2016, 88, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Arantes, V.; Jellison, J.; Goodell, B. Peculiarities of brown-rot fungi and biochemical Fenton reaction with regard to their potential as a model for bioprocessing biomass. Appl. Microbiol. Biotechnol. 2012, 94, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Elliot, A.J. Rate constant for reaction of hydroxyl radicals with bicarbonate ions. Radiat. Phys. Chem. 1986, 27, 241–243. [Google Scholar] [CrossRef]
- Hussain, R.S.; Cillard, J.; Cillard, P. Hydroxyl radical scavenging activity of flavonoids. Phytochemistry 1987, 26, 2489–2491. [Google Scholar] [CrossRef]
- Heijnen, C.G.; Haenen, G.R.; van Acker, F.A.; van der Vijgh, W.J.; Bast, A. Flavonoids as peroxynitrite scavengers: The role of the hydroxyl groups. Toxicol. In Vitro 2001, 15, 3–6. [Google Scholar] [CrossRef]
- Jung, H.A.; Jung, M.J.; Kim, J.Y.; Chung, H.Y.; Choi, J.S. Inhibitory activity of flavonoids from Prunus davidiana and other flavonoids on total ROS and hydroxyl radical generation. Arch. Pharm. Res. 2003, 26, 809–815. [Google Scholar] [CrossRef] [PubMed]
- De Graft-Johnson, J.; Nowak, D. Effect of selected plant phenolics on Fe2+-EDTA-H2O2 system mediated deoxyribose oxidation: Molecular structure-derived relationships of anti- and pro-oxidant actions. Molecules 2016, 22, 59. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Satsu, H.; Bae, M.J.; Totsuka, M.; Shimizu, M. Catechol groups enable reactive oxygen species scavenging-mediated suppression of PKD-NFkappaB-IL-8 signaling pathway by chlorogenic and caffeic acids in human intestinal cells. Nutrients 2017, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.; Perluigi, M.; Sultana, R.; Agrippino, R.; Calabrese, V.; Butterfield, D.A. In vivo protection of synaptosomes by ferulic acid ethyl ester (FAEE) from oxidative stress mediated by 2,2-azobis(2-amidino-propane)dihydrochloride (AAPH) or Fe(2+)/H(2)O(2): Insight into mechanisms of neuroprotection and relevance to oxidative stress-related neurodegenerative disorders. Neurochem. Int. 2006, 48, 318–327. [Google Scholar] [PubMed]
- Kanski, J.; Aksenova, M.; Stoyanova, A.; Butterfield, D.A. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: Structure-activity studies. J. Nutr. Biochem. 2002, 13, 273–281. [Google Scholar] [CrossRef]
- Ogiwara, T.; Satoh, K.; Kadoma, Y.; Murakami, Y.; Unten, S.; Atsumi, T.; Sakagami, H.; Fujisawa, S. Radical scavenging activity and cytotoxicity of ferulic acid. Anticancer Res. 2002, 22, 2711–2717. [Google Scholar] [PubMed]
- Kono, Y.; Kashine, S.; Yoneyama, T.; Sakamoto, Y.; Matsui, Y.; Shibata, H. Iron chelation by chlorogenic acid as a natural antioxidant. Biosci. Biotechnol. Biochem. 1998, 62, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Adjimani, J.P.; Asare, P. Antioxidant and free radical scavenging activity of iron chelators. Toxicol. Rep. 2015, 2, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Granieri, L.; Del Pino, A.M.; Mazzoni, M.; Mancinelli, L.; Proietti, P.; Perretti, G.; Palmerini, C.A. Chelating properties of beer: Implications on calcium homeostasis in PE/CA-PJ15 cells. J. Nutr. Intermed. Metab. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Minakata, K.; Fukushima, K.; Nakamura, M.; Iwahashi, H. Effect of some naturally occurring iron ion chelators on the formation of radicals in the reaction mixtures of rat liver microsomes with ADP, Fe and NADPH. J. Clin. Biochem. Nutr. 2011, 49, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- DeGraft-Johnson, J.; Kolodziejczyk, K.; Krol, M.; Nowak, P.; Krol, B.; Nowak, D. Ferric-reducing ability power of selected plant polyphenols and their metabolites: Implications for clinical studies on the antioxidant effects of fruits and vegetable consumption. Basic Clin. Pharmacol. Toxicol. 2007, 100, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.; Frei, B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999, 13, 1007–1024. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.C.; Bode, A.M. Biology of free radical scavengers: An evaluation of ascorbate. FASEB J. 1993, 7, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am. J. Clin. Nutr. 1991, 54 (Suppl. 6), 1119S–1124S. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.; Piasecka, G.; Antczak, A.; Pietras, T. Effect of ascorbic acid on hydroxyl radical generation by chemical, enzymatic and cellular systems. Importance for antioxidant prevention of pulmonary emphysema. Biomed. Biochim. Acta 1991, 50, 265–272. [Google Scholar] [PubMed]
