Microwave-Assisted Oxalic Acid Pretreatment for the Enhancing of Enzyme Hydrolysis in the Production of Xylose and Arabinose from Bagasse
Abstract
:1. Introduction
2. Results and Discussion
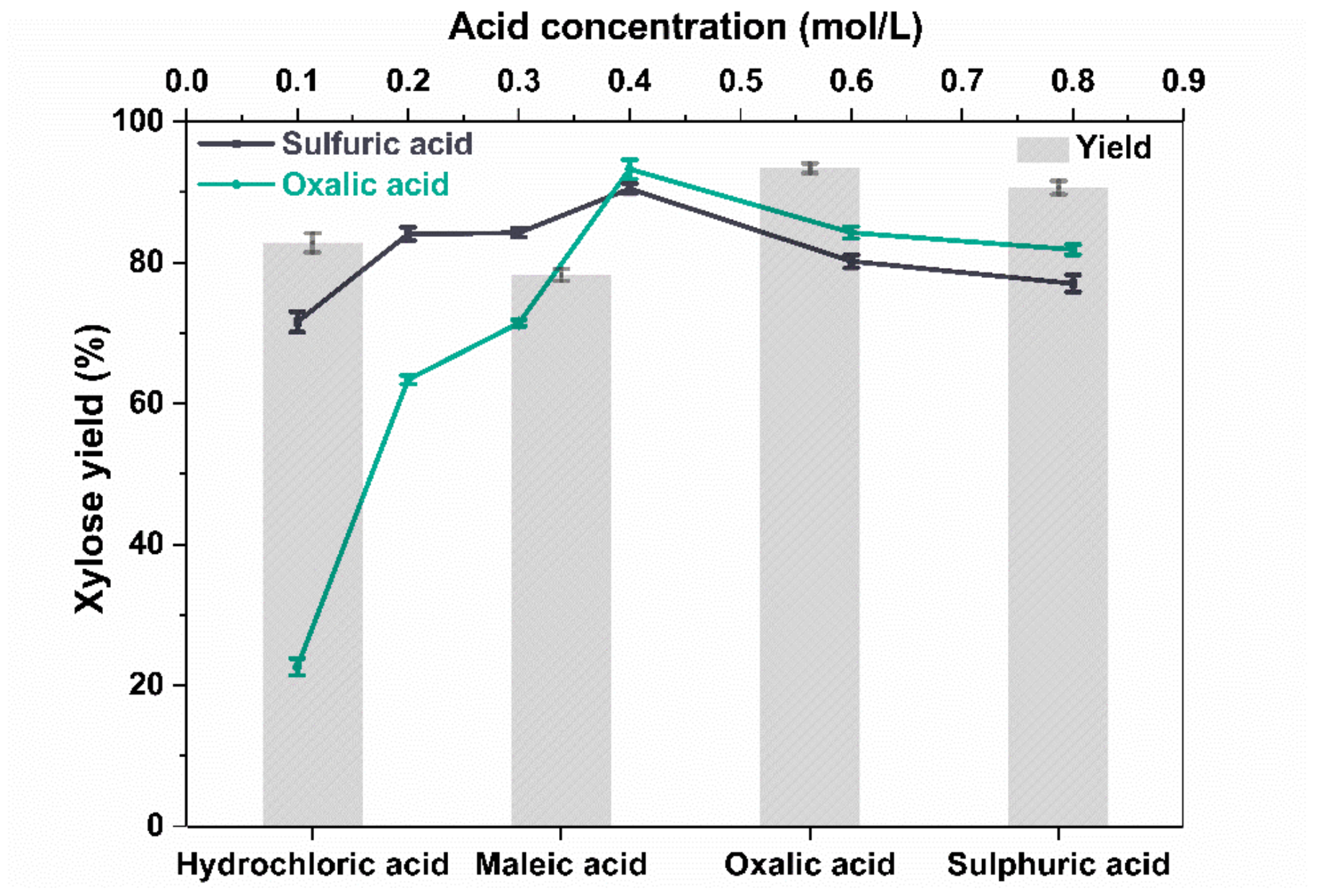
2.1. Influence of Different Acids on Xylose Yield
