A Co-Precursor Approach Coupled with a Supercritical Modification Method for Constructing Highly Transparent and Superhydrophobic Polymethylsilsesquioxane Aerogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Silica Aerogels
2.3. Characterization of Materials
3. Results and Discussion
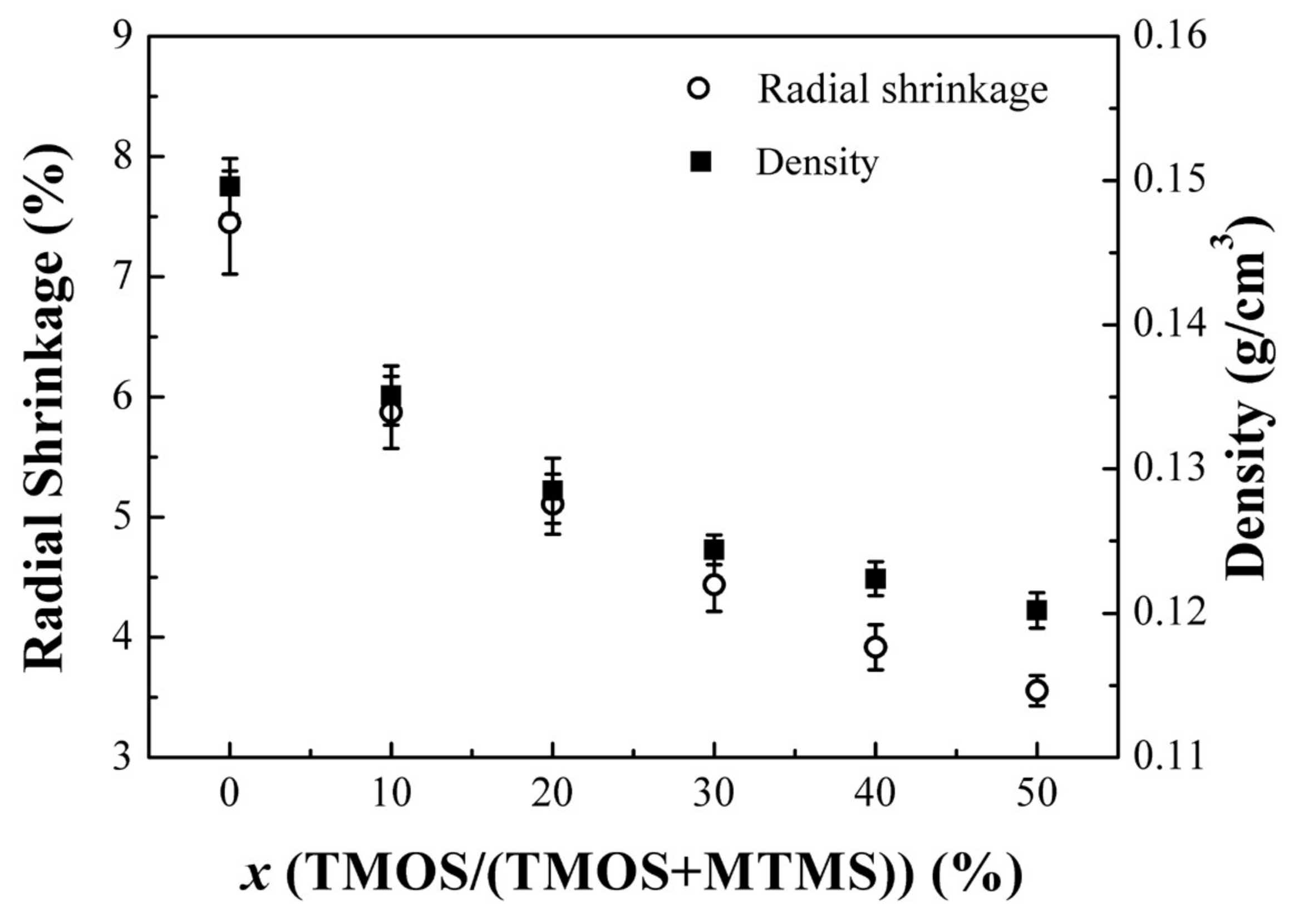
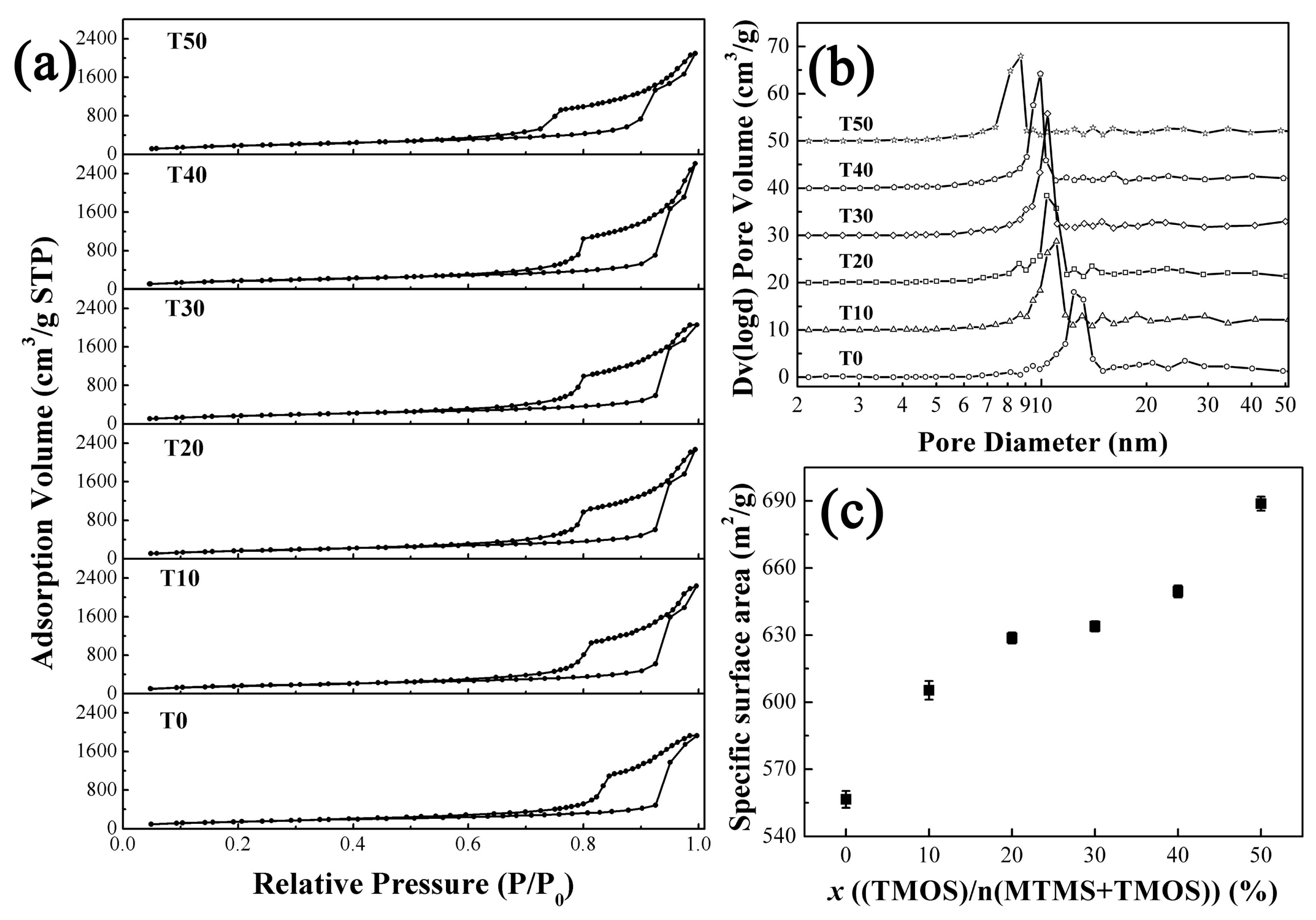
3.1. Structural Characterization of the PMSQ Aerogels Prepared by the Co-Precursor
3.2. The Properties of the PMSQ Aerogels Prepared by the Co-Precursor
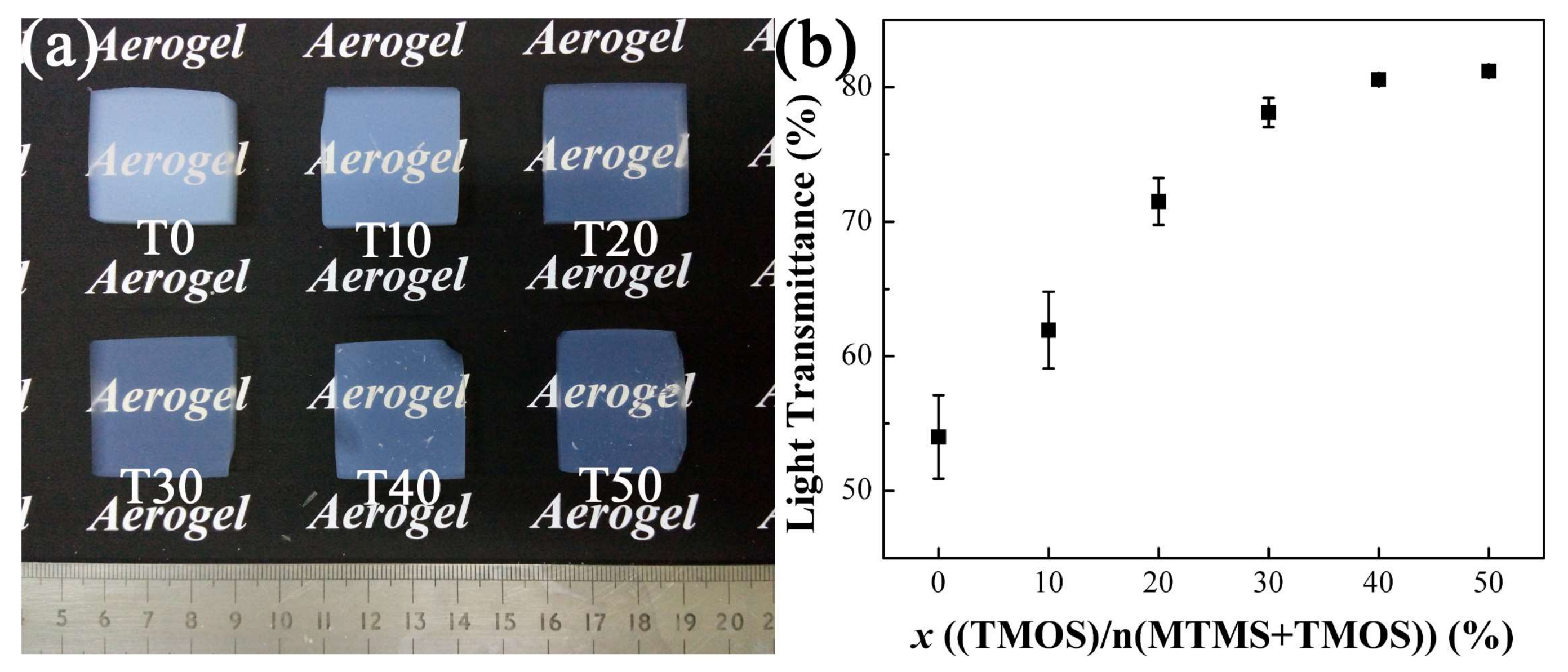
3.2.1. The Transparency of Aerogels with Varied TMOS Content
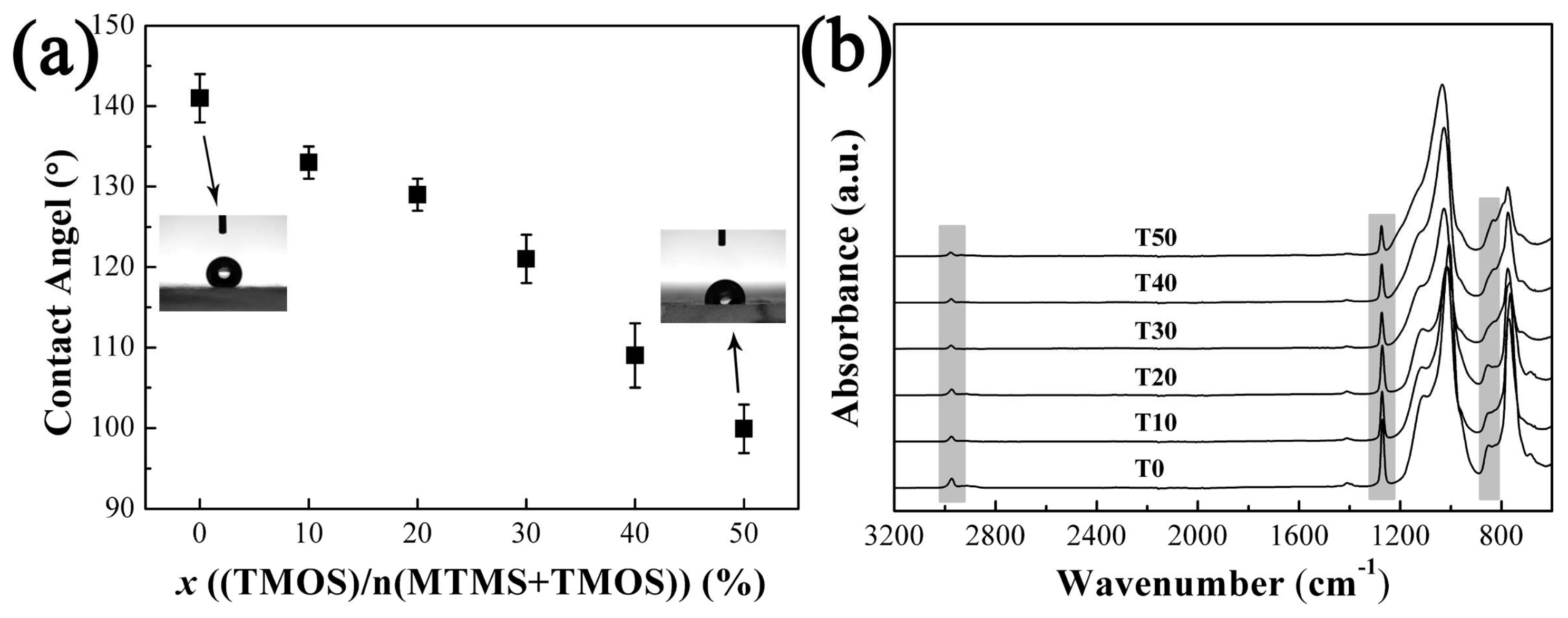
3.2.2. The Hydrophobicity of Aerogels with Varied TMOS Content
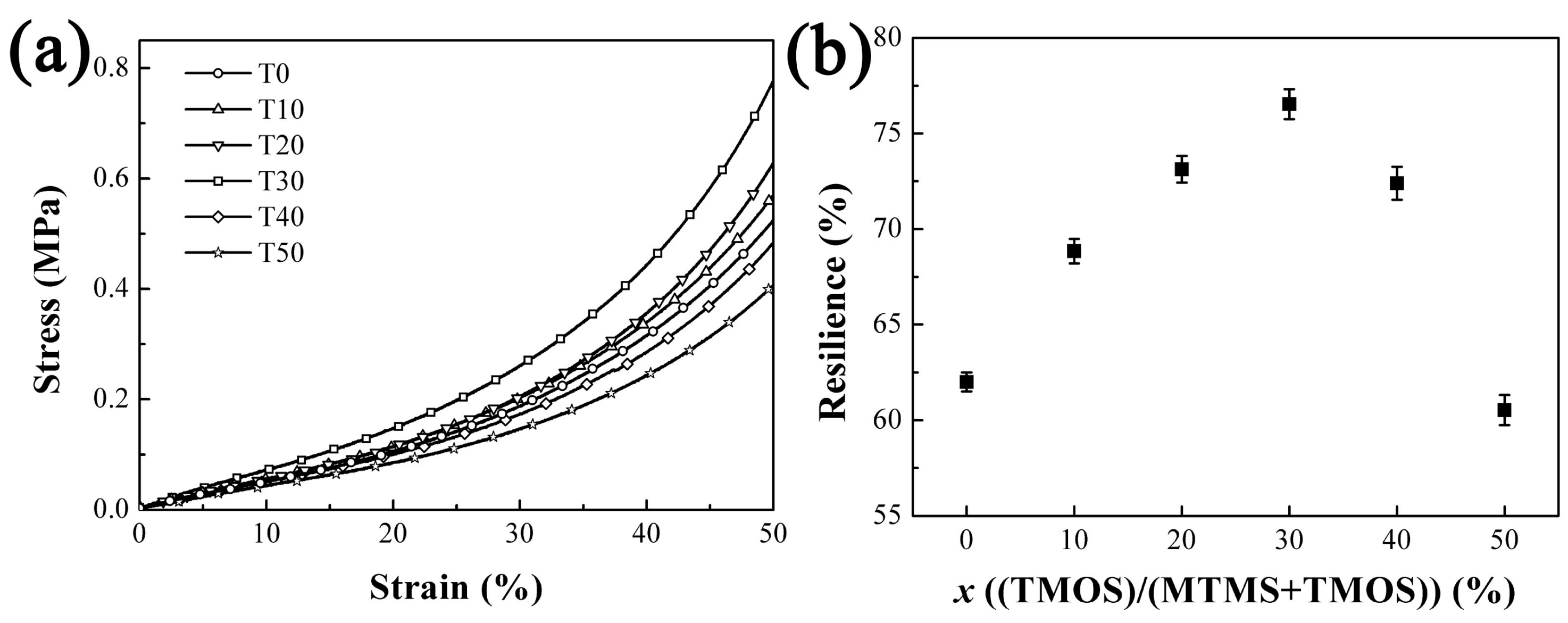
3.2.3. The Mechanical Properties of Aerogels with Varied TMOS Content
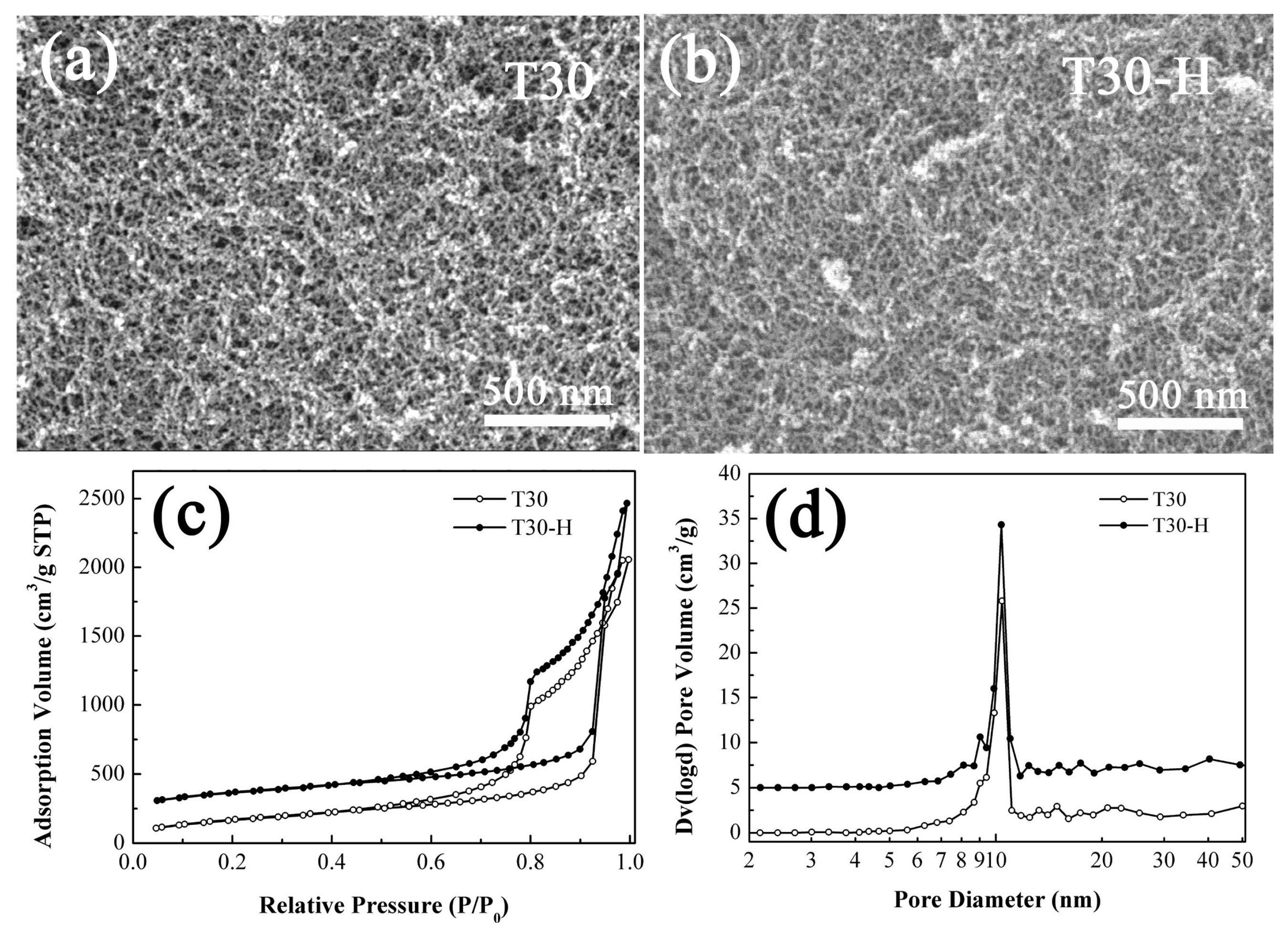
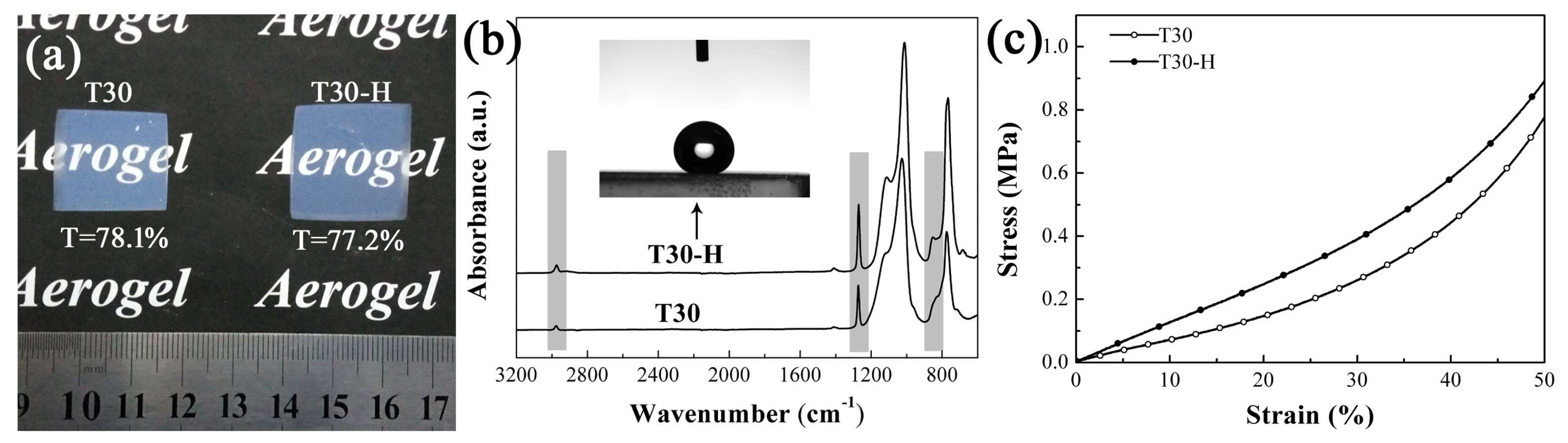
3.3. The Supercritical Modification of the PMSQ Aerogel
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Soleimani Dorcheh, A.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, X.D.; He, S.; Shi, X.J.; Yang, H.; Zhang, H.P. Tailoring thermal properties of ambient pressure dried MTMS/TEOS co-precursor aerogels. Mater. Lett. 2016, 171, 91–94. [Google Scholar] [CrossRef]
