1. Introduction
Leishmaniasis is classified as one of the neglected tropical diseases by the World Health Organization which estimates that 350 million people are at risk of contracting this infection, while nearly two million new cases occur annually [
1]. The infection is caused by more than 20
Leishmania species, which are transmitted by inoculation of promastigote forms in humans through the bite of infected female phlebotomine sandflies. In the mammalian host, these parasites differentiate into amastigote forms inside cells and affect skin, mucosa, and cartilage, causing cutaneous leishmaniasis (CL). However; some species can infect internal tissues and organs, such as the liver, spleen, and bone marrow, causing visceral leishmaniasis (VL). Mucosal leishmaniasis (ML) is a metastatic outcome of the cutaneous form in which the parasites become disseminated to the oropharyngeal mucosa [
2]. The epidemiology of leishmaniasis depends on the characteristics of the parasite species, the ecological features of the transmission sites, and the degree of current or past exposure of the population to the parasite. Furthermore, the risk factors of transmission are linked to socioeconomic and environmental patterns, which can make disease control more difficult [
3].
Despite some important recent advances in the diagnosis, treatment and cost reduction of key drugs, both mortality and morbidity show a worrying increasing trend worldwide. This can be attributed to several factors, including lack of a vaccine, ineffective vector control and limitations of current drugs used to treat the infection [
4,
5].
Pentavalent antimonials, such as meglumine antimoniate (
Figure 1), have been used since the 1940s and remain the first-choice drugs to treat all clinical forms of leishmaniasis, due to the even higher risks of toxicity associated with the second line drugs amphotericin B and pentamidine. These second line drugs are only used when there is a contraindication, intolerability or resistance to the first line drugs [
6]. Nevertheless, pentavalent antimonials are frequently associated with high frequencies of mild to severe adverse effects, including musculoskeletal pain, gastrointestinal disorders, headache and anorexia, as well as cardiac, hepatic and pancreatic toxicity, leading in some cases to death [
7]. At the same time, the large pharmaceutical companies have made little investment in research to develop therapeutic alternatives for leishmaniasis which explains the paucity of compounds and formulations with low toxicity and proven effectiveness in clinical use.
Consequently, the search for plant products is gaining special attention because they are theoretically more accessible, usually cheap and can be made accessible to lower income population who are the most affected by the disease [
8]. A variety of natural products obtained from plant extracts has proved to be active against
Leishmania species. Among these, the 1,4-naphthoquinones are considered attractive structures in medicinal chemistry due to their biological activities and chemical properties [
9]. Examples of 1,4-naphthoquinones that have shown activity against
Leishmania species and
Trypanosoma cruzi are lapachol, isolated from Brazilian trees belonging to the genus
Tabebuia and its derivatives α-lapachone and β-lapachone [
10]. Most of the lapachone derivatives however, exhibit significant toxicity that limits their potential as new drugs [
11].
In a search for less toxic derivatives for mammalian cells, chemical modification of the quinonoid center of α-lapachone and 2-hydroxy-1,4-naphthoquinone (lawsone) followed by an epoxidation, generated the oxiranes epoxy-α-lapachone and epoxymethoxylawsone (
Figure 1), respectively [
12]. We have demonstrated that epoxy-α-lapachone was capable to kill promastigote forms of
Leishmania (
Viannia)
braziliensis and
Leishmania (
Leishmania)
amazonensis and intracellular amastigotes in human macrophages [
13]. Furthermore, reduction of the lesion size in the paw of BALB/c mice infected was observed after four weeks of treatment [
14].
Previous data showed that epoxymethoxylawsone has a significant effect on control of BALB/c mice paw lesion caused by
L. (
L.)
amazonensis [
16]. In the present study, unequivocal evidence is presented of the antileishmanial activity of this oxirane compound on intracellular amastigotes and promastigote forms as well as in the control of the paw lesion caused by
L. (
L.)
amazonensis.
