The Molecular Targets and Anti-Invasive Effects of 2,6-bis-(4-hydroxyl-3methoxybenzylidine) cyclohexanone or BHMC in MDA-MB-231 Human Breast Cancer Cells
Abstract
:1. Introduction
2. Results
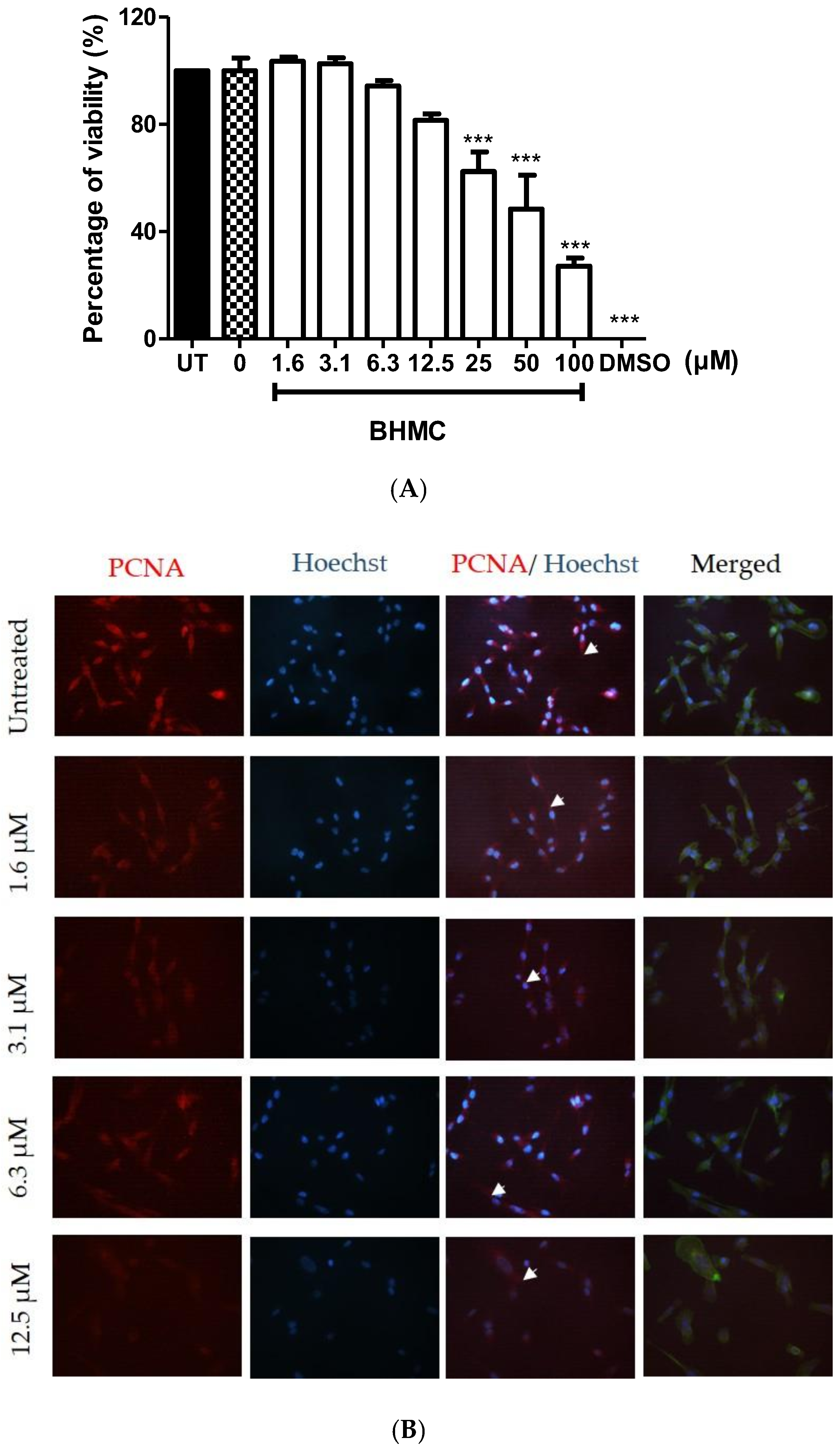
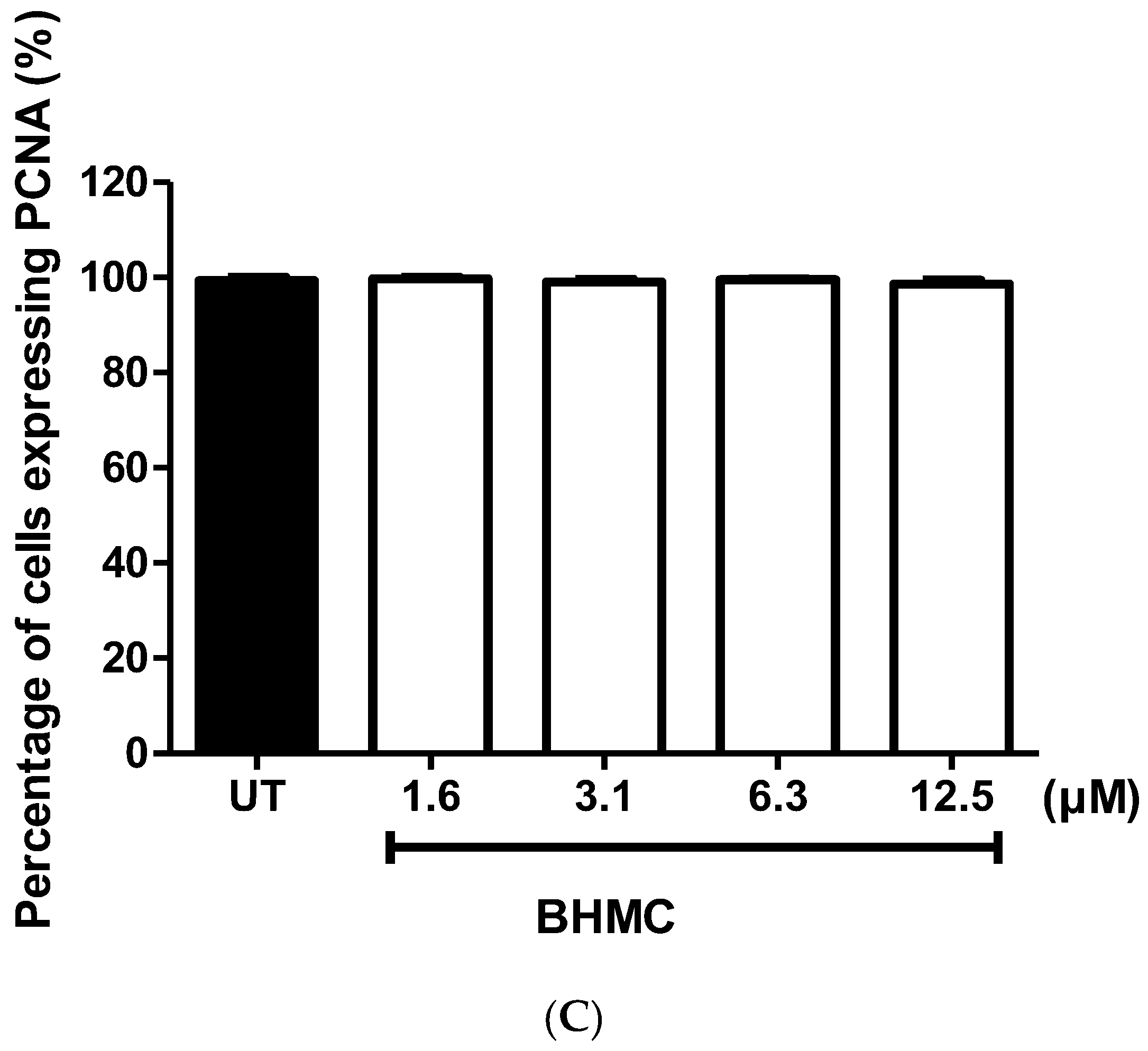