- Feng, J.D.; Le, D.; Nguyen, S.T.; Nien, V.T.C.; Jewell, D.; Duong, H.M. Silica–cellulose hybrid aerogels for thermal and acoustic insulation applications. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 298–305. [Google Scholar] [CrossRef]
- Cuce, E.; Cuce, P.M.; Wood, C.J.; Riffat, S.B. Toward aerogel based thermal superinsulation in buildings: A comprehensive review. Renew. Sustain. Energy Rev. 2014, 34, 273–299. [Google Scholar] [CrossRef]
- Zu, G.Q.; Shen, J.; Wang, W.Q.; Zou, L.P.; Lian, Y.; Zhang, Z.H. Silica-titania composite aerogel photocatalysts by chemical liquid deposition of titania onto nanoporous silica scaffolds. ACS Appl. Mater. Interfaces 2015, 7, 5400–5409. [Google Scholar] [CrossRef] [PubMed]
- Alnaief, M.; Smirnova, I. Effect of surface functionalization of silica aerogel on their adsorptive and release properties. J. Non-Cryst. Solids 2010, 356, 1644–1649. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Beland, F.; Ilharco, L.M.; Pagliaro, M. The sol-gel route to advanced silica-based materials and recent applications. Chem. Rev. 2013, 113, 6592–6620. