1-(Acylamino)alkylphosphonic Acids—Alkaline Deacylation
Abstract
:1. Introduction
2. Results and Discussion
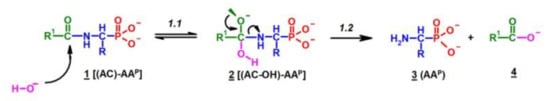
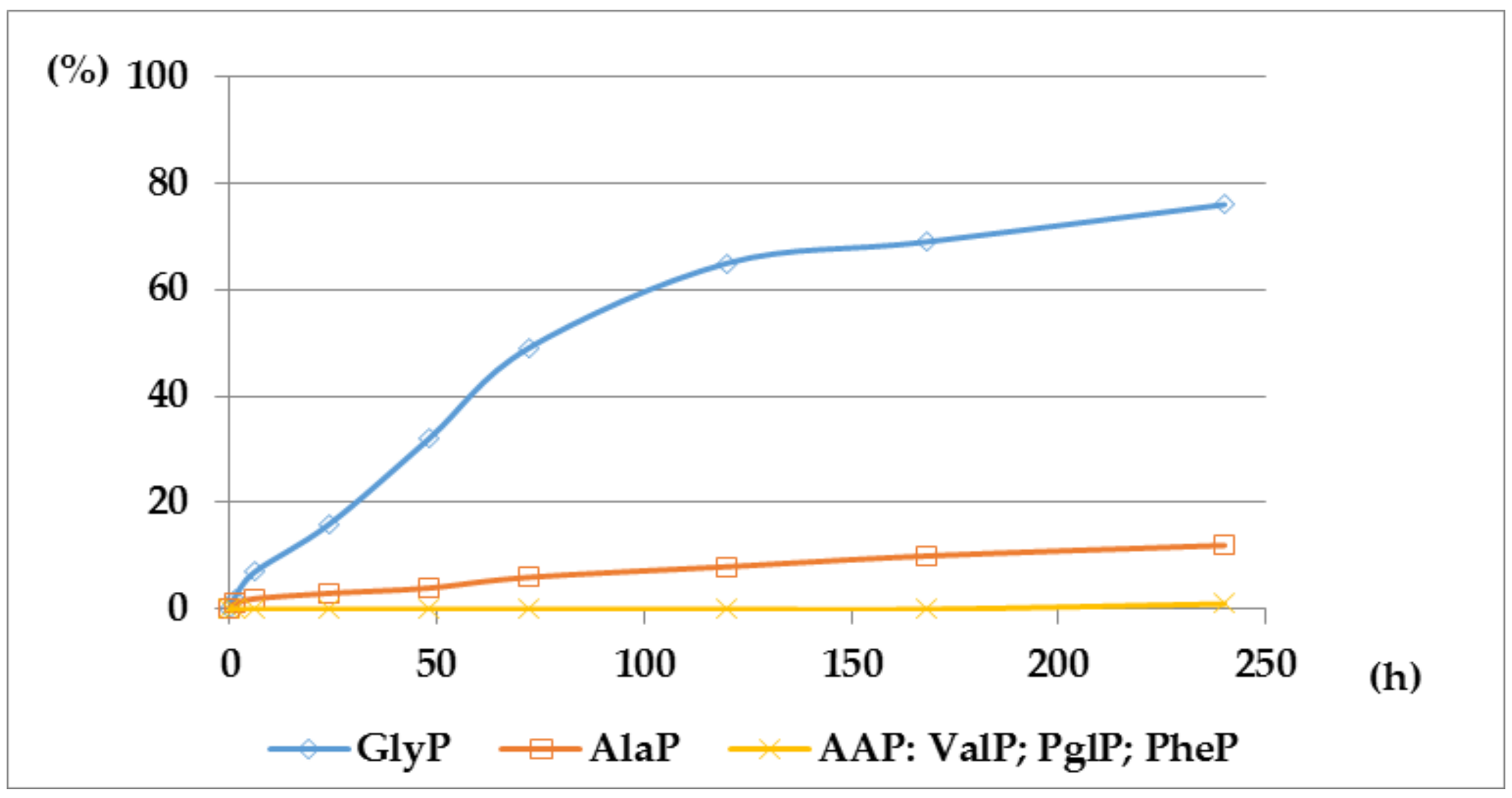
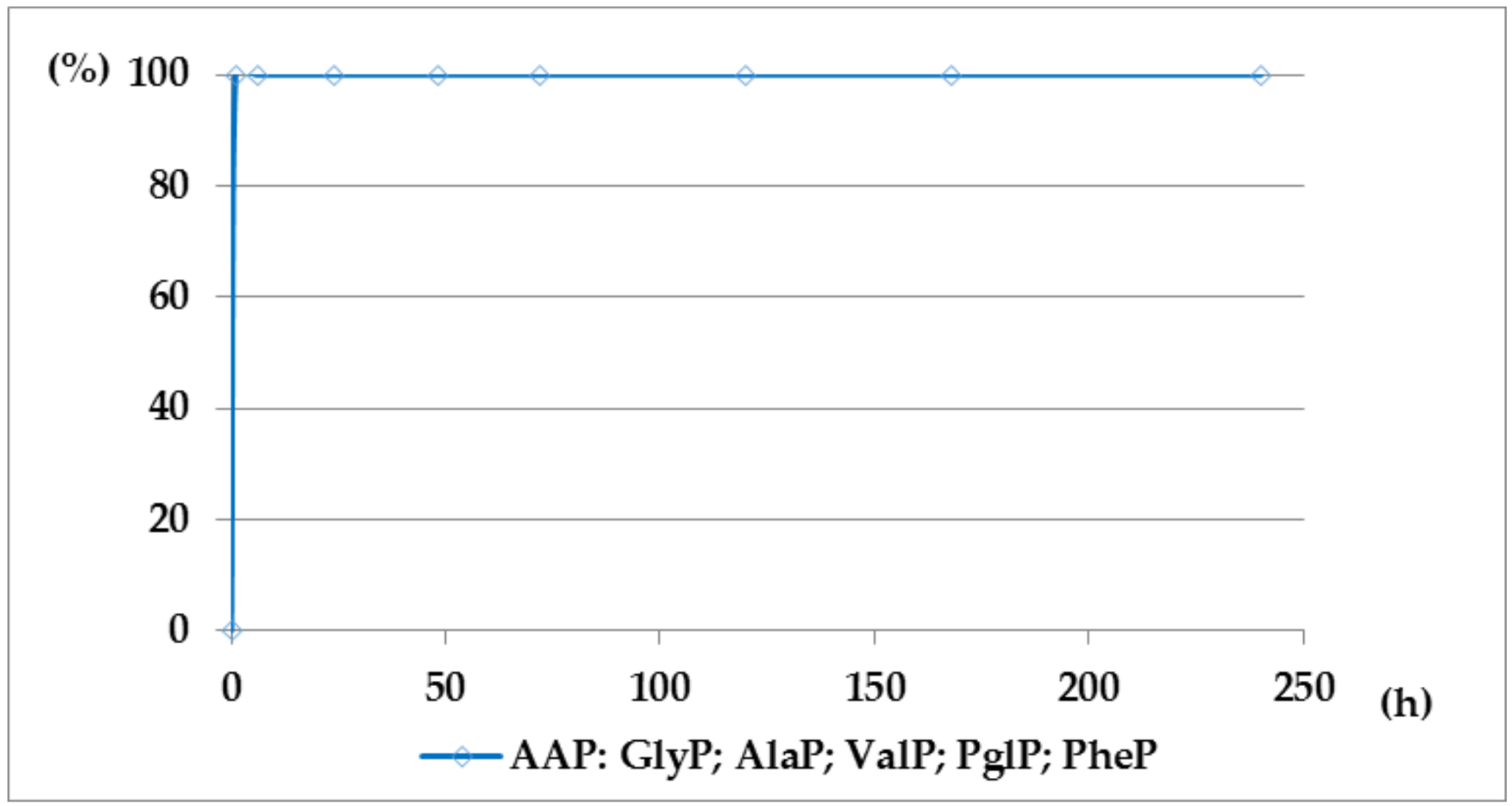
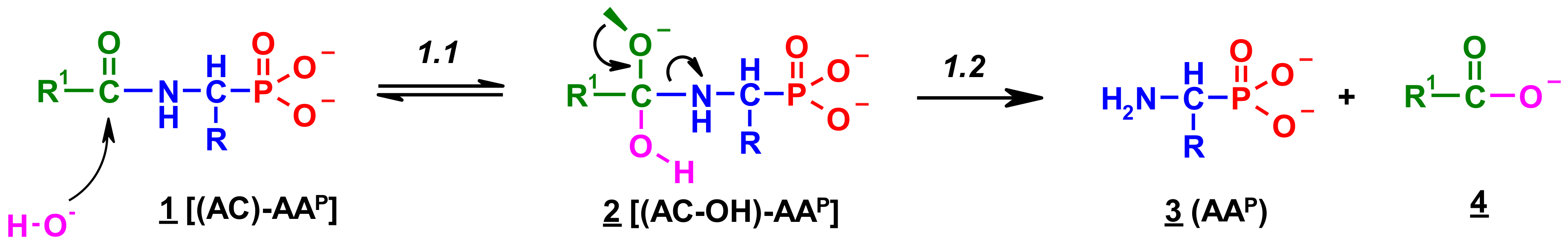
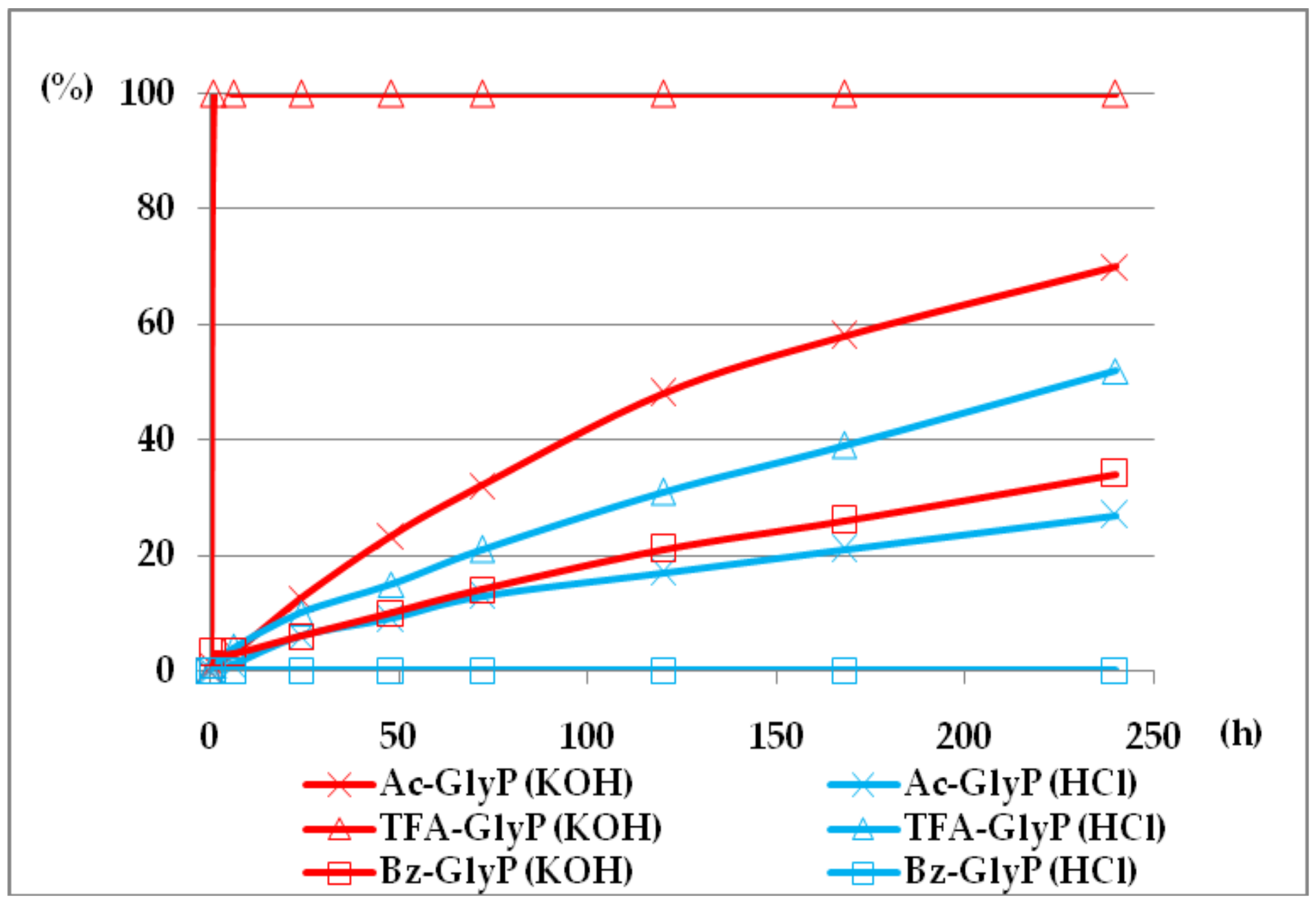
2.1. Investigations on the Deacylation Course of 1-(acylamino)alkylphosphonic Acids
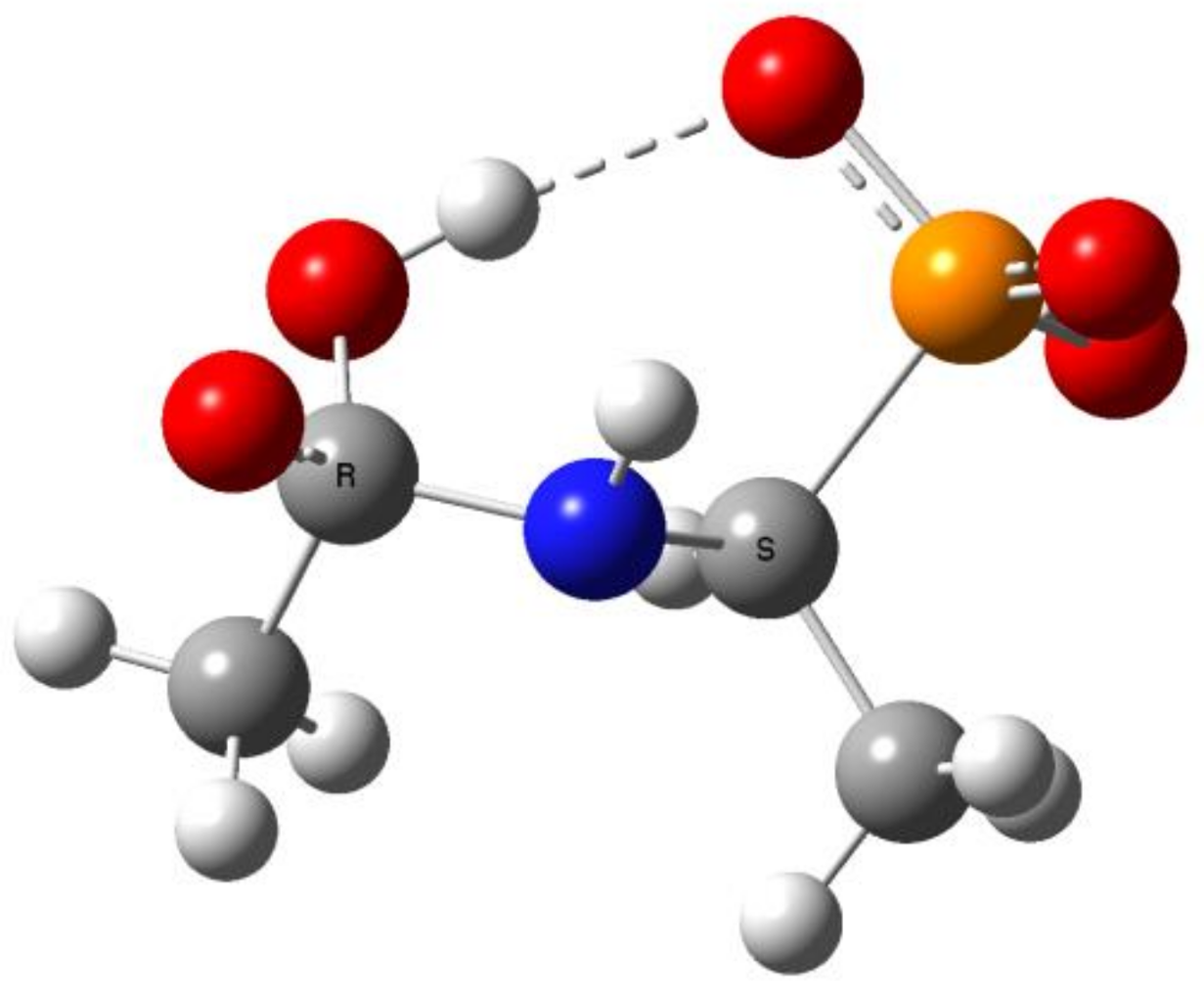
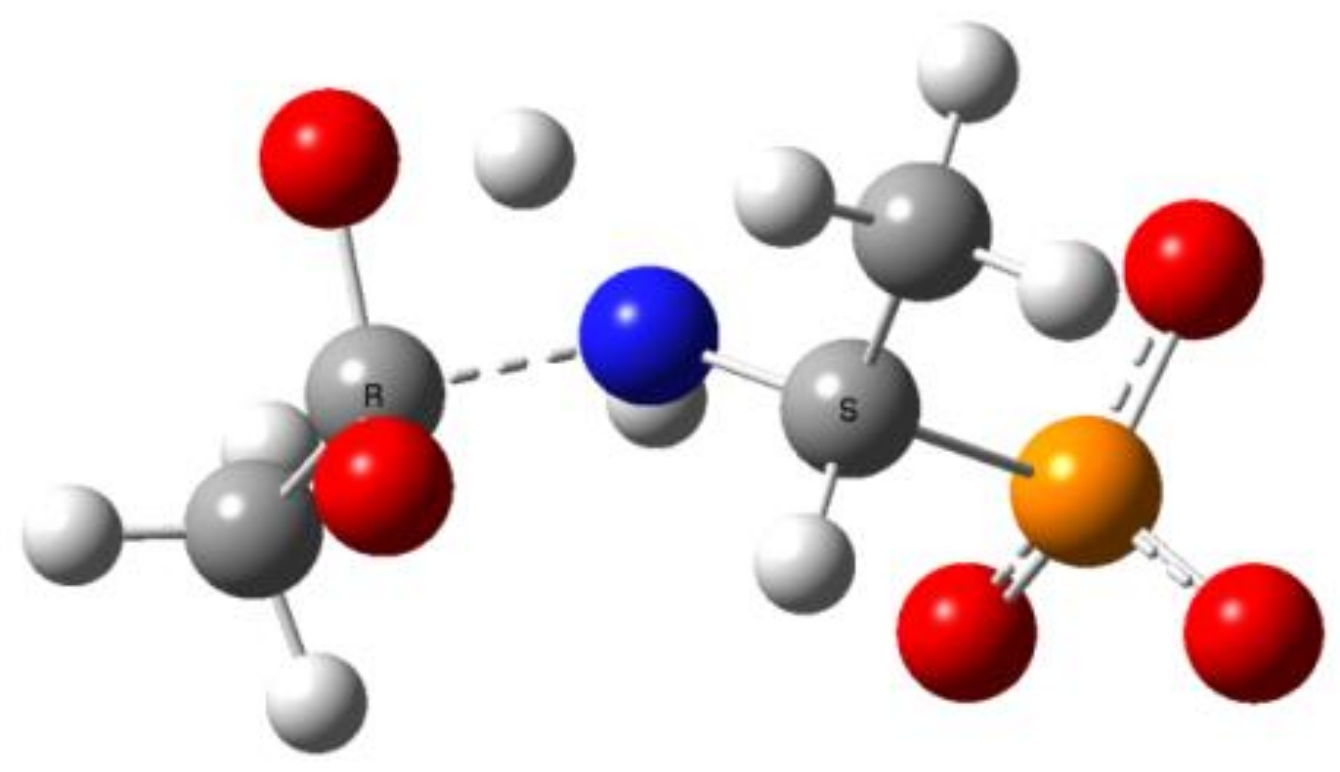
2.2. Quantum Chemical Calculations for Deacylation of (AC)-AAP in Basic Conditions
3. Materials and Methods
3.1. General Information
3.2. Investigated Compounds
3.3. Reaction of Deacylation of 1-(acylamino)alkylphosphonic Acids
3.4. Computational Methods
4. Conclusions
- The alkaline deacylation of (AC)-AAP occurs through the hydroxyl adduct intermediates, which was observed on 31P NMR spectra of the reaction mixtures;
- The deacylation ability of investigated (AC)-AAP derivatives exhibited strong dependence of electron-acceptor character of the acyl group, with CF3-C(O)- > CH3-C(O)-;
- The 1-(acylamino)alkylphosphonic acids are present in aqueous solutions with substantial stability at ambient temperatures, which decreases with temperature elevation;
- The deacylation of (AC)-AAP increases substantially in basic (2 M KOH) solutions;
- The lowest deacylation ability (highest stability) was found for the 1-(benzoylamino acids Bz-AAP;
- For the same type of 1-(acylamino)-derivatives (AC)-AAP, the highest stability was found in(AC)-ValP and (AC)-PheP; lower stability in (AC)-AlaP; and the lowest in (AC)-GlyP and (AC)-PglP;
- All examined 31P NMR spectra of deacylation mixtures did not reveal any trace of H3PO3 and/orH3PO4, which represent the products of dephosphonylation or oxidative dephosphonylation of (AC)-AAP.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kukhar, V.P.; Hudson, H.R. (Eds.) Aminophosphonic and Aminophosphinic Acids: Chemistry and Biological Activity; J. Wiley & Sons, Ltd.: Chichester, UK; New York, NY, USA; Weinheim, Germany; Brisbane, Australia; Singapore; Toronto, ON, Canada, 2000; ISBN 0-471-89149-5. [Google Scholar]