2.1. Cytotoxicity and Effects of BHMC on the Proliferation of MDA-MB-231 Cells
2.2. Inhibition of BHMC on the Migration and Invasion of MDA-MB-231 Cells
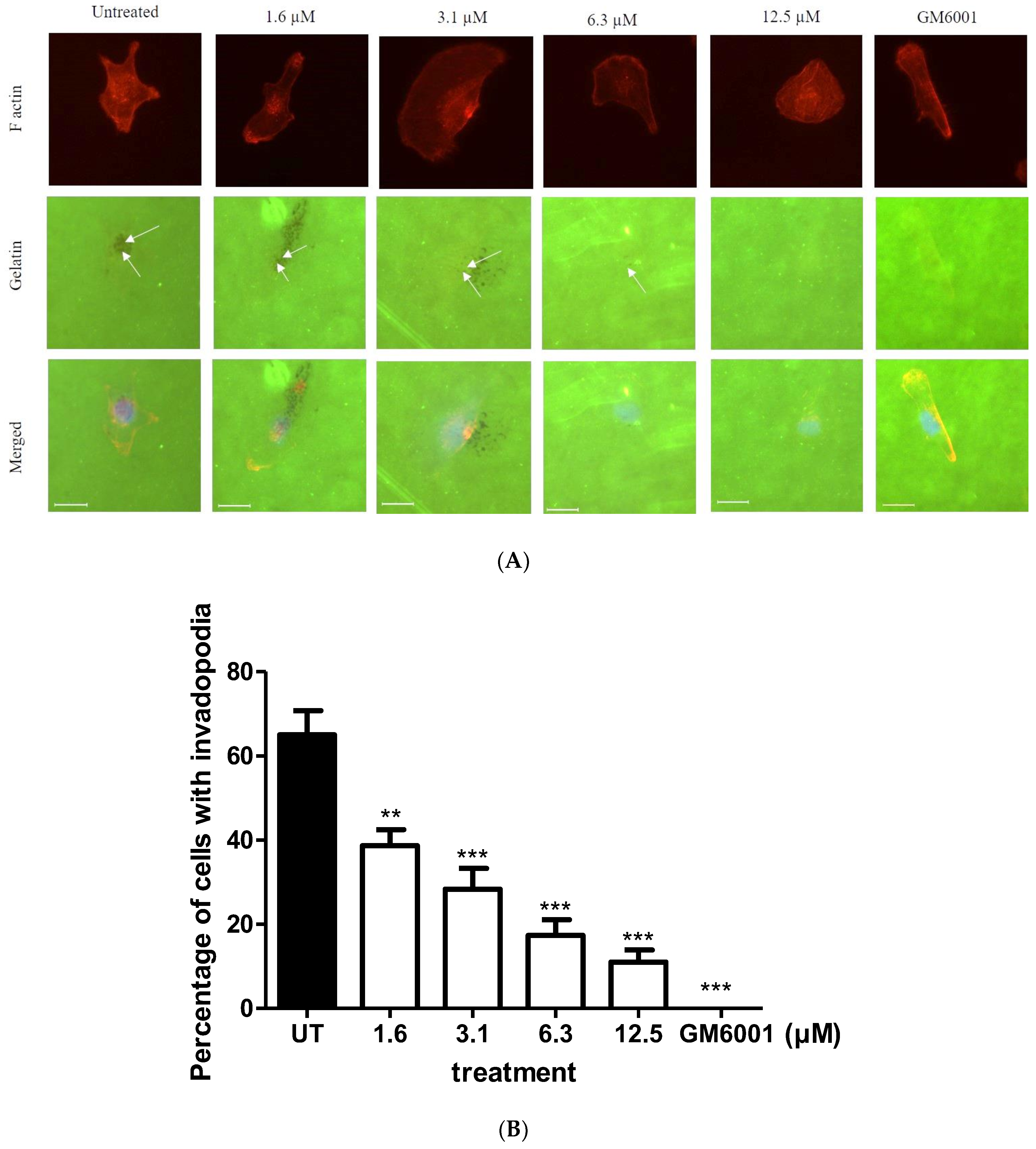
2.3. BHMC Effects on the Number of Cells Forming Invadopodia
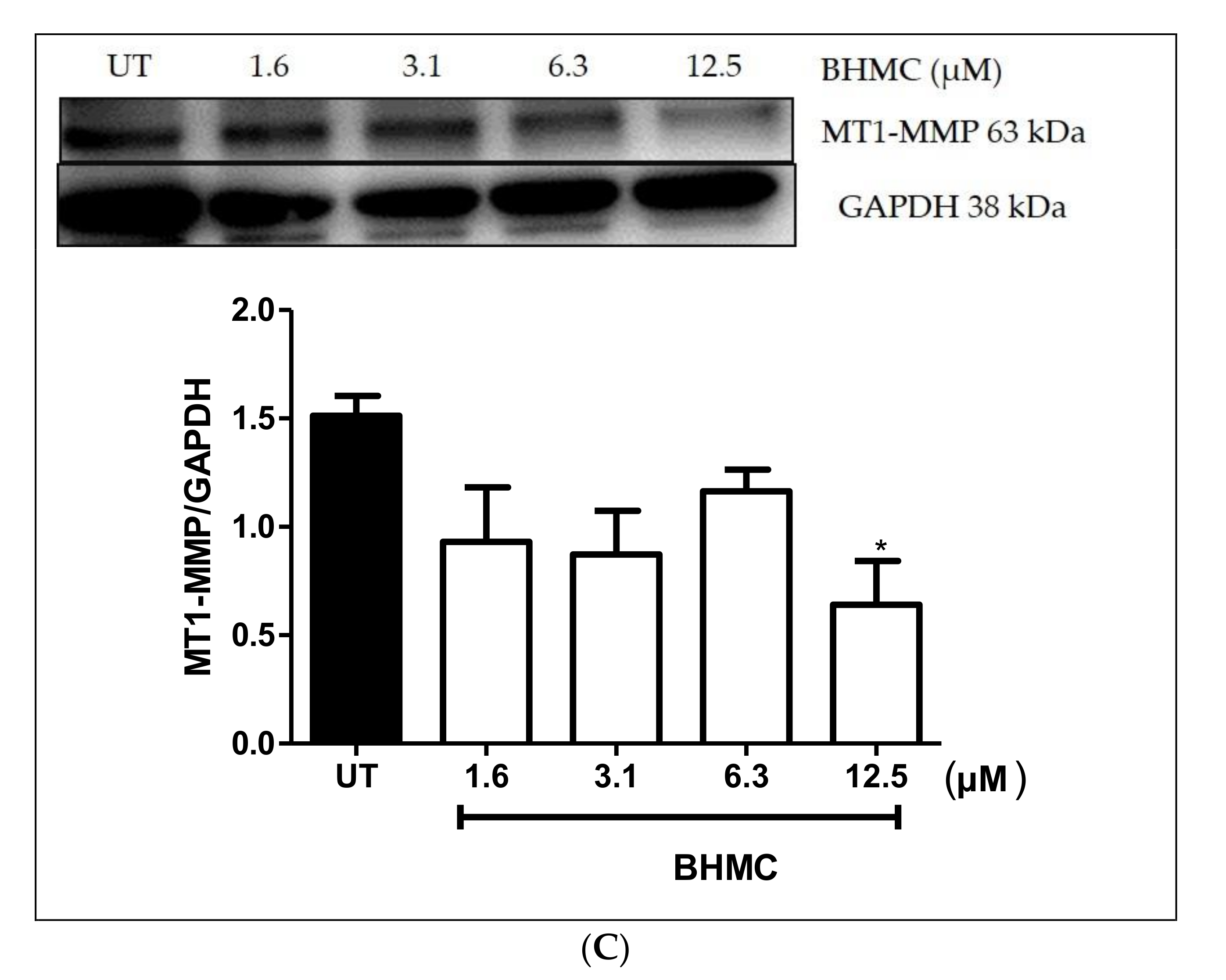
2.4. Inhibition of β-PIX, MMP-9, and MT1-MMP Protein Expression on MDA-MB-231 Cells upon BHMC Treatment
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. MTT Assay
4.4. Proliferation Assay
4.5. Scratch Migration Assay
4.6. Transwell Migration and Invasion Assays
4.7. Gelatin Degradation Assay
4.8. Immunoblotting
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Soejomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. World Cancer Research Fund International Worldwide Data. Cancer Incidence and Mortality Worldwide. Available online: http://www.wcrf.org/int/cancer-facts-figures/ worldwide-data (accessed on 8 August 2017).