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.C.H.; Kaymak, H.; Brunner, S.; Koebel, M.M. Mechanical properties of monolithic silica aerogels made from polyethoxydisiloxanes. Microporous Mesoporous Mater. 2014, 183, 23–29. [Google Scholar] [CrossRef]
- Liu, G.W.; Zhou, B.; Du, A.; Shen, J.; Wu, G.M. Greatly strengthened silica aerogels via co-gelation of binary sols with different concentrations: A method to control the microstructure of the colloids. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 763–774. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, Z.; Wu, J.J.; Zhao, N.; Xu, J. Vacuum-dried robust bridged silsesquioxane aerogels. Adv. Mater. 2013, 25, 4494–4497. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Luo, H.J.; Gao, Y.F. Low-density, hydrophobic, highly flexible ambient-pressure-dried monolithic bridged silsesquioxane aerogels. J. Mater. Chem. A 2015, 3, 3390–3398. [Google Scholar] [CrossRef]
- Rao, A.V.; Kulkarni, M.M.; Amalnerkar, D.P.; Seth, T. Superhydrophobic silica aerogels based on methyltrimethoxysilane precursor. J. Non-Cryst. Solids 2003, 330, 187–195. [Google Scholar]
- Cai, L.; Shan, G.R. Elastic silica aerogel using methyltrimethoxysilane precusor via ambient pressure drying. J. Porous Mater. 2015, 22, 1455–1463. [Google Scholar] [CrossRef]