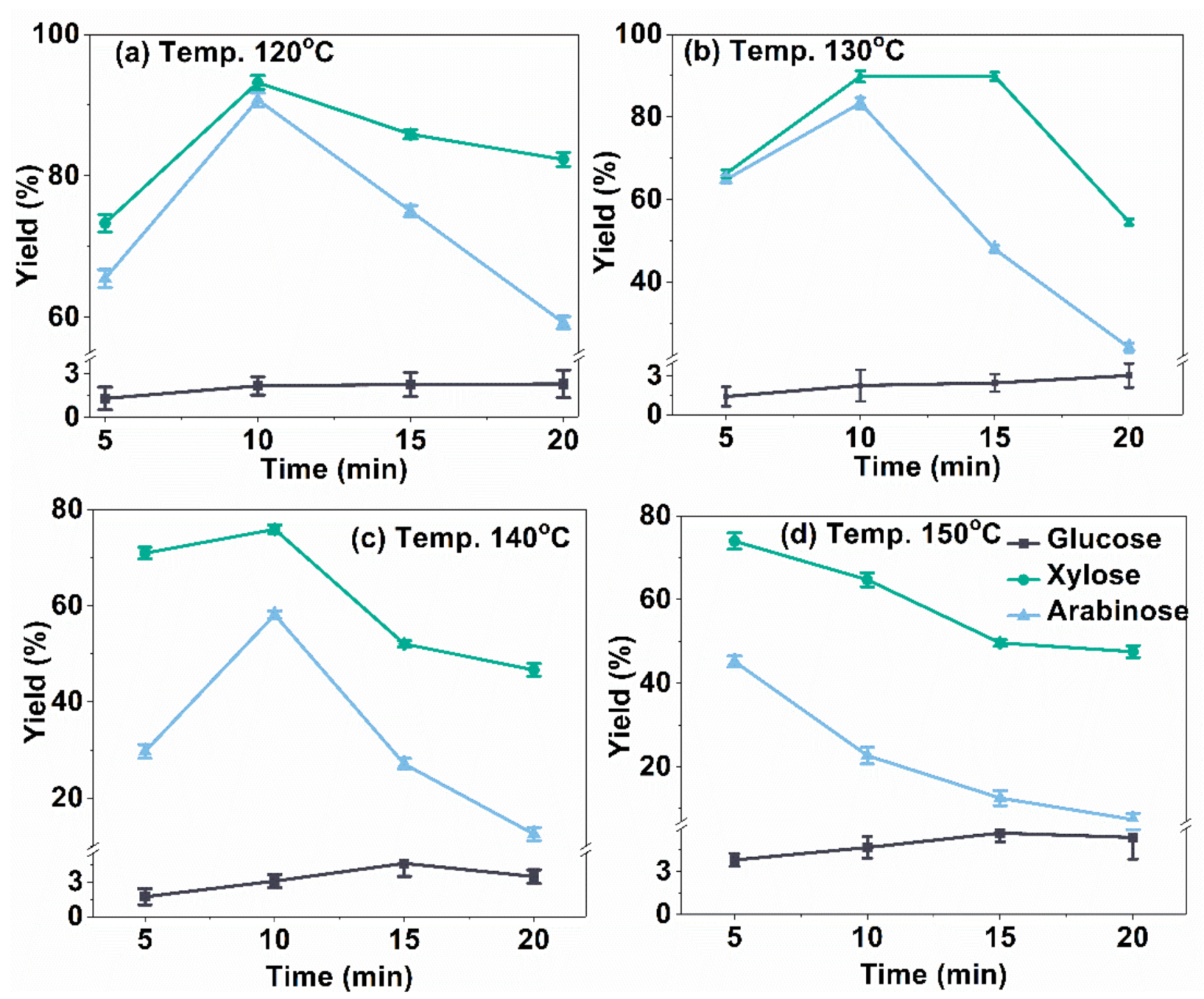
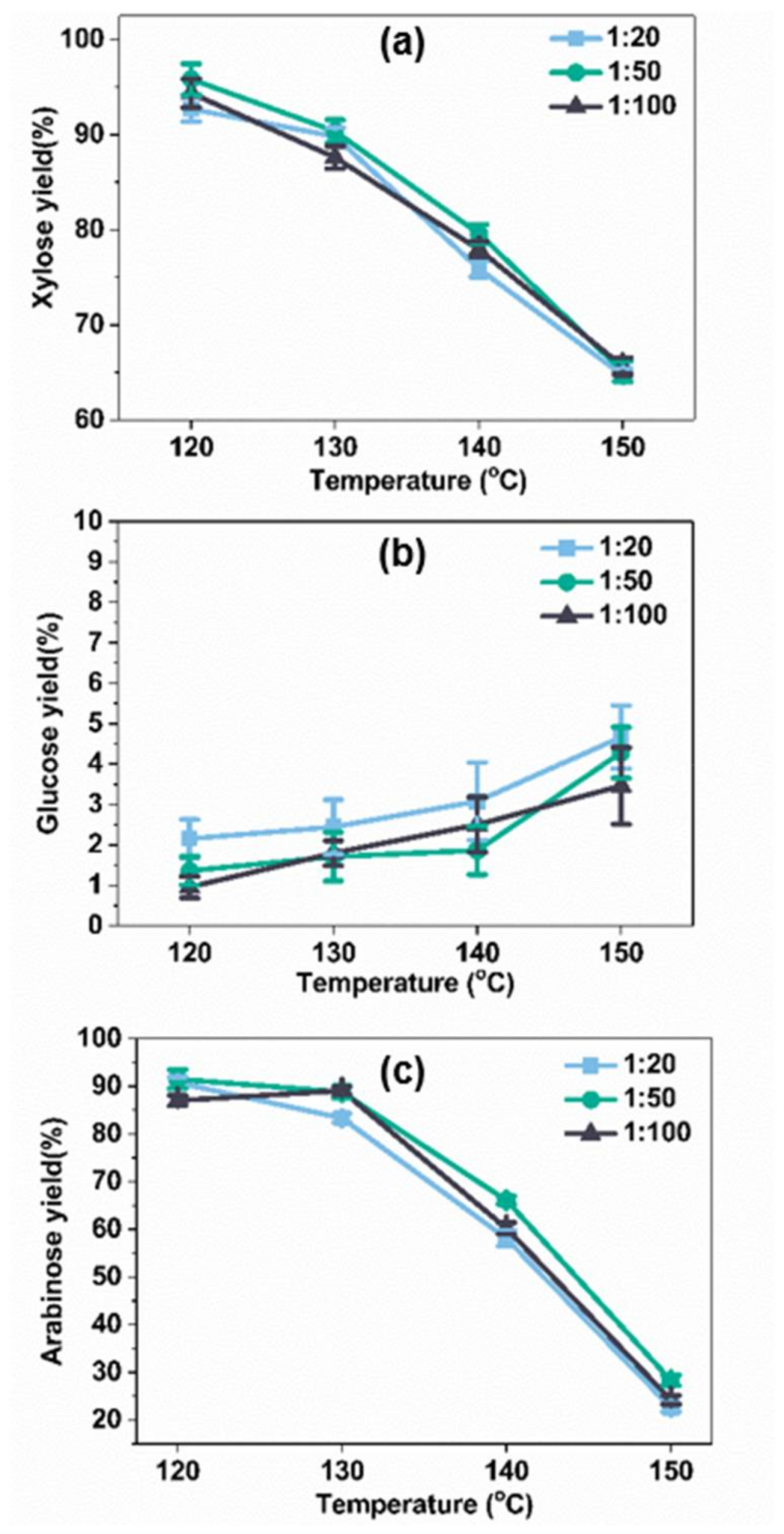
2.2. Influence of Temperature and Time on C5 and C6 Sugars Yield
2.3. Characterizations of the Untreated and Pretreated Bagasse
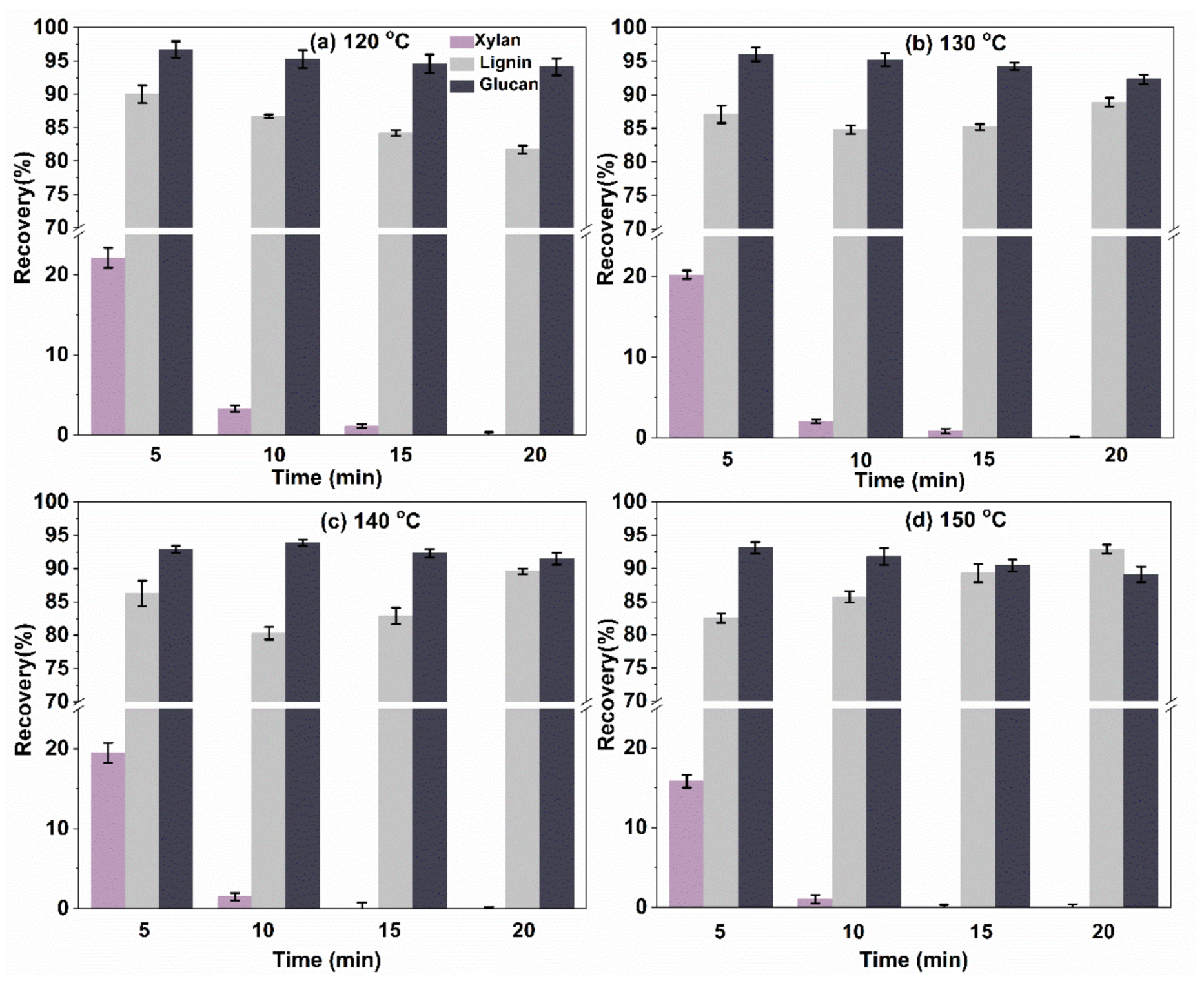