- Azizah, A.M.; Ibrahim, N.S.; Abdullah, N.H. Malaysian National Cancer Registry Report 2007–2011. Minist. Health Malays. 2016. [Google Scholar]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef]
- Anders, C.; Carey, L.A. Understanding and treating triple-negative breast cancer. Oncology 2008, 22, 1233. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. Cancer Metastasis. Br. Med. Bull. 1991, 47, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Buccione, R.; Orth, J.D.; McNiven, M.A. Foot and mouth: Podosomes, invadopodia and circular dorsal ruffles. Nat. Rev. Mol. Cell Biol. 2004, 5, 647–657. [Google Scholar] [CrossRef]
- Murphy, D.A.; Courtneidge, S.A. The “ins” and “outs” of podosomes and invadopodia: Characteristics, formation and function. Nat. Rev. Mol. Cell Biol. 2011, 12, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-T. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J. Exp. Zool. 1989, 251, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Leong, H.S.; Robertson, A.E.; Stoletov, K.; Leith, S.J.; Chin, C.A.; Chien, A.E.; Hague, M.N.; Ablack, A.; Carmine-Simmen, K.; McPherson, V.A.; et al. Invadopodia Are Required for Cancer Cell Extravasation and Are a Therapeutic Target for Metastasis. Cell Rep. 2014, 8, 1558–1570. [Google Scholar] [CrossRef]
- Hoskin, V.; Szeto, A.; Ghaffari, A.; Greer, P.A.; Cote, G.P.; Elliott, B.E. Ezrin regulates focal adhesion and invadopodia dynamics by altering calpain activity to promote breast cancer cell invasion. Mol. Biol. Cell 2015, 26, 3464–3479. [Google Scholar] [CrossRef]
- Hoshino, D.; Branch, K.M.; Weaver, A.M. Signaling inputs to invadopodia and podosomes. J. Cell Sci. 2013, 126, 2979–2989. [Google Scholar] [CrossRef] [PubMed]
- Ten Klooster, J.P.; Jaffer, Z.M.; Chernoff, J.; Hordijk, P.L. Targeting and activation of Rac1 are mediated by the exchange factor β-Pix. J. Cell Biol. 2006, 172, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Marchesin, V.; Castro-Castro, A.; Lodillinsky, C.; Castagnino, A.; Cyrta, J.; Bonsang-Kitzis, H.; Reyal, F. ARF6-JIP3/4 regulate endosomal tubules for MT1-MMP exocytosis in cancer invasion. J. Cell Biol. 2015, 211, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Weaver, A.M. Invadopodia: Specialized cell structures for cancer invasion. Clin. Exp. Metastasis 2006, 23, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Jing, J.; Lee, J.; Schedin, P.; Gilbert, S.M.; Peden, A.A.; Junutula, J.R.; Prekeris, R. Rab40b regulates MMP2 and MMP9 trafficking during invadopodia formation and breast cancer cell invasion. J. Cell Sci. 2013, 2013. [Google Scholar] [CrossRef]
- Ichikawa, K. Synergistic effect of blocking cancer cell invasion revealed by computer simulations. Math. Biosci. Eng. 2015, 12, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Peterse, J.L.; Van’t Veer, L.J. Breast cancer metastasis: Markers and models. Nat. Rev. Cancer 2005, 5, 591–602. [Google Scholar] [CrossRef]
- Volk-Draper, L.; Hall, K.; Griggs, C.; Rajput, S.; Kohio, P.; DeNardo, D.; Ran, S. Paclitaxel therapy promotes breast cancer metastasis in a TLR4-dependent manner. Cancer Res. 2014, 74, 5421–5434. [Google Scholar] [CrossRef] [PubMed]
- Quintavalle, M.; Elia, L.; Price, J.H.; Heynen-Genel, S.; Courtneidge, S.A. A cell-based high-content screening assay reveals activators and inhibitors of cancer cell invasion. Sci. Signal. 2011, 4. [Google Scholar] [CrossRef] [PubMed]
- Krausz, A.E.; Adler, B.L.; Cabral, V.; Navati, M.; Doerner, J.; Charafeddine, R.A.; Chandra, D.; Liang, H.; Gunther, L.; Clendaniel, A.; et al. Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.F.; Shen, L. Interactions of curcumin with the PfATP6 model and the implications for its antimalarial mechanism. Bioorganic Med. Chem. Lett. 2009, 19, 2453–2455. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Foresti, R.; Bassi, R.; Green, C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic. Biol. Med. 2000, 28, 1303–1312. [Google Scholar] [CrossRef]