- Kanamori, K.; Aizawa, M.; Nakanishi, K.; Hanada, T. New transparent methylsilsesquioxane aerogels and xerogels with improved mechanical properties. Adv. Mater. 2007, 19, 1589–1593. [Google Scholar] [CrossRef]
- Kanamori, K. Monolithic silsesquioxane materials with well-defined pore structure. J. Mater. Res. 2014, 20, 2773–2786. [Google Scholar] [CrossRef]
- Hayase, G.; Kugimiya, K.; Ogawa, M.; Kodera, Y.; Kanamori, K.; Nakanishi, K. The thermal conductivity of polymethylsilsesquioxane aerogels and xerogels with varied pore sizes for practical application as thermal superinsulators. J. Mater. Chem. A 2014, 2, 6525–6531. [Google Scholar] [CrossRef]
- Lei, C.S.; Hu, Z.J.; Zhang, Y.; Yang, H.L.; Li, J.N.; Hu, S.B. Tailoring structural and physical properties of polymethylsilsesquioxane aerogels by adjusting NH3·H2O concentration. Microporous Mesoporous Mater. 2018, 258, 236–243. [Google Scholar] [CrossRef]
- Ingale, S.V.; Wagh, P.B.; Tripathi, A.K.; Kamble, V.S.; Kumar, R.; Gupta, S.C. Physico-chemical properties of silica aerogels prepared from TMOS/MTMS mixtures. J. Porous Mater. 2011, 18, 567–572. [Google Scholar] [CrossRef]