2.3.1. Composition Analysis of the Pretreated Solid Residual
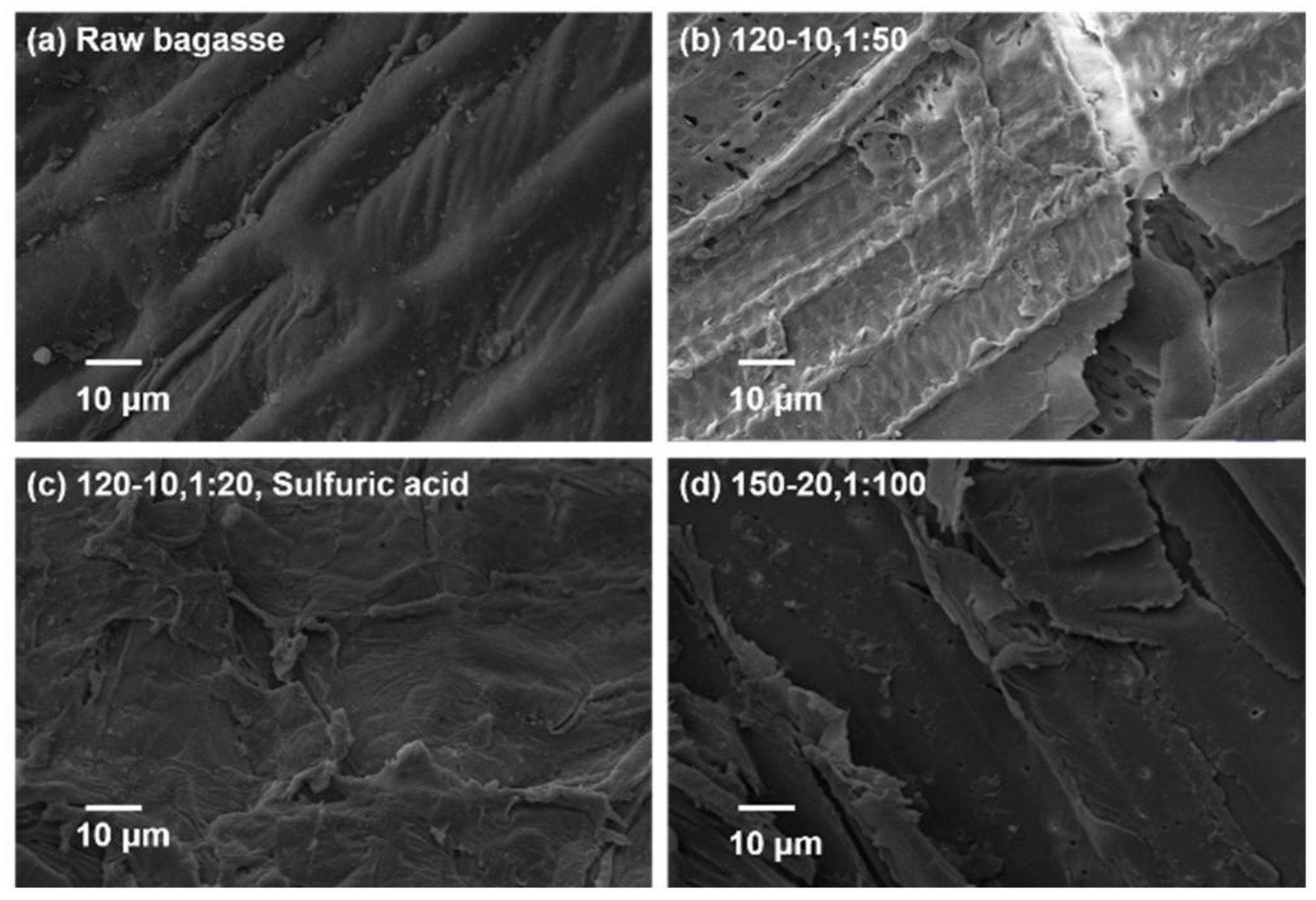
2.3.2. SEM Analysis
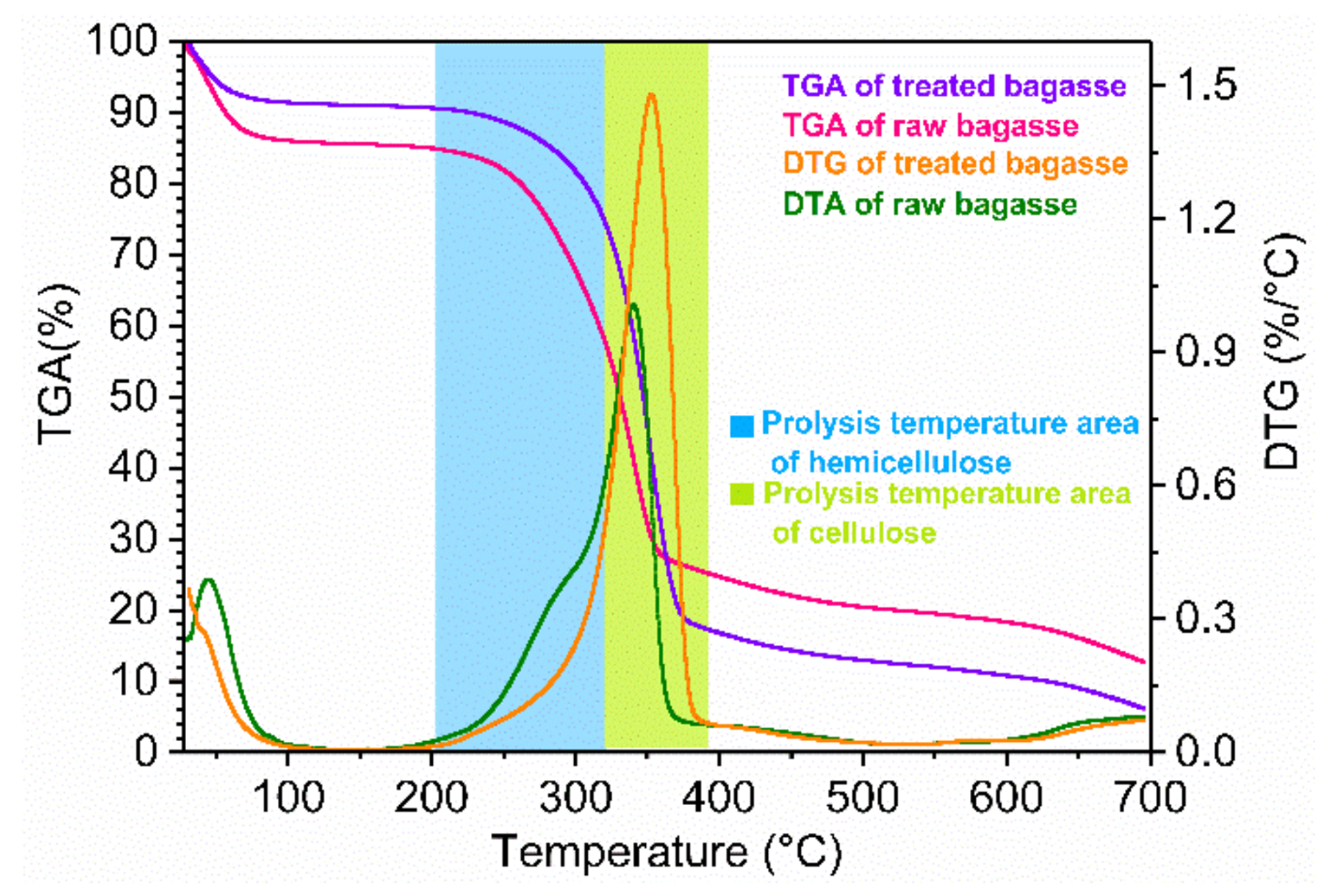
2.3.3. TGA and DTG Analysis
2.4. Enzymatic Hydrolysis Analysis
3. Materials and Methods
3.1. Materials
3.2. Microwave-Assisted Acid Pretreatment of Sugarcane Bagasse
3.3. Enzymatic Hydrolysis of Pretreated Bagasse
3.4. Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mou, H.Y.; Orblin, E.; Kruus, K.; Fardim, P. Topochemical pretreatment of wood biomass to enhance enzymatic hydrolysis of polysaccharides to sugars. Bioresour. Technol. 2013, 142, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). Available online: http://faostat.fao.org/site/567/desktopdefault.aspx#ancor (accessed on 30 March 2018).