3. Discussion
Leishmaniasis remains as one of the most neglected tropical diseases, affecting mainly the poorer populations in developing countries. Current treatment, based on pentavalent antimony, is associated with severe side effects, including cardiotoxicity, hepatotoxicity and pancreatic toxicity [
7]. Furthermore, high cost and technological dependence should be considered by endemic countries, increasing the need for new, more efficient and less toxic drugs. In this scenario, we highlight plant-derived compounds such as the naphthoquinones and its derivatives. Recently studies have demonstrated antileishmanial activity of epoxy-α-lapachone on macrophage infection and in the treatment of experimental murine infection with low cytotoxicity in mammalian cells [
13]. Here, we tested epoxymethoxylawsone, a new oxirane derivative that also exhibited lower toxicity on mammalian cell against both in vitro and in vivo infection.
The approach proposed here to evaluate leishmanicidal effects of epoxymethoxylawsone was successful since we have proved its in vitro action on the multiplication rate of intracellular amastigotes and promastigotes growth, as well as in vivo ability to control the experimental mice paw lesion infection induced by L. (L.) amazonensis. In this study, we administrated meglumine antimoniate as reference control due to be a first-choice drug for all forms of human leishmaniasis treatment. Our results showed a similar selectivity index for both drugs, and their IC50 values were slightly different. Besides that, reference drug showed higher cytotoxicity on macrophages than oxirane compound (1.7-fold). Selectivity index is a key parameter to consider when developing antimicrobial compound and the higher reach this value the safer it will be, in theory, the therapeutic use of the drug, as exhibited for epoxymethoxylawsone here.
Recently, a series of recommendations on criteria for selecting hit and lead compounds in drug discovery was published [
17]. Selectivity values may vary considerably according to the microorganism and its environment in the host, but general recommendation is that this value should be greater than 10-fold using a mammalian cell line, as HepG2 or Vero cells [
17]. Indeed, one of the most crucial elements for the success of drug screening is to apply assays that faithfully reproduce the microenvironment of a pathogen causing the disease. For this reason, we used murine macrophages due to this type of cell is the main target of
Leishmania spp infection. Additionally, epoxymethoxylawsone demonstrated other relevant parameters for the
Leishmania life cycle that is the specificity by the intracellular stage. It should be noted that epoxymethoxylawsone showed higher potency than epoxy-α-lapachone in infected macrophage cultures, represented by an IC
50 value about five-fold lower for 24 h of exposure (7.41 μM compared to 37 μM), with similar cytotoxicity profile [
13].
We have evidence that epoxymethoxylawsone reduced of lesion size in BALB/c mice paws due decreasing the parasite load, but not by reduction of the inflammatory process only, since was not possible to distinguish the lesion reduction effects among the three doses tested. In addition, this idea is reinforced by the observation that both epoxymethoxylawsone and meglumine antimoniate did not induce nitric oxide production in BALB/c mice macrophages infection. It is important to emphasize that drug efficacy measured solely by changes in lesion size can be misleading since a typical lesion is composed by a complex profile of inflammatory cells and amastigotes contained in vacuoles within macrophages of the skin [
18].