- Lee, W.H.; Loo, C.Y.; Young, P.M.; Rohanizadeh, R.; Traini, D. Curcumin Nanoparticles Attenuate Production of Pro-inflammatory Markers in Lipopolysaccharide-Induced Macrophages. Pharm. Res. 2016, 33, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.L.; Fan, C.C.; Chen, Y.A.; Cheng, C.W.; Sung, Y.J.; Hsu, C.P.; Kao, T.Y. Curcumin Inhibits Invasiveness and Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma Through Reducing Matrix Metalloproteinase 2, 9 and Modulating p53-E-Cadherin Pathway. Integr. Cancer Ther. 2015, 14, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.-L.; Su, C.C. Curcumin inhibits proliferation and migration by increasing the Bax to Bcl-2 ratio and decreasing NF-kBp65 expression in breast cancer MDA-MB-231 cells. Int. J. Mol. Med. 2009, 23, 469–475. [Google Scholar]
- Menon, L.G.; Kuttan, R.; Kuttan, G. Anti-metastatic activity of curcumin and catechin. Cancer Lett. 1999, 141, 159–165. [Google Scholar] [CrossRef]
- Tham, C.L.; Liew, C.Y.; Lam, K.W.; Mohamad, A.S.; Kim, M.K.; Cheah, Y.K.; Zakaria, Z.A.; Sulaiman, M.R.; Lajis, N.H.; Israf, D.A. A synthetic curcuminoid derivative inhibits nitric oxide and proinflammatory cytokine synthesis. Eur. J. Pharmacol. 2010, 628, 247–254. [Google Scholar] [CrossRef]
- Tham, C.L.; Lam, K.W.; Rajajendram, R.; Tham, C.L.; Lam, K.W.; Rajajendram, R.; Cheah, Y.K.; Sulaiman, M.R.; Lajis, N.H.; Kim, M.; Israf, D.A. The effects of a synthetic curcuminoid analogue, 2,6-bis-(4-hydroxyl-3-methoxybenzylidine)cyclohexanone on proinflammatory signaling pathways and CLP-induced lethal sepsis in mice. Eur. J. Pharmacol. 2011, 652, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Ming-Tatt, L.; Khalivulla, S.I.; Akhtar, M.N.; Lajis, N.; Perimal, E.K.; Akira, A.; Sulaiman, M.R. Anti-Hyperalgesic effect of a benzilidine-cyclohexanone analogue on a mouse model of chronic constriction injury-induced neuropathic pain: Participation of the κ-Opioid receptor and KATP. Pharmacol. Biochem. Behav. 2013, 114, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Razak, N.A.; Akhtar, M.N.; Abu, N.; Ho, W.Y.; Tan, S.W.; Zareen, S.; Taj-ud-dinb, S.N.B.; Long, K.; Alitheen, N.B.; Yeap, S.K. The in vivo anti-tumor effect of curcumin derivative (2E,6E)-2,6-bis(4-hydroxy-3-methoxybenzylidene)cyclohexanone (BHMC) on 4T1 breast cancer cells. RSC Adv. 2017, 7, 36185–36192. [Google Scholar] [CrossRef]
- Gupta, G.P.; Massagué, J. Cancer Metastasis: Building a Framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liang, X.; Cai, M.; Du, G. Analysis of invadopodia formation in breast cancer cells. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2016; Volume 1406, pp. 203–210. [Google Scholar] [CrossRef]
- Maity, G.; Choudhury, P.R.; Sen, T.; Ganguly, K.K.; Sil, H.; Chatterjee, A. Culture of human breast cancer cell line (MDA-MB-231) on fibronectin-coated surface induces pro-matrix metalloproteinase-9 expression and activity. Tumor Biol. 2011, 32, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; Wang, B.; Zheng, T.; Li, X.; Zheng, L.; Hu, J.; Zhang, Y.; Xing, Y.; Xi, T. MALAT1 induced migration and invasion of human breast cancer cells by competitively binding MIR-1 with cdc42. Biochem. Biophys. Res. Commun. 2016, 472, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Zhou, C.-X.; Gao, Y. Podoplanin promotes the invasion of oral squamous cell carcinoma in coordination with MT1-MMP and Rho GTPases. Am. J. Cancer Res. 2015, 5, 514–529. [Google Scholar] [PubMed]