- Kittredge, J.S.; Roberts, E. A carbon-phosphorus compounds in nature. Science 1969, 164, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kafarski, P.; Mastalerz, P. Aminophosphonates, Natural Occurrence, Biochemistry and Biological Properties; Beiträge zur Wirkstofforschung; Institut für Wirkstofforschung: Berlin, Germany, 1984; Volume 24, pp. 1–110. [Google Scholar]
- Horiguchi, M.; Kandatsu, M. Isolation of 2-aminoethyl phosphonic amid from rumen protozoa. Nature 1959, 184, 901–902. [Google Scholar] [CrossRef] [PubMed]
- Kittredge, J.S.; Hughes, R.R. Occurence of α-amino-β-phosphono-propionic acid in the zoanthid, zoanthussociatus, and the ciliate, Tetrahymena pyrifornis. Biochemistry 1964, 3, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Korn, E.D.; Deaborn, D.G.; Falles, H.M.; Sokoloski, E.A. A major polysaccharide constituents of the amoeba plasma membrane contains 2-aminoethylphosphonic acid and 1-hydroxy-2-aminoethyl-phosphonic acid. J. Biol. Chem. 1973, 248, 2257–2259. [Google Scholar] [PubMed]
- Kido, Y.; Hamakado, T.; Anno, M.; Miyagawa, E.; Motoki, Y.; Wakamiya, T.; Shiba, T. Isolation and characterization of 15112, a new phosphorus containing inhibitor of angiotensin I converting enzyme produced by Actinomadura sp. J. Antibiot. 1984, 37, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Bayer, E.; Gugel, K.H.; Haegele, K.; Hagenmaier, H.; Jessipov, S.; Koenig, W.A.; Zaehner, H. Metabolic products of microorganisms. 98. Phosphinothricine and phosphinothricylo-alanylo-alanine. Helv. Chim. Acta 1972, 55, 224–239. [Google Scholar] [CrossRef] [PubMed]
- Franz, J.E. Herbicidal compositions and methods employing esters of N-phosphonoglycine. U.S. Patent US 3997860, 14 December 1976. [Google Scholar]
- Mastalerz, P. Inhibition of glutamine synthetase by phosphonic analogs of glutamic acid. Arch. Immun. Ter. Dośw. 1959, 7, 201–210. [Google Scholar]