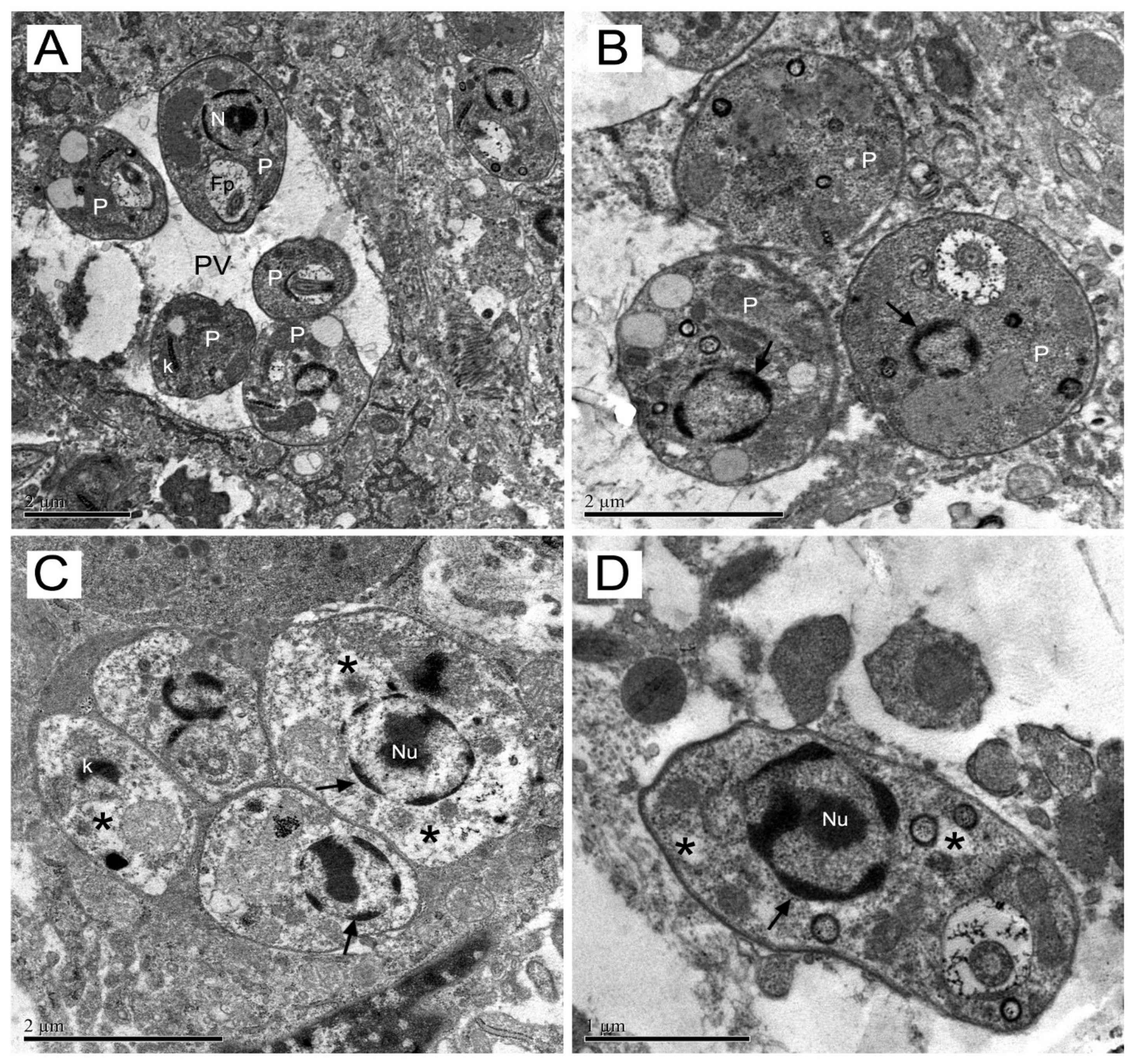
In addition, the effects of drugs were perceived by the presence of vacuoles organelles with remaining parasite after elimination, mainly in mice groups treated with meglumine antimoniate as result of lesions treated by an effective drug. On the other hand, in the epoxymethoxylawsone groups, lesion tissues showed a lower number of vacuoles. These depleted organelles were extensively detected, indicating a drastic reduction of parasite load.
To prove the drastic effect of treatments on the parasites from paw lesions, we decide to investigate ultrastructural alterations suffered by amastigotes to assess the integrity of parasites in skin tissue. Transmission electron microscopy analysis applied here revealed drastic changes in amastigotes ultrastructure which clearly affected parasite integrity. Epoxymethoxy-lawsone caused more pronounced changes than the reference drug.
Some of these alterations, such as those found in the amastigotes nuclei, are indicative of apoptosis events [
19]. We are sure that those disorders found are in accordance with amastigotes inability to multiply in the macrophage mice lesions thus decreasing the paw lesion size in treated mice.
The data presented here suggest that epoxymethoxylawsone is capable of crossing the plasma membrane of the macrophages and acts by directly killing amastigotes, but the mode of action of the drug on
L. (
L.)
amazonensis is not well known yet. As this compound is also derived from the α-lapachone molecule by an epoxidation reaction, and shares most of chemical and structural features with epoxy-α-lapachone, is possible that the new oxirane also acts by inhibiting proteases from
Leishmania sp. and other trypanosomatids, as previously reported [
12,
14,
20,
21], however further studies are needed to prove this hypothesis.