- Artym, V.V.; Zhang, Y.; Seillier-Moiseiwitsch, F.; Yamada, K.M.; Mueller, S.C. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: Defining the stages of invadopodia formation and function. Cancer Res. 2006, 66, 3034–3043. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Baird, D.; Peng, X.; Wang, J.; Ly, T.; Guan, J.L.; Cerione, R.A. Cool-1 functions as an essential regulatory node for EGF receptor- and Src-mediated cell growth. Nat. Cell Biol. 2006, 8, 945–956. [Google Scholar] [CrossRef]
- Md Hashim, N.F.; Nicholas, N.S.; Dart, A.E.; Kiriakidis, S.; Paleolog, E.; Wells, C.M. Hypoxia-induced invadopodia formation: A role for β-PIX. Open Biol. 2013, 3, 120159. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.D.; Ha, J.H.; Jayaraman, M.; Dhanasekaran, D.N. LPA-mediated migration of ovarian cancer cells involves translocalization of Gαi2 to invadopodia and association with Src and β-PIX. Cancer Lett. 2015, 356, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Fidler, I.J. Selection of successive tumour lines for metastasis. Nat. New Biol. 1973, 242, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Rockwell, S.; Dobrucki, I.T.; Kim, E.Y.; Marrison, S.T.; Vu, V.T. Hypoxia and radiation therapy: past history, ongoing research, and future promise. Curr. Mol. Med. 2009, 9, 442–458. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E. Mechanisms of cancer cell invasion. Curr. Opin. Genet. Dev. 2005, 15, 87–96. [Google Scholar] [CrossRef]
- Wang, S.C. PCNA: A silent housekeeper or a potential therapeutic target? Trends Pharmacol. Sci. 2014, 35, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, G.L.; Pfander, B.; Jentsch, S. PCNA, the Maestro of the Replication Fork. Cell 2007, 129, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Naryzhny, S.N.; Lee, H. Proliferating cell nuclear antigen in the cytoplasm interacts with components of glycolysis and cancer. FEBS Lett. 2010, 584, 4292–4298. [Google Scholar] [CrossRef] [PubMed]
- Poste, G.; Fidler, I.J. The pathogenesis of cancer metastasis. Nature 1980, 283, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.-M.; Shen, Z.-Z.; Liu, C.-H.; Sartippour, M.R.; Go, V.L.; Heber, D.; Nguyen, M. Curcumin exerts multiple suppressive effects on human breast carcinoma cells. Int. J. Cancer 2002, 98, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Lorenz, M.; Kempiak, S.; Sarmiento, C.; Coniglio, S.; Symons, M.; Segall, J.; Eddy, R.; Miki, H.; Takenawa, T.; et al. Molecular mechanisms of invadopodium formation: The role of the N-WASP–Arp2/3 complex pathway and cofilin. J. Cell Biol. 2005, 168, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Wu, R.; Li, Y.; Zhang, L.; Tang, X.; Tu, J.; Zhou, W.; Wang, J.; Shou, Q. Safflower Yellow Prevents Pulmonary Metastasis of Breast Cancer by Inhibiting Tumor Cell Invadopodia. Am. J. Chin. Med. 2016, 44, 1491–1506. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, E.; Gao, Y.; Wang, Y.; Guo, Z.; He, J.; Zhang, J.; Gao, Z.; Wang, Q. Study on Invadopodia Formation for Lung Carcinoma Invasion with a Microfluidic 3D Culture Device. PLoS ONE 2013, 8, 56448. [Google Scholar] [CrossRef] [PubMed]
- Ayala, I.; Baldassarre, M.; Giacchetti, G.; Caldieri, G.; Tetè, S.; Luini, A.; Buccione, R. Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J. Cell Sci. 2008, 121, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.C.; Han, X.; Hsiao, C.T.; Yates, J.R.; Waterman, C.M. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat. Cell Biol. 2011, 13, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.C.; Huang, J.; Ali, S.; Lowry, W.; Huang, X.Y. Src tyrosine kinase is a novel direct effector of G proteins. Cell 2000, 102, 635–646. [Google Scholar] [CrossRef]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011, 41, 271–290. [Google Scholar] [CrossRef]