- Engelmann, M.; Pikl, J. Phosphonic Acids Derived from Organic Acylamidomethyl Compounds. U.S. Patent 2304156, 8 December 1942. [Google Scholar]
- Allen, J.G.; Atherton, F.R.; Hall, M.J.; Hassal, C.H.; Holmes, S.W.; Lambert, R.W.; Nisbet, L.J.; Ringrose, P.S. Phosphonopeptides, a new class of synthetic antibacterial agents. Nature 1978, 272, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, N.E.; Bartlett, P.A. A phosphonamidate dipeptide analog as an inhibitor of carboxypeptidase A. J. Am. Chem. Soc. 1981, 103, 654–657. [Google Scholar] [CrossRef]
- McLeod, D.A.; Brinkworth, R.; Ashley, J.A.; Janda, K.D.; Wirsching, P. Phosphonamidates and phosphonamidate esters as HIV-1 protease inhibitors. Bioorg. Med. Chem. Lett. 1991, 1, 653–658. [Google Scholar] [CrossRef]
- Drabowicz, J.; Jakubowski, H.; Kudzin, M.H.; Kudzin, Z.H. The nomenclature of 1-aminoalkylphosphonic acids and derivatives: Evolution of the code system. Acta Biochim. Pol. 2015, 62, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Kabachnik, M.I.; Medved, T.Y.; Dyatlova, N.M.; Archipova, O.G.; Rudomino, M.W. Phosphoroorganic complexones. Usp. Khim. 1974, 43, 1554–1574. [Google Scholar] [CrossRef]
- Rizkalla, E.N. Metal chelates of phosphonate-containing ligands. Rev. Inorg. Chem. 1983, 5, 223–304. [Google Scholar]
- Nowack, B. Environmental chemistry of phosphonates. Water Res. 2003, 37, 2533–2546. [Google Scholar] [CrossRef]
- Kafarski, P.; Lejczak, B. Aminophosphonic acids of potential medical importance. Curr. Med. Chem. Anti-Cancer Agents 2001, 1, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Lejczak, B.; Kafarski, P. Biological activity of aminophosphonic acids and their short peptides. Top. Heterocycl. Chem. 2009, 20, 31–63. [Google Scholar] [CrossRef]
- Orsini, F.; Sello, G.; Sisti, M. Aminophosphonic acids and derivatives. Synthesis and biological applications. Curr. Med. Chem. 2010, 17, 264–289. [Google Scholar] [CrossRef] [PubMed]