- Rao, A.V.; Pajonk, G.M. Effect of methyltrimethoxysilane as a co-precursor on the optical properties of silica aerogels. J. Non-Cryst. Solids 2001, 285, 202–209. [Google Scholar]
- Cheng, X.L.; Chen, D.R.; Liu, Y.J. Mechanisms of silicon alkoxide hydrolysis–oligomerization reactions: A DFT investigation. Chem. Phys. Chem. 2012, 13, 2392–2404. [Google Scholar] [CrossRef] [PubMed]
- Tamon, H.; Sone, T.; Okazaki, M. Control of mesoporous structure of silica aerogel prepared from TMOS. J. Colloid Interface Sci. 1997, 188, 162–167. [Google Scholar] [CrossRef]
- Wu, G.Y.; Yu, Y.X.; Cheng, X.; Zhang, Y. Preparation and surface modification mechanism of silica aerogels via ambient. Mater. Chem. Phys. 2011, 129, 308–314. [Google Scholar] [CrossRef]
- Sanli, D.; Erkey, C. Monolithic composites of silica aerogels by reactive supercritical deposition of hydroxy-terminated poly(dimethylsiloxane). ACS Appl. Mater. Interfaces 2013, 5, 11708–11717. [Google Scholar] [CrossRef] [PubMed]
- Zu, G.Q.; Shen, J.; Zou, L.P.; Wang, W.Q.; Lian, Y.; Zhang, Z.H.; Du, A. Nanoengineering super heat-resistant, strong alumina aerogels. Chem. Mater. 2013, 25, 4757–4764. [Google Scholar] [CrossRef]
- Bhagat, S.D.; Kim, Y.-H.; Ahn, Y.-S.; Yeo, J.-G. Rapid synthesis of water-glass based aerogels by in situ surface modification of the hydrogels. Appl. Surf. Sci. 2007, 253, 3231–3236. [Google Scholar] [CrossRef]