- Hu, X.M.; Xiao, Y.B.; Niu, K.; Zhao, Y.; Zhang, B.X.; Hu, B.Z. Functional ionic liquids for hydrolysis of lignocellulose. Carbohydr. Polym. 2013, 97, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, L.; Wang, L.; Zhan, W.; Zhou, H. Irradiation pretreatment facilitates the achievement of high total sugars concentration from lignocellulose biomass. Bioresour. Technol. 2017, 232, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M. Enzymatic Conversion of Lignocellulose into Sugars. Prog. Chem. 2009, 21, 1070–1074. [Google Scholar]
- Guragain, Y.N.; Wang, D.; Vadlani, P.V. Appropriate biorefining strategies for multiple feedstocks: Critical evaluation for pretreatment methods, and hydrolysis with high solids loading. Renew. Energy 2016, 96, 832–842. [Google Scholar] [CrossRef]
- Xie, S.X.; Qin, X.; Cheng, Y.B.; Laskar, D.; Qiao, W.C.; Sun, S.; Reyes, L.H.; Wang, X.; Dai, S.Y.; Sattler, S.E. Simultaneous conversion of all cell wall components by an oleaginous fungus without chemi-physical pretreatment. Green Chem. 2015, 17, 1657–1667. [Google Scholar] [CrossRef]
- Yan, Y.H.; Li, H.L.; Ren, J.L.; Lin, Q.X.; Peng, F.; Sun, R.C.; Chen, K.F. Xylo-sugars production by microwave-induced hydrothermal treatment of corncob: Trace sodium hydroxide addition for suppression of side effects. Ind. Crops Prod. 2017, 101, 36–45. [Google Scholar] [CrossRef]
- Ertas, M.; Han, Q.; Jameel, H. Acid-catalyzed autohydrolysis of wheat straw to improve sugar recovery. Bioresour. Technol. 2014, 169, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, T.A.; Wyman, C.E. Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis of the remaining solids. Bioresour. Technol. 2005, 96, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Jeffries, T.W. Efficiencies of acid catalysts in the hydrolysis of lignocellulosic biomass over a range of combined severity factors. Bioresour. Technol. 2011, 102, 5884–5890. [Google Scholar] [CrossRef] [PubMed]
- Lawford, H.G.; Rousseau, J.D. Cellulosic fuel ethanol—Alternative fermentation process designs with wild-type and recombinant zymomonas mobilis. Appl. Biochem. Biotechnol. 2003, 106, 457–469. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladisch, M.R.; Lee, Y.Y. Coordinated development of leading biomass pretreatment technologies. Bioresour. Technol. 2005, 96, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Nimlos, M.R.; Davis, M.; Johnson, D.K.; Himmel, M.E. Ab initio molecular dynamics simulations of beta-d-glucose and beta-d-xylose degradation mechanisms in acidic aqueous solution. Carbohydr. Res. 2005, 340, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Vom Stein, T.; Grande, P.M.; Kayser, H.; Sibilla, F.; Leitner, W.; Dominguez de Maria, P. From biomass to feedstock: One-step fractionation of lignocellulose components by the selective organic acid-catalyzed depolymerization of hemicellulose in a biphasic system. Green Chem. 2011, 13, 1772–1777. [Google Scholar] [CrossRef]
- Zhang, T.; Kumar, R.; Wyman, C.E. Sugar yields from dilute oxalic acid pretreatment of maple wood compared to those with other dilute acids and hot water. Carbohydr. Polym. 2013, 92, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Rodrigues, R.C.L.B.; Jeffries, T.W. Simultaneous saccharification and ethanol fermentation of oxalic acid pretreated corncob assessed with response surface methodology. Bioresour. Technol. 2009, 100, 6307–6311. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wang, Y.; Wang, C.; Ma, D.; Fang, X. Combination use of microwave irradiation and ionic liquid in enzymatic isomerization of xylose to xylulose. J. Mol. Catal. B Enzym. 2012, 79, 8–14. [Google Scholar] [CrossRef]
- Hernouxvillière, A.; Lassi, U.; Hu, T.; Paquet, A.; Rinaldi, L.; Cravotto, G.; Molinaboisseau, S.; Marais, M.F.; Lévêque, J.M. Simultaneous Microwave/Ultrasound-Assisted Hydrolysis of Starch-Based Industrial Waste into Reducing Sugars. ACS Sustain. Chem. Eng. 2013, 1, 418–429. [Google Scholar]