- Mucha, A.; Kafarski, P.; Berlicki, L. Remarkable potential of the α-amino-phosphonate/phosphinate structural motif in medicinal chemistry. J. Med. Chem. 2011, 54, 5955–5980. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, J.A.; Logush, E.W. Aliphatic carbon-phosphorus compound as herbicides. In Handbook in Organophosphorus Chemistry; Engel, R., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1988; Volume Chapter 15, pp. 737–806. [Google Scholar]
- Maier, L. Phosphoroorganic detergents. Chimia 1969, 23, 323–330. [Google Scholar]
- Petrov, K.A.; Chauzov, V.A.; Erokhina, T.E. Aminoalkyl organo-phosphorus compounds. Usp. Khim. 1974, 43, 2045–2087. [Google Scholar] [CrossRef]
- Palacios, F.; Alonso, C.; de los Santos, J.M. Synthesis of β-amino-phosphonates and -phosphinates. Chem. Rev. 2005, 105, 899–931. [Google Scholar] [CrossRef] [PubMed]
- Kudzin, Z.H.; Kudzin, M.H.; Drabowicz, J.; Stevens, C. Aminophosphonic acids—Phosphorus analogues of natural amino acids. P. 1: Syntheses of α-amino-phosphonic acids. Curr. Org. Chem. 2011, 15, 2015–2071. [Google Scholar] [CrossRef]
- Kudzin, Z.H.; Kudzin, M.H.; Drabowicz, J. Thioureidoalkylphosphonates in the synthesis of 1-aminoalkylphosphonic acids. The Ptc-aminophosphonate method. Arkivoc 2011, 6, 227–269. [Google Scholar] [CrossRef]
- Ma, J.-N. Catalytic asymmetric synthesis of α- and β-amino phosphonic acid derivatives. Chem. Soc. Rev. 2006, 35, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, M.; Rojas-Cabrera, H.; Cativiela, C. An overview of stereoselective synthesis of α-aminophosphonic acids and derivatives. Tetrahedron 2009, 65, 17–49. [Google Scholar] [CrossRef] [PubMed]
- Kukhar, V.P.; Solodenko, V.A. The phosphorus analogs of aminocarboxylic acids. Usp. Khim. 1987, 56, 1504–1532. [Google Scholar] [CrossRef]
- Kudzin, Z.H.; Mokrzan, J.; Skowroński, R. Long chain 1-aminothia-alkanephosphonates, their sulphinyl and sulphonyl derivatives. A new class of complexane type surfactants. Phosphorus Sulfur Silicon Relat. Elem. 1989, 42, 41–46. [Google Scholar] [CrossRef]
- Kudzin, Z.H.; Skrzypek, S.W.; Skowroński, R.; Ciesielski, W.; Cristau, H.J.; Plenat, F. Polarographic investigations of functionalized alkanephosphonic acids. Part II. Phosphorus Sulfur Silicon Relat. Elem. 1996, 119, 201–207. [Google Scholar] [CrossRef]