- Yan, C.; Boyd, D.D. Regulation of matrix metalloproteinase gene expression. J. Cell Physiol. 2007, 211, 19–26. [Google Scholar] [CrossRef]
- Legrand, C.; Gilles, C.; Zahm, J.M.; Polette, M.; Buisson, A.; Kaplan, H.; Birembaut, P.; Tournier, J. Airway epithelial cell migration dynamics. MMP-9 role in cell-extracellular matrix remodeling. J. Cell Biol. 1999, 146, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.S.; Zhao, Z.; Zhu, H.P. Hispolon inhibits TPA-induced invasion by reducing MMP-9 expression through the NF-κB signaling pathway in MDA-MB-231 human breast cancer cells. Oncol. Lett. 2015, 10, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.S.; Jung, S.H.; Kim, S.Y.; Hyun, J.; Ko, K.; Kim, W.; Kim, H. Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKC to MAPK signaling pathways in human astroglioma cells. Biochem. Biophys. Res. Commun. 2005, 335, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.S.; Whigham, A.S.; Yarbrough, W.G.; Weaver, A.M. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007, 67, 4227–4235. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.J. Cortactin signalling and dynamic actin networks. Biochem. J. 2004, 382, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Wang, Z.; Deng, Z.; Ren, H.; Li, X. Curcumin inhibits lung cancer invasion and metastasis by attenuating GLUT1/MT1-MMP/MMP2 pathway. Int. J. Clin. Exp. Med. 2015, 8, 8948–8957. [Google Scholar] [PubMed]
- Yang, J.; Wang, C.; Zhang, Z.; Chen, X.; Jia, Y.; Wang, B.; Kong, T. Curcumin inhibits the survival and metastasis of prostate cancer cells via the Notch-1 signaling pathway. Apmis 2017, 125, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Jiao, D.M.; Yao, Q.H.; Yan, J.; Song, J.; Chen, F.Y.; Lu, G.; Zhou, J.Y. Expression analysis of Cdc42 in lung cancer and modulation of its expression by curcumin in lung cancer cell lines. Int. J. Oncol. 2012, 40, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zheng, Y.; Jiao, D.; Chen, F.Y.; Hu, H.Z.; Wu, Y.Q.; Song, J.; Yan, J.; Wu, L.J.; Lv, G.Y. Curcumin inhibits lung cancer cell migration and invasion through Rac1-dependent signaling pathway. J. Nutr. Biochem. 2014, 25, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y. Scratch Wound Healing Assay. J. Chem. Inf. Model. 2013, 53, 1689–1699. [Google Scholar] [CrossRef]
- Albini, A.; Iwamoto, Y.; Kleinman, H.K.; Martin, G.R.; Aaronson, S.A.; Kozlowski, J.M.; McEwan, R.N. A Rapid in Vitro Assay for Quantitating the Invasive Potential of Tumor Cells. Cancer Res. 1987, 47, 3239–3245. [Google Scholar] [CrossRef] [PubMed]
- Diaz, B. Invadopodia Detection and Gelatin Degradation Assay. Bio-protocol 2013, 3. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from Nur Fariesha Md Hashim. |



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harun, S.N.A.; Israf, D.A.; Tham, C.L.; Lam, K.W.; Cheema, M.S.; Md Hashim, N.F. The Molecular Targets and Anti-Invasive Effects of 2,6-bis-(4-hydroxyl-3methoxybenzylidine) cyclohexanone or BHMC in MDA-MB-231 Human Breast Cancer Cells. Molecules 2018, 23, 865. https://doi.org/10.3390/molecules23040865
Harun SNA, Israf DA, Tham CL, Lam KW, Cheema MS, Md Hashim NF. The Molecular Targets and Anti-Invasive Effects of 2,6-bis-(4-hydroxyl-3methoxybenzylidine) cyclohexanone or BHMC in MDA-MB-231 Human Breast Cancer Cells. Molecules. 2018; 23(4):865. https://doi.org/10.3390/molecules23040865
Chicago/Turabian StyleHarun, Siti Nor Aini, Daud Ahmad Israf, Chau Ling Tham, Kok Wai Lam, Manraj Singh Cheema, and Nur Fariesha Md Hashim. 2018. "The Molecular Targets and Anti-Invasive Effects of 2,6-bis-(4-hydroxyl-3methoxybenzylidine) cyclohexanone or BHMC in MDA-MB-231 Human Breast Cancer Cells" Molecules 23, no. 4: 865. https://doi.org/10.3390/molecules23040865