4. Materials and Methods
4.1. Chemicals and Culture Material
Dimethyl sulfoxide (DMSO), osmium tetroxide solution (OsO4), Epoxy Embedding Medium kit (Epon), penicillin, streptomycin, Lab-Tek chamber slides, Greiner CELLSTAR® 96 well plates, RPMI 1640 medium and Schneider’s Drosophila medium were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Fetal calf serum (FCS) was acquired from Cultilab S/A (São Paulo, Brazil). CellTiter-Glo® luminescent cell viability assay was acquired from Promega Corporation (Madison, WI, USA). Meglumine antimoniate (Glucantime®) was kindly provided by Dr. Armando de Oliveira Schubach team (INI/Fiocruz). Propylene glycol was obtained from Vetec Quimica (Rio de Janeiro, Brasil). The epoxymethoxylawsone compound was synthesized by the Department of Organic Chemistry of the Instituto de Química, Universidade Federal Fluminense and the powder was stored at 2 to 8 °C until its further use in assays.
4.2. Cell Culture
Peritoneal macrophages were harvested from BALB/c mice as previously described [
22]. Cells were recovered after centrifugation (2 ×, 1800 ×
g, 10 min, 4 °C) in RPMI 1640 medium containing 10% FCS. Subsequently, cells were seeded at a density of 5 × 10
5 cells/well in Lab-Tek chamber slides and maintained at 37 °C in a 5% of CO
2 atmosphere for 24 h. Non-adherent cells were removed by washing the culture plates with RPMI 1640 medium. Monolayers of murine peritoneal macrophages were used in leshmanicidal assays.
4.3. Parasite Cultures
Leishmania (Leishmania) amazonensis (strain MHOM/BR/73/LTB0016) was obtained from the Leishmania collection (Coleção de Leishmania do Instituto Oswaldo Cruz—CLIOC) of the Instituto Oswaldo Cruz (IOC). In vitro promastigote cultures were maintained at 28 °C in Schneider’s medium (pH 7.2) containing 1 mM l-glutamine, 10% FCS, 100 IU/mL penicillin, and 100 µg/mL streptomycin, with frequent subpassages to maintain the parasites in the logarithmic growth phase.
4.4. Activity against Promastigotes
Parasites were seeded on 96-well plates (1 × 10
5 per well) in Schneider’s medium, were treated for 24 h at 28 °C with epoxymethoxylawsone in a concentration ranging from 1.6 µM to 100 µM. Parasite viability was assessed by measuring ATP production using CellTiter-Glo
® (50 µL/well) and the luminescent signal was measured using a FlexStation 3 reader (Molecular Devices, Sunnyvale, CA, USA) [
23]. Drug efficacy determined by half maximal inhibitory concentration (IC
50) was calculated by linear regression.