- Chęcińska, L.; Kudzin, Z.H.; Małecka, M.; Nazarski, R.B.; Okruszek, A. [(Diphenoxyphosphinyl)methylidene]triphenylphosphorane—The double P+-stabilisedcarboanion: A crystallographic, computational and solution NMR comperative study on the ylidic bonding. Tetrahedron 2003, 59, 7681–7693. [Google Scholar] [CrossRef]
- Kudzin, Z.H.; Saganiak, M.; Andrijewski, G.; Drabowicz, J. Oxidation of phosphonocysteine and phosphonohomocysteine. Synthesis of phosphonocysteic and phosphonohomocysteic acids. Pol. J. Chem. 2005, 79, 529–539. [Google Scholar]
- Kudzin, Z.H.; Depczyński, R.; Andrijewski, G.; Drabowicz, J.; Łuczak, J. 1-(N-acylamino)alkanephosphonates. IV. N-acylation of 1-aminoalkanephosphonic acids. Pol. J. Chem. 2005, 79, 499–513. [Google Scholar]
- Kudzin, Z.H.; Depczyński, R.; Kudzin, M.H.; Drabowicz, J.; Łuczak, J. 1-(N-Trifluoroacetylamino)alkylphosphonic acids. Synthesis and properties. Amino Acids 2007, 33, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Kudzin, Z.H.; Depczyński, R.; Kudzin, M.H.; Drabowicz, J. 1-(N-Chloroacetylamino)alkylphosphonic acids—Synthetic precursors of glycylophosphono-peptides and related compounds. Amino Acids 2008, 34, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Drabowicz, J.; Jordan, F.; Kudzin, M.H; Kudzin, Z.H.; Urbaniak, P.; Stevens, C. Reactivity of aminophosphonic acids. oxidative dephosphonylation of 1-aminoalkylphosphonic acids by aqueous halogens. Dalton Trans. 2016, 45, 2308–2317. [Google Scholar] [CrossRef] [PubMed]
- Gancarz, R.; Kiersnowska, A.D. Associative vs. dissociative mechanism of P–C bond breaking in α-aminophosphonates leading to phosphoric acid [P(V)] derivatives. Arkivoc 2017, 2, 285–302. [Google Scholar] [CrossRef]
- Aldrich-Sigma Catalog 2016. Aminophosphonic Self-Assembled Monolayers Agents (e.g., 795798, SIK 7701-10; SIK 7701-101). Available online: https://www.sigmaaldrich.com/china-mainland.html/ (accessed on 9 April 2018).