- Wang, W.Q.; Zhang, Z.H.; Zu, G.Q.; Shen, J.; Zou, L.P.; Lian, Y.; Liu, B.; Zhang, F. Trimethylethoxysilane-modified super heat-resistant alumina aerogels for high-temperature thermal insulation and adsorption applications. RSC Adv. 2014, 4, 54864–54871. [Google Scholar] [CrossRef]
- Zu, G.Q.; Shen, J.; Zou, L.P.; Zou, W.B.; Guan, D.Y.; Wu, Y.; Zhang, Y.W. Highly thermally stable zirconia/silica composite aerogels prepared by supercritical deposition. Microporous Mesoporous Mater. 2017, 238, 90–96. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, X.D.; He, S.; Huang, D.M.; Bi, H.J.; Yang, H. Preparation of ambient pressure dried MTMS/TEOS co-precursor silica aerogel by adjusting NH4OH concentration. Mater. Lett. 2014, 129, 12–15. [Google Scholar] [CrossRef]
- Chang, K.-J.; Wang, Y.-Z.; Peng, K.-C.; Tsai, H.-S.; Chen, J.-R.; Huang, C.-T.; Ho, K.-S.; Lien, W.-F. Preparation of silica aerogel/polyurethane composites for the application of thermal insulation. J. Polym. Res. 2014, 21, 1–9. [Google Scholar] [CrossRef]