4.5. Activity against Intracellular Amastigotes
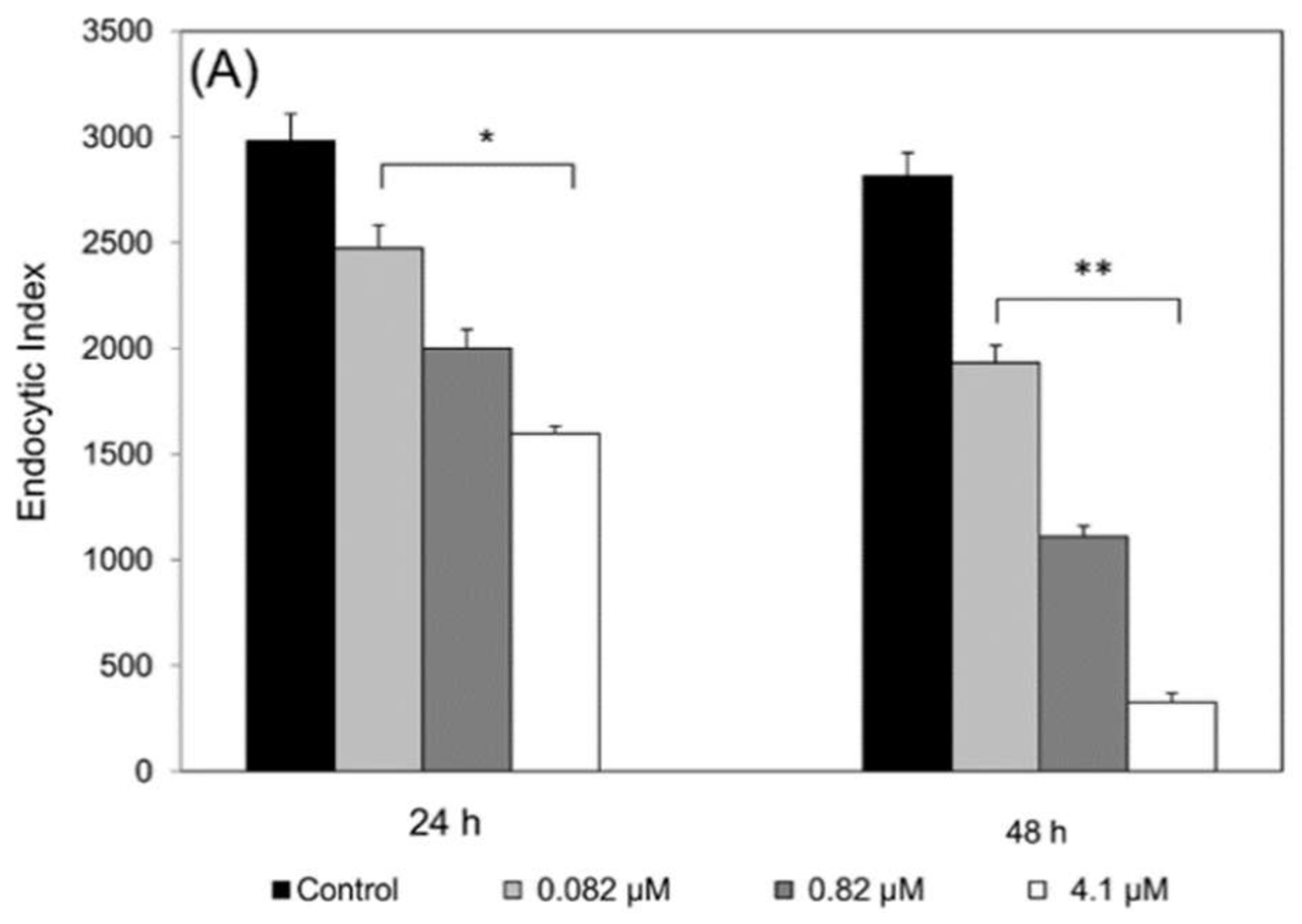
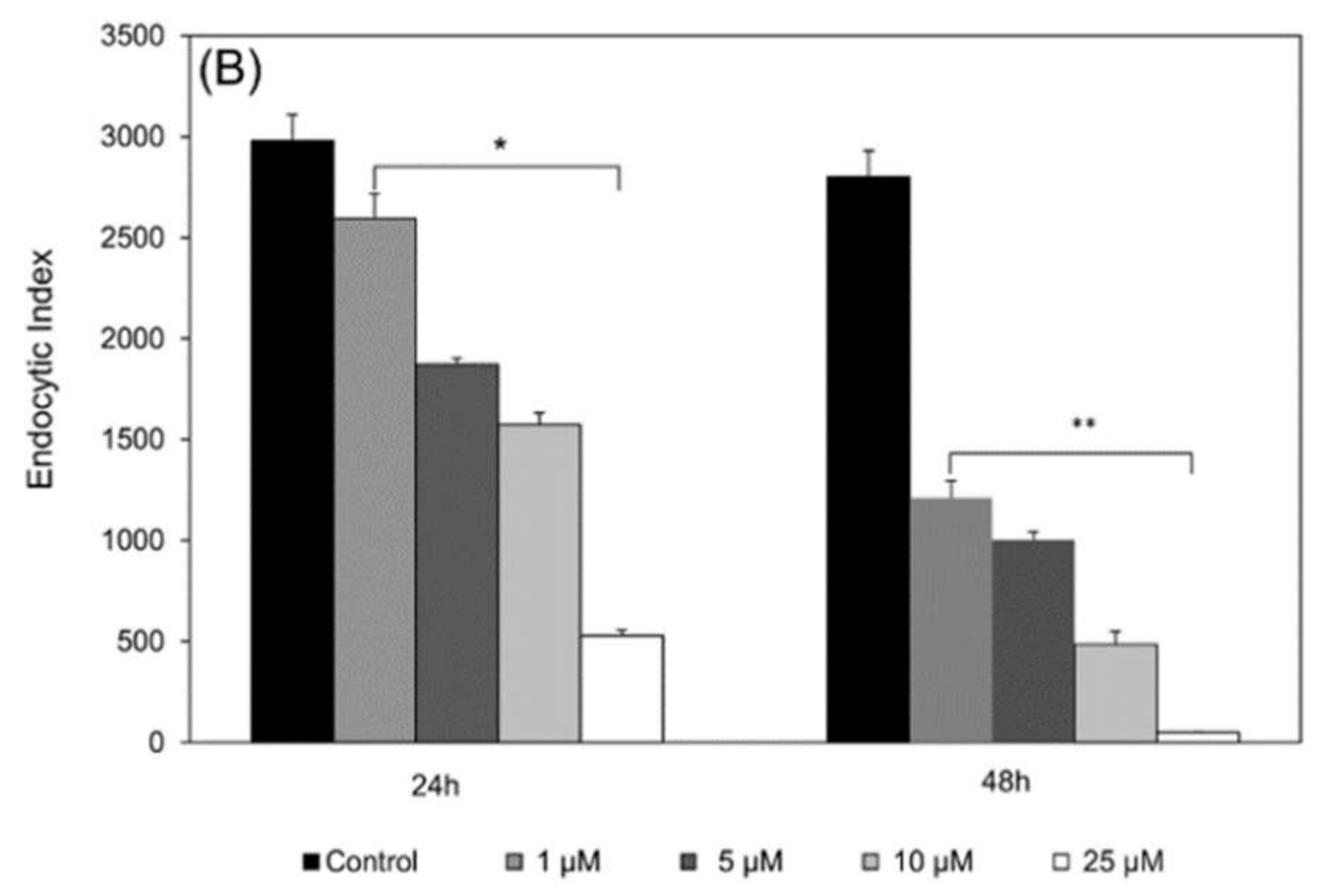
To evaluate the activity of compounds against intracellular amastigotes, macrophages were infected by promastigotes in a proportion of 10:1 (parasite:cell) for 4 h of interaction, at 37 °C followed by washing with PBS and addition of RPMI medium containing 5% FCS. After 4 h of infection, the cultures were treated for 24 h or 48 h at 37 °C with epoxymethoxylawsone (1 to 25 µM) and meglumine antimoniate (0.082 to 4.1 µM) and then, fixed with 100% methanol and Giemsa-stained. The level of infection and number of intracellular parasites was determined by random counting of at least 300 cells. The endocytic index was calculated by multiplying the percentage of infected cells by the mean number of parasites per infected cell. Drug inhibition concentrations (IC50) were calculated by regression analysis of dose-response curves. Selectivity index (SI) was calculated by the following formula: SI = CC50 (macrophage cytotoxicity) divided by IC50 (antiparasitic activity) for both drugs. The experiments were carried out in triplicate.
4.6. Toxicity to Mammalian Cells
BALB/c mice macrophages grown on 96-well plates were treated with epoxymethoxy-lawsone (1.6 to 100 µM) and meglumine antimoniate (4.1–200.0 µM of Sb5+) for 72 h at 37 °C. Then, macrophages viability was determined by incubation with CellTiter-Glo® (20 μL/well) for 3 minutes at room temperature under agitation. Luminescence was measured using a FlexStation 3 reader (Molecular Devices). The CC50, concentration of compound that reduces 50% of mammalian cell viability was determined by linear regression. Control was incubated with dimethyl sulfoxide (DMSO) in concentrations ≤1%.