- Kudzin, Z.H.; Łuczak, J. A facile conversion of aminoalkanephosphonic acids into O,O-dialkyl 1-(N-acylamino)alkanephosphonate derivatives. Synthesis 1995, 509–511. [Google Scholar] [CrossRef]
- Kudzin, Z.H.; Łyżwa, P.; Łuczak, J.; Andrijewski, G. Aminoalkanephosphonates. P.II. Facile conversion of 1-aminoalkanephosphonic acids their O,O-diester derivatives. Synthesis 1997, 44–47. [Google Scholar] [CrossRef]
- Svedas, V.K.; Kozlova, E.V.; Mironenko, D.A.; Kukhar, V.P.; Kasheva, T.N.; Solodenko, V.A.; Belozersky, A.N. Enzymatic hydrolysis of N-acylated 1-aminophosphonic acids. Phosphorus Sulfur Silicon Relat. Elem. 1990, 51, 411. [Google Scholar] [CrossRef]
- Solodenko, V.; Kasheva, T.; Kukhar, V.; Kozlova, E.; Mironenko, D.; Svedas, V. Preparation of optically active 1-aminoalkylphosphonic acids by stereoselective enzymatic hydrolysis of racemic N-acylated 1-aminoalkyl-phosphonic acids. Tetrahedron 1991, 47, 3989–3998. [Google Scholar] [CrossRef]
- Solodenko, V.; Belik, M.; Galushko, S.; Kukhar, V.; Kozlova, E.; Mironenko, D.; Svedas, V. Enzymatic preparation of both l- and d-enantiomers of phosphonic and phosphonous analogues of alanine using penicillin acylase. Tetrahedron Asymmetry 1993, 4, 1965–1968. [Google Scholar] [CrossRef]
- Zielinska, K.; Mazurkiewicz, R.; Szymanska, K.; Jarzebski, A.; Magierac, S.; Erfurt, K. Penicillin G acylase-mediated kinetic resolution of racemic 1-(N-acylamino)alkylphosphonic and 1-(N-acylamino)alkylphosphinic acids and their esters. J. Mol. Catal. B Enzym. 2016, 132, 31–40. [Google Scholar] [CrossRef]
- Zielinska, K.; Szymanska, K.; Mazurkiewicz, R.; Jarzebski, A. Batch and in-flow kinetic resolution of racemic 1-(N-acylamino)alkylphosphonic and 1-(N-acylamino)alkylphosphinic acids and their esters using immobilized penicillin G acylase. Tetrahedron Asymmetry 2017, 28, 146–152. [Google Scholar] [CrossRef]
- Oleksyszyn, J. An amidoalkylation of trivalent phosphorous compounds with P(O)H functions including acetic acid solutions of PCl3, RPCl2 or R2PCl, diesters of phosphorous acid and phosphorous-III-acids. J. Prakt. Chem. 1987, 329, 19–29. [Google Scholar] [CrossRef]
- Soroka, M. The synthesis of 1-aminoalkylphosphonic acids. A revised mechanism of the reaction of phosphorus trichloride, amides and aldehydes or ketones in acetic acid solution (Oleksyszyn reaction). Liebigs Ann. Chem. 1990, 4, 331–334. [Google Scholar] [CrossRef]
- Cypryk, M.; Drabowicz, J.; Gostynski, B.; Kudzin, M.H.; Kudzin, Z.H.; Urbaniak, P. 1-(N-Acylamino)-alkylphosphonic acids. Deacylation in aqueous solutions. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 651–658. [Google Scholar] [CrossRef]
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; Volume Chapters 16–60, pp. 1408–1411. ISBN 13-978-0-471-72091-1. [Google Scholar]
- O’Connor, C. Acidic and basic amide hydrolysis. Q. Rev. Chem. Soc. 1970, 24, 553–564. [Google Scholar] [CrossRef]
- Brown, R.S.; Bennet, A.J.; Slebocka-Tilk, H. Recent perspectives concerning the mechanism of H3O+ and OH− promoted amide hydrolysis. Acc. Chem. Res. 1992, 25, 481–488. [Google Scholar] [CrossRef]
- Bagno, A.; Lovato, G.; Scorrano, G. Thermodynamics of protonation and hydration of aliphatic amides. J. Chem. Soc. Perkin Trans. 1993, 2, 1091–1098. [Google Scholar] [CrossRef]
- Zahn, D. On the role of water in amide hydrolysis. Eur. J. Org. Chem. 2004, 4020–4023. [Google Scholar] [CrossRef]
- Kahne, D.; Still, W.C. Hydrolysis of a peptide bond in neutral water. J. Am. Chem. Soc. 1988, 110, 7529–7534. [Google Scholar] [CrossRef]
- DeWolfe, R.H.; Newcombe, R.C. Hydrolysis of formanilides in alkaline solutions. J. Org. Chem. 1971, 36, 3870–3878. [Google Scholar] [CrossRef]
- Desai, S.D.; Kirsch, L.E. The ortho effect on the acidic and alkaline hydrolysis of substituted formanilides. Int. J. Chem. Kinet. 2015, 47, 471–488. [Google Scholar] [CrossRef]