- Nowak, D.; Piasecka, G.; Pietras, T.; Antczak, A. Effect of ascorbic acid on killing of lymphocytes and macrophages by hydrogen peroxide. Biomed. Biochim. Acta 1991, 50, 1079–1086. [Google Scholar] [PubMed]
- Noble, R.W.; Gibson, Q.H. The reaction of ferrous horseradish peroxidase with hydrogen peroxide. J. Biol. Chem. 1970, 245, 2409–2413. [Google Scholar] [PubMed]
- Rosenblum, W.I.; El-Sabban, F. Dimethyl sulfoxide (DMSO) and glycerol, hydroxyl radical scavengers, impair platelet aggregation within and eliminate the accompanying vasodilation of injured mouse pial arterioles. Stroke 1982, 13, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Jensen, R.G.; Bohnert, H.J. Mannitol protects against oxidation by hydroxyl radicals. Plant Physiol. 1997, 115, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Bancirova, M. Sodium azide as a specific quencher of singlet oxygen during chemiluminescent detection by luminol and Cypridina luciferin analogues. Luminescence 2011, 26, 685–688. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the ferulic acid, chlorogenic acid and caffeic acid are available from the authors. |
| Total Light Emission [RLU] | ||||||
|---|---|---|---|---|---|---|
| Experiment | Sample Composition in Phosphate Buffered Saline (pH = 7.4) | |||||
| Fe2+-EGTA-H2O2 | Fe2+-H2O2 | EGTA-H2O2 | H2O2 | Fe2+-EGTA-H2O | H2O | |
| A (n = 4) | 1351 ± 178 * (1343;212) | 594 ± 58 (602;86) | 612 ± 68 (607;70) | 543 ± 37 (547;26) | 578 ± 70 (574;66) | 530 ± 38 (523;32) |
| B (n = 5) | 1533 ± 76 * (1552;139) | 522 ± 36 (515;53) | 507 ± 14 (512;13) | 522 ± 43 (525;34) | 489 ± 9 (486;14) | 472 ± 12 (472;15) |
| C (n = 10) | 4319 ± 755 *† (4355;1127) | 645 ± 100 ** (628;117) | 600 ± 80 (594;80) | 565 ± 63 (556;74) | 545 ± 77 (515;95) | 521 ± 64 (497;70) |
| D (n = 8) | 6278 ± 502 *† (6070;296) ‡ | 609 ± 77 ** (610;62) | 549 ± 55 (559;85) | 526 ± 50 (507;71) | 495 ± 40 (495;53) | 497 ± 38 (488;66) |
| Compound Concentration (µmol/L) | % Inhibition of Light Emission from Fe2+-EGTA-H2O2 System | ||
|---|---|---|---|
| Ferulic Acid | Chlorogenic Acid | Caffeic Acid | |
| 87 | 90 ± 4 (90;7) * | 90 ± 5 (90;8) | 97 ± 2 (97;3) |
| 174 | 90 ± 3 (90;5) * | 94 ± 3 (94;5) | 98 ± 1 (98;1) |
| 870 | 90 ± 5 (88;9) * | 91 ± 5 (88;8) * | 98 ± 3 (98;2) |
| No | Sample | Volumes of Working Solutions Added to Luminometer Tube (µL) | ||||
|---|---|---|---|---|---|---|
| A | B * | C ** | D | E | ||
| PBS | EGTA | FeSO4 | H2O2 | H2O | ||
| 1 | Complete system | 940 | 20 | 20 | 100 | - |
| 2 | Incomplete system I | 960 | - | 20 | 100 | - |
| 3 | Incomplete system II | 960 | 20 | - | 100 | - |
| 4 | H2O2 alone | 980 | - | - | 100 | - |
| Additional controls | ||||||
| 5 | Fe2+-EGTA without H2O2 | 940 | 20 | 20 | - | 100 |
| 6 | Medium alone | 980 | - | - | - | 100 |
| No | Sample | Volumes of Working Solutions Added to Luminometer Tube (µL) | ||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | ||
| PBS | Polyphenol | EGTA | FeSO4 | ROS Scavenger | H2O2 | H2O | ||
| 1 | Complete system | 940 | - | 20 | 20 | - | 100 | - |
| 2 | Complete system + polyphenol | - | 940 | 20 | 20 | - | 100 | - |
| 3 | Complete system + ROS scavenger | 920 | - | 20 | 20 | 20 | 100 | - |
| 4 | Incomplete system I | 960 | - | - | 20 | - | 100 | - |
| 5 | Incomplete system I + polyphenol | 20 | 940 | - | 20 | - | 100 | - |
| 6 | Incomplete system I + ROS scavenger | 940 | - | - | 20 | 20 | 100 | - |
| Additional controls | ||||||||
| 7 | Fe2+-EGTA without H2O2 | 940 | - | 20 | 20 | - | - | 100 |
| 8 | Fe2+-EGTA without H2O2 + polyphenol | - | 940 | 20 | 20 | - | - | 100 |
| 9 | Fe2+-EGTA without H2O2 + ROS scavenger | 920 | - | 20 | 20 | 20 | - | 100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, M.; Tryniszewski, W.; Sarniak, A.; Wlodarczyk, A.; Nowak, P.J.; Nowak, D. Light Emission from the Fe2+-EGTA-H2O2 System: Possible Application for the Determination of Antioxidant Activity of Plant Phenolics. Molecules 2018, 23, 866. https://doi.org/10.3390/molecules23040866
Nowak M, Tryniszewski W, Sarniak A, Wlodarczyk A, Nowak PJ, Nowak D. Light Emission from the Fe2+-EGTA-H2O2 System: Possible Application for the Determination of Antioxidant Activity of Plant Phenolics. Molecules. 2018; 23(4):866. https://doi.org/10.3390/molecules23040866
Chicago/Turabian StyleNowak, Michal, Wieslaw Tryniszewski, Agata Sarniak, Anna Wlodarczyk, Piotr J. Nowak, and Dariusz Nowak. 2018. "Light Emission from the Fe2+-EGTA-H2O2 System: Possible Application for the Determination of Antioxidant Activity of Plant Phenolics" Molecules 23, no. 4: 866. https://doi.org/10.3390/molecules23040866