- Bundhoo, Z.M.A. Microwave-assisted conversion of biomass and waste materials to biofuels. Renew. Sustain. Energy Rev. 2018, 82, 1149–1177. [Google Scholar] [CrossRef]
- Da, V.S.L.; Lópezsotelo, J.B.; Correaguimarães, A.; Hernándeznavarro, S.; Sánchezbascones, M.; Navasgracia, L.M.; Martínramos, P.; Pérezlebeña, E.; Martíngil, J. A kinetic study on microwave-assisted conversion of cellulose and lignocellulosic waste into hydroxymethylfurfural/furfural. Bioresour. Technol. 2015, 180, 88–96. [Google Scholar]
- Zuin, V.G.; Ramin, L.Z. Green and sustainable separation of natural products from agro-industrial waste: Challenges, potentialities, and perspectives on emerging approaches. Top. Curr. Chem. 2018, 376, 3–57. [Google Scholar] [CrossRef] [PubMed]
- Pratt, C.W.; Cornely, K. Essential Biochemistry 3E with 1 Semester Sapling RC Set; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Lee, J.; Rodrigues, R.; Kim, H.; Choi, I.; Jeffries, T. The roles of xylan and lignin in oxalic acid pretreated corncob during separate enzymatic hydrolysis and ethanol fermentation. Bioresour. Technol. 2010, 101, 4379–4385. [Google Scholar] [CrossRef] [PubMed]
- Kolthoff, I.M.; Elving, P.J. Treatise on Analytical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 1962; p. 294. [Google Scholar]
- Umbreit, W. Data for Biochemical Research; Clarendon Press: Oxford, UK, 1986. [Google Scholar]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Dias, A.S.; Lima, S.; Carriazo, D.; Rives, V.; Pillinger, M.; Valente, A.A. Exfoliated titanate, niobate and titanoniobate nanosheets as solid acid catalysts for the liquid-phase dehydration of d-xylose into furfural. J. Catal. 2006, 244, 230–237. [Google Scholar] [CrossRef]
- Na, B.I.; Lee, J.W. Kinetic study on the dilute acid catalyzed hydrolysis of waste mushroom medium. J. Ind. Eng. Chem. 2014, 25, 176–179. [Google Scholar] [CrossRef]
- Weingarten, R.; Tompsett, G.A.; Conner, W.C., Jr.; Huber, G.W. Design of solid acid catalysts for aqueous-phase dehydration of carbohydrates: The role of Lewis and Brønsted acid sites. J. Catal. 2011, 279, 174–182. [Google Scholar] [CrossRef]
- Deng, A.; Ren, J.; Wang, W.; Li, H.; Lin, Q.; Yan, Y.; Sun, R.; Liu, G. Production of xylo-sugars from corncob by oxalic acid-assisted ball milling and microwave-induced hydrothermal treatments. Ind. Crops Prod. 2016, 79, 137–145. [Google Scholar] [CrossRef]
- Li, H.; Deng, A.; Ren, J.; Liu, C.; Qi, L.; Zhong, L.; Feng, P.; Sun, R. Catalytic hydrothermal pretreatment of corncob into xylose and furfural via solid acid catalyst. Bioresour. Technol. 2014, 158, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.L.; Geng, Z.C.; Liu, C.F.; Xu, F.; Sun, J.X.; Sun, R.C. Fractional isolation and structural characterisation of hemicellulosic polymers from delignified and ultrasonic irradiated sugarcane bagasse. E-Polymers 2006, 6, 855–866. [Google Scholar] [CrossRef]
- Xu, F.; Sun, J.X.; Liu, C.F.; Sun, R.C. Comparative study of alkali- and acidic organic solvent-soluble hemicellulosic polysaccharides from sugarcane bagasse. Carbohydr. Res. 2006, 341, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Mou, H.-Y.; Heikkilä, E.; Fardim, P. Topochemistry of environmentally friendly pretreatments to enhance enzymatic hydrolysis of sugar cane bagasse to fermentable sugar. J. Agric. Food Chem. 2014, 62, 3619. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.R.; Morinelly, J.E. Effects of dilute acid pretreatment conditions on enzymatic hydrolysis monomer and oligomer sugar yields for aspen, balsam, and switchgrass. Bioresour. Technol. 2010, 101, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wu, S.; Liu, L. Combination of liquid hot water pretreatment and wet disk milling to improve the efficiency of the enzymatic hydrolysis of eucalyptus. Bioresour. Technol. 2013, 128, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Barisik, G.; Isci, A.; Kutlu, N.; Bagder Elmaci, S.; Akay, B. Optimization of organic acid pretreatment of wheat straw. Biotechnol. Prog. 2016, 32, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; De, S.; Saha, B.; Alam, M.I. ChemInform Abstract: Advances in Conversion of Hemicellulosic Biomass to Furfural and Upgrading to Biofuels. Catal. Sci. Technol. 2012, 2, 2025–2036. [Google Scholar] [CrossRef]