- Bowden, K.; Bromley, K. Reactions of carbonyl compounds in basic solutions. Part 14. The alkaline hydrolysis of substituted N-methylformanilides, N-methylacetanilides, 1-phenylazetidin-2-ones, 1-phenyl-2-pyrrolidones, and 1-phenyl-2-piperidones. J. Chem. Soc. Perkin Trans. 1990, 2, 2103–2109. [Google Scholar] [CrossRef]
- Hibbert, F.; Malana, M.A. Kinetics of the alkaline hydrolysis of 1,8-bis(trifluoroacetylamino)-naphthalene to 1-amino-8-trifluoroacetylaminonaphthalene in 70%, 80% and 90%(v/v) Me2SO–H2O. J. Chem. Soc. Perkin Trans. 1992, 2, 755–759. [Google Scholar] [CrossRef]
- Cheshmedzhieva, D.; Ilieva, S.; Hadjieva, B.; Galabov, B. The mechanism of alkaline hydrolysis of amides: A comparative computational and experimental study of the hydrolysis of N-methylacetamide, N-methylbenzamide, and acetanilide. J. Phys. Org. Chem. 2009, 22, 619–631. [Google Scholar] [CrossRef]
- Theodorou, V.; Paraskevopoulos, G.; Skobridis, K. A mild alkaline hydrolysis of N- and N,N-substituted amides and nitriles. Arkivoc 2015, 7, 101–112. [Google Scholar] [CrossRef]
- Soroka, M. Comments on the synthesis of aminomethylphosphonic acid. Synthesis 1989, 547–548. [Google Scholar] [CrossRef]
- Kudzin, Z.H.; Stec, W.J. Synthesis of 1-aminoalkanephosphonic acids via thioureidoalkanephosphonates. Synthesis 1978, 469–472. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all 1-(acylamino)phosphonic acids discussed in this paper are available from the authors. Calculated geometries of all modeled species are available upon request. |






 |  |  |  |  |
| AAC | ω-AAC | AAP | ω-AAP | AAP–R |
| Naturally occurring aminophosphonic acids. | ||||
 |  |  |  |  |
| β-AlaP [4] | Aspβ–P [5] | IserP [6] | TyrP [7] | Glu−γ–P(Me) [8] |
| Representative, biologically active aminophosphonates. | ||||
 |  |  |  |  |
| PMG [9] | Gluγ–P [10] | (AC)-AAP[R=H, (AC)-GlyP] [11] | AAC-AAP(R=R1=Me, Ala-AlaP) [12] | AAP-AAC [13,14] |
| Abbreviation of AAP and (AC)-AAP follow the general rules elaborated by Kudzin et al. [15]. | ||||
| (A) | |||||||||||
| Ac-GlyP | Ac-AlaP | Ac-NvaP | Ac-ValP | Ac-PglP | |||||||
| Ac-GlyP | Ac(OH)-GlyP | Ac-AlaP | Ac(OH)-AlaP | Ac-NvaP | Ac(OH)-NvaP | Ac-ValP | Ac(OH)-ValP | Ac-PglP | Ac(OH)-PglP | ||
| δ | 13.4 | 12.8 | 17.0 | 16.5 | 15.9 | 15.2 | 16.8 | 16.1 | 13.3 | 12.7 | |
| % | 97.1 | 2.9 | 97.1 | 2.9 | 96.3 | 3.7 | 97.0 | 3.0 | 97.0 | 3.0 | |
| (B) | |||||||||||
| Ac-GlyP | Prp-GlyP | Bz-GlyP | |||||||||
| GlyP | Ac-GlyP | Ac(OH)-GlyP | GlyP | Prp-GlyP | Prp(OH)-GlyP | GlyP | Bz-GlyP | Bz(OH)-GlyP | |||
| δ | 19.1 | 13.4 | 12.8 | 19.3 | 13.4 | 12.8 | 19.3 | 13.5 | 13.2 | ||
| % | - | 97.1 | 2.9 | - | 98.3 | 1.7 | - | 99.5 | <0.5 | ||
 | ||||
|---|---|---|---|---|
| (AC)-AAP (1Xx) | R | R1-C(O)- (1Xx) | ||
| CH3- (1Ax) | CF3- (1Bx) | Ph- (1Cx) | ||
| (AC)-GlyP (1Xa) | H- | 139.43 | 123.16 | 126.87 |
| (AC)-AlaP (1Xb) | CH3- | 135.19 | 119.26 | 124.82 |
| (AC)-ValP (1Xc) | (CH3)2CH- | 133.61 | 117.48 | 132.28 |
| (AC)-PglP (1Xd) | Ph- | 125.63 | 121.43 | 117.08 |
| (AC)-PheP (1Xe) | PhCH2- | 139.31 | 122.06 | 129.57 |
 | ||||
|---|---|---|---|---|
| (AC)-AAP (1Xx) | R | R1-C(O)- (1Xx) | ||
| CH3- (1Ax) | CF3- (1Bx) | Ph- (1Cx) | ||
| (AC)-GlyP (1Xa) | H- | 26.70 | 11.77 | 25.70 |
| (AC)-AlaP (1Xb) | CH3- | 26.26 | 10.54 | 25.45 |
| (AC)-ValP (1Xc) | (CH3)2CH- | 26.15 | 10.90 | 26.57 |
| (AC)-PglP (1Xd) | Ph- | 25.47 | 12.27 | 24.96 |
| (AC)-PheP (1Xe) | PhCH2- | 24.22 | 7.73 | 24.53 |
 | ||||
|---|---|---|---|---|
| (AC)-AAP (1Xx) | R | R1-C(O)- (1Xx) | ||
| CH3- (1Ax) | CF3- (1Bx) | Ph- (1Cx) | ||
| (AC)-GlyP (1Xa) | H- | 29.99 | 19.51 | 25.70 |
| (AC)-AlaP (1Xb) | CH3- | 30.85 | 19.12 | 26.75 |
| (AC)-ValP (1Xc) | (CH3)2CH- | 31.40 | 18.47 | 29.68 |
| (AC)-PglP (1Xd) | Ph- | 28.57 | 17.60 | 28.44 |
| (AC)-PheP (1Xe) | PhCH2- | 28.85 | 18.66 | 30.23 |
 | ||||
|---|---|---|---|---|
| (AC-OH)-AAP (2-Xx) | R | R1-C(O−)(OH)- (2Xx) | ||