4.7. Experimental Murine Infection
Experimental infection was conducted with 5- to 7-week-old BALB/c mice weighing approximately 22 g. Mice were inoculated in the footpad of the left hind limb with 1.0 × 104 promastigotes of L. (L.) amazonensis in the stationary growth phase (after 5 days of culture in Schneider’s medium) in a total volume of 50 μL of phosphate-buffered saline (PBS) at 10 mM.
4.8. Mice Treatment Schedules
The experimental treatments were performed with either antimoniate meglumine, as a comparative control for treatment efficacy and epoxymethoxylawsone diluted in a mixture of DMSO/propylene glycol/saline (1:9:10, defined as vehicle) in the following doses: 22.7 mg of Sb(V)Kg/day, 2.27 mg of Sb(V)/Kg/day and 0.23 mg of Sb(V)/Kg/day (corresponding to 4.1 µM, 0.41 µM and 0.041 µM of Sb(V)) and 11.4 mg/Kg/day, 1.14 mg/Kg/day and 0.11 mg/Kg/day (corresponding to 1.2 µM, 0.12 µM, and 0.012 µM of oxirane compound). Drugs were administered subcutaneously in the dorsal region of each mouse in a dose of 100 µL per animal. Treatments were carried out for four weeks with daily injections (five consecutive days with a two-day pause until 20 doses), starting four weeks after challenge infection, when the paw lesions had already become noticeable. Negative-control groups were treated with vehicle used to dissolve the oxirane compound. The lesions were evaluated weekly by measuring the height and width of the paw and calculating lesion areas (obtained by multiplying these measures in mm2) with a digital caliper.
4.9. Processing of Samples for Transmission Electron Microscopy
Lesions were excised with a surgical scissors after 20 days of treatment (50 days post infection), washed in PBS and fixed in 2.5% glutaraldehyde in 0.1M sodium cacodylate buffer pH 7.2 with 3.5% sucrose for 1h/4 °C. Samples were post-fixed with 1% OsO4 (1 h/4 °C) in cacodylate buffer and, after washing, the samples were dehydrated in serial acetone concentrations (30%, 50%, 70%, 90% and 100%). Finally, samples were embedded in PolyBed 812 resin and polymerized at 72 h/ 60 °C. After polymerization, semi-thin sections were made in an Ultracut S ultramicrotome (Leica, Vienna, Austria) stained with toluidine blue and eosin and analyzed under a light microscope - Axio imager 2 (Zeiss, Göttingen, Germany). After selecting the areas of interest, ultra-thin sections were obtained in Leica Ultracut S ultramicrotome, collected in 300 mesh copper grids, contrasted with 5% and lead citrate and analyzed in the transmission electron microscope - JEOL JEM-1011 (Boston, MA, USA).
4.10. Semiquantitative Analysis of Fragments
All fragments were semiquantitatively assessed based on the intensity and focal or diffuse character of the infection, considering presence of vacuoles and quantity of amastigotes and the results were plotted as the media of both amastigote number and vacuoles in macrophages. For each fragment a parameter was assigned with a numerical value between + and ++++, according to the intensity and extent of the infection: + = mild, ++ = moderate, +++ = severe, and ++++ = severe-diffuse. The samples were analyzed under light microscope (Zeiss Axio imager m2).
4.11. Ethical Aspects
Mice experimental procedures performed here were approved by the Committee for the Ethical Use of Animals of Instituto Oswaldo Cruz (L-052/2015). The animals were obtained from the animal breeding center of Fundação Oswaldo Cruz (Fiocruz). The additional assays with human cells from healthy donors were approved by the Committee of Ethics Fiocruz (C.E. Fiocruz protocol number 535/09).
4.12. Statistical Analysis
To compare results, Student’s test was applied; data matrices were considered statistically different when the P value was less than 0.05. Statistical analyses were performed using GraphPad Prism version 5.03 (GraphPad Software, San Diego, CA, USA).