- Kumar, V.; Satyanarayana, T. Generation of xylooligosaccharides from microwave irradiated agroresidues using recombinant thermo-alkali-stable endoxylanase of the polyextremophilic bacterium Bacillus halodurans expressed in Pichia pastoris. Bioresour. Technol. 2015, 179, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qi, W.; Liu, R.; Su, R.; Wu, S.; He, Z. Fractionating lignocellulose by formic acid: Characterization of major components. Biomass Bioenergy 2010, 34, 525–532. [Google Scholar] [CrossRef]
- Chen, W.H.; Ye, S.C.; Sheen, H.K. Hydrolysis characteristics of sugarcane bagasse pretreated by dilute acid solution in a microwave irradiation environment. Appl. Energy 2012, 93, 237–244. [Google Scholar] [CrossRef]
- Tominaka, S.; Momma, T.; Scrosati, B.; Osaka, T. Sulfated zirconia as a proton conductor for fuel cells: Stability to hydrolysis and influence on catalysts. J. Power Sources 2010, 195, 4065–4071. [Google Scholar] [CrossRef]
- Yu, G.; Li, B.; Liu, C.; Zhang, Y.; Wang, H.; Mu, X. Fractionation of the main components of corn stover by formic acid and enzymatic saccharification of solid residue. Ind. Crops Prod. 2013, 50, 750–757. [Google Scholar] [CrossRef]
- Avci, A.; Saha, B.C.; Kennedy, G.J.; Cotta, M.A. Dilute sulfuric acid pretreatment of corn stover for enzymatic hydrolysis and efficient ethanol production by recombinant Escherichia coli FBR5 without detoxification. Bioresour. Technol. 2013, 142, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Alvira, P.; Negro, M.J.; Ballesteros, M. Effect of endoxylanase and α-l-arabinofuranosidase supplementation on the enzymatic hydrolysis of steam exploded wheat straw. Bioresour. Technol. 2011, 102, 4552–4558. [Google Scholar] [CrossRef] [PubMed]
- Rollin, J.A.; Zhu, Z.; Sathitsuksanoh, N.; Zhang, Y.H. Increasing cellulose accessibility is more important than removing lignin: A comparison of cellulose solvent-based lignocellulose fractionation and soaking in aqueous ammonia. Biotechnol. Bioeng. 2011, 108, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.B.; Wang, L.; Liu, D.H. Effect of several factors on peracetic acid pretreatment of sugarcane bagasse for enzymatic hydrolysis. J. Chem. Technol. Biotechnol. 2010, 82, 1115–1121. [Google Scholar] [CrossRef]
- Liu, L.; Sun, J.; Li, M.; Wang, S.; Pei, H.; Zhang, J. Enhanced enzymatic hydrolysis and structural features of corn stover by FeCl3 pretreatment. Bioresour. Technol. 2009, 100, 5853–5858. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wu, S.; Xu, S. Enhancement of enzymatic saccharification of bagasse by ethanol-based organosolv auto-catalyzed pretreatment. J. Chem. Technol. Biotechnol. 2016, 92, 570–577. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Zhang, C.; Lin, Q.; Wang, X.; Cheng, B.; Li, H.; Ren, J. Microwave-Assisted Oxalic Acid Pretreatment for the Enhancing of Enzyme Hydrolysis in the Production of Xylose and Arabinose from Bagasse. Molecules 2018, 23, 862. https://doi.org/10.3390/molecules23040862
Yan Y, Zhang C, Lin Q, Wang X, Cheng B, Li H, Ren J. Microwave-Assisted Oxalic Acid Pretreatment for the Enhancing of Enzyme Hydrolysis in the Production of Xylose and Arabinose from Bagasse. Molecules. 2018; 23(4):862. https://doi.org/10.3390/molecules23040862
Chicago/Turabian StyleYan, Yuhuan, Chunhui Zhang, Qixuan Lin, Xiaohui Wang, Banggui Cheng, Huiling Li, and Junli Ren. 2018. "Microwave-Assisted Oxalic Acid Pretreatment for the Enhancing of Enzyme Hydrolysis in the Production of Xylose and Arabinose from Bagasse" Molecules 23, no. 4: 862. https://doi.org/10.3390/molecules23040862