| CH3- (2Ax) | CF3- (2Bx) | Ph- (2Cx) | ||
| (AC-OH)-GlyP (2Xa) | H- | −165.22 | −153.75 | −152.91 |
| (AC-OH)-AlaP (2Xb) | CH3- | −159.50 | −149.37 | −149.31 |
| (AC-OH)-ValP (2Xc) | (CH3)2CH- | −162.42 | −152.74 | −161.46 |
| (AC-OH)-PglP (2Xd) | Ph- | −157.24 | −160.78 | −150.14 |
| (AC-OH)-PheP (2Xe) | PhCH2- | −168.00 | −158.21 | −159.56 |
 | ||||
|---|---|---|---|---|
| (AC-OH)-AAP (2-Xx) | R | R1-C(O−)(OH)- (2Xx) | ||
| CH3- (2Ax) | CF3- (2Bx) | Ph- (2Cx) | ||
| (AC-OH)-GlyP (2Xa) | H- | −46.41 | −40.32 | −48.51 |
| (AC-OH)-AlaP (2Xb) | CH3- | −46.62 | −38.58 | −48.56 |
| (AC-OH)-ValP (2Xc) | (CH3)2CH- | −44.45 | −37.28 | −47.29 |
| (AC-OH)-PglP (2Xd) | Ph- | −45.60 | −40.90 | −47.44 |
| (AC-OH)-PheP (2Xe) | PhCH2- | −47.74 | −39.80 | −50.89 |
 | ||||
|---|---|---|---|---|
| (AC-OH)-AAP (2-Xx) | R | R1-C(O−)(OH)- (2Xx) | ||
| CH3- (2Ax) | CF3- (2Bx) | Ph- (2Cx) | ||
| (AC-OH)-GlyP (2Xa) | H- | 20.76 | 21.54 | 16.91 |
| (AC-OH)-AlaP (2Xb) | CH3- | 21.47 | 23.43 | 19.00 |
| (AC-OH)-ValP (2Xc) | (CH3)2CH- | 23.73 | 24.91 | 20.56 |
| (AC-OH)-PglP (2Xd) | Ph- | 20.05 | 21.74 | 18.51 |
| (AC-OH)-PheP (2Xe) | PhCH2- | 22.50 | 25.00 | 18.99 |
 | ||||
|---|---|---|---|---|
| (AC)-AAP (1Xx) | R | R1-C(O)- (1Xx) | ||
| CH3- (1Ax) | CF3- (1Bx) | Ph- (1Cx) | ||
| (AC)-GlyP (1Xa) | H- | −19.72 (47.5) | −28.56 (31.3) | −22.82 (42.6) |
| (AC)-AlaP (1Xb) | CH3- | −20.36 (47.7) | −28.04 (34.0) | −23.11 (44.5) |
| (AC)-ValP (1Xc) | (CH3)2CH- | −18.30 (49.9) | −26.38 (35.8) | −20.72 (47.1) |
| (AC)-PglP (1Xd) | Ph- | −20.13 (45.5) | −28.63 (34.0) | −22.48 (43.5) |
| (AC)-PheP (1Xe) | PhCH2- | −23.53 (46.7) | −32.08 (32.7) | −26.36 (43.5) |
| Name | Trivial Name | Abbr./(No) | Ref. | ||
 | Aminoalkyl-phosphonic acid | Phosphono-Amino Acid | AAP (3) | ||
| R | |||||
| H | Aminomethyl-phosphonic acid | Phosphono-glycine | GlyP (3a) | [64] | |
| Me | 1-Aminoethyl-phosphonic acid | Phosphono-alanine | AlaP (3b) | [65] | |
| iPr | 1-Amino-1-methylethyl-phosphonic acid | Phosphono-valine | ValP (3c) | ||
| Ph | 1-Amino-1-phenylmethyl-phosphonic acid | Phosphono-phenyl-glycine | PglP (3d) | ||
| PhCH2 | 1-Amino-1-phenylethyl-phosphonic acid | Phosphono-phenyl-alanine | PheP (3e) | ||
 | 1-(Acylamino)alkyl-phosphonic acid | Acylamino-Phosphono Acid | (AC)-AAP (2) | ||
| R1 | R | ||||
| CH3 | H, Me, iPr, Ph, PhCH2 | 1-(Acetylamino)alkyl-phosphonic acid | Acetylamino-Phosphono Acid | Ac-AAP (2A) | [36] |
| CH3 | H | 1-(Acetylamino)methyl-phosphonic acid | Phosphono-Acetylamino Glycine | Ac-GlyP (2Aa) | |
| CH3 | Ph | 1-(Acetylamino)-1-phenyl-methyl-phosphonic acid | Phosphono-Acetyl-amino-PhenylGlycine | Ac-PglP (2Ad) | |
| CF3 | H, Me, iPr, Ph, PhCH2 | 1-(Trifluoroacetylamino)-alkyl-phosphonic acid | Phosphono-Trifluoroacetyl-amino Acid | TFA-AAP (2B) | [37] |
| CF3 | iPr | 1-(Trifluoroacetylamino)-2-methylethyl-phosphonic acid | Phosphono-Trifluoroacetyl-Valine | TFA-ValP (2Bc) | |
| Ph | H, Me, iPr, Ph, PhCH2 | 1-(Benzoylamino)alkyl-phosphonic acid | Phosphono-Benzoylamino Acid | Bz-AAP (2C) | [36] |
| Ph | PhCH2 | 1-(Benzoylamino)-2-phenyl-ethyl-phosphonic acid | Phosphono-Benzoyl-Phenyl-alanine | Bz-PheP (2Ce) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cypryk, M.; Drabowicz, J.; Gostynski, B.; Kudzin, M.H.; Kudzin, Z.H.; Urbaniak, P. 1-(Acylamino)alkylphosphonic Acids—Alkaline Deacylation. Molecules 2018, 23, 859. https://doi.org/10.3390/molecules23040859
Cypryk M, Drabowicz J, Gostynski B, Kudzin MH, Kudzin ZH, Urbaniak P. 1-(Acylamino)alkylphosphonic Acids—Alkaline Deacylation. Molecules. 2018; 23(4):859. https://doi.org/10.3390/molecules23040859
Chicago/Turabian StyleCypryk, Marek, Jozef Drabowicz, Bartlomiej Gostynski, Marcin H. Kudzin, Zbigniew H. Kudzin, and Pawel Urbaniak. 2018. "1-(Acylamino)alkylphosphonic Acids—Alkaline Deacylation" Molecules 23, no. 4: 859. https://doi.org/10.3390/molecules23040859