- Mazraeh-shahi, Z.T.; Shoushtari, A.M.; Abdouss, M.; Bahramian, A.R. Relationship analysis of processing parameters with micro and macro structure of silica aerogel dried at ambient pressure. J. Non-Cryst. Solids 2013, 376, 30–37. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Hsu, S.-H. Solvent-resistant CTAB-modified polymethylsilsesquioxane aerogels for organic solvent and oil adsorption. J. Colloid Interface Sci. 2017, 485, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Li, T.M.; Zhou, B.; Du, A.; Xiang, Y.L.; Wu, S.; Liu, M.F.; Ding, W.H.; Shen, J.; Zhang, Z.H. Microstructure control of the silica aerogels via pinhole drying. J. Sol-Gel Sci. Technol. 2017, 84, 96–103. [Google Scholar] [CrossRef]
- Yokogawa, H. Transparent silica aerogel blocks for high-energy physics research. In Aerogels Handbook, 28th ed.; Aegerter, M.A., Levetis, N., Koebel, M.M., Eds.; Springer: New York, NY, USA, 2011; pp. 651–664. [Google Scholar]
- Zhao, S.Y.; Zhang, Z.; Sèbe, G.; Wu, R.; Virtudazo, R.V.R.; Tingaut, P.; Koebel, M.M. Multiscale assembly of superinsulating silica aerogels within silylated nanocellulosic scaffolds: Improved mechanical properties promoted by nanoscale chemical compatibilization. Adv. Funct. Mater. 2015, 25, 2326–2334. [Google Scholar] [CrossRef]
- Sai, H.Z.; Fu, R.; Xing, L.; Xiang, J.H.; Li, Z.Y.; Li, F.; Zhang, T. Surface modification of bacterial cellulose aerogels’ web-like skeleton for oil/water separation. ACS Appl. Mater. Interfaces 2015, 7, 7373–7381. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the PMSQ aerogels and the modified samples are available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, C.; Li, J.; Sun, C.; Yang, H.; Xia, T.; Hu, Z.; Zhang, Y. A Co-Precursor Approach Coupled with a Supercritical Modification Method for Constructing Highly Transparent and Superhydrophobic Polymethylsilsesquioxane Aerogels. Molecules 2018, 23, 797. https://doi.org/10.3390/molecules23040797
Lei C, Li J, Sun C, Yang H, Xia T, Hu Z, Zhang Y. A Co-Precursor Approach Coupled with a Supercritical Modification Method for Constructing Highly Transparent and Superhydrophobic Polymethylsilsesquioxane Aerogels. Molecules. 2018; 23(4):797. https://doi.org/10.3390/molecules23040797
Chicago/Turabian StyleLei, Chaoshuai, Junning Li, Chencheng Sun, Hailong Yang, Tao Xia, Zijun Hu, and Yue Zhang. 2018. "A Co-Precursor Approach Coupled with a Supercritical Modification Method for Constructing Highly Transparent and Superhydrophobic Polymethylsilsesquioxane Aerogels" Molecules 23, no. 4: 797. https://doi.org/10.3390/molecules23040797