Molecular Mechanisms of Melatonin Protection from Gastric Mucosal Apoptotic Injury in Experimental Burns
Abstract
:1. Introduction
2. Results
2.1. The Effect of Melatonin on 4-HNE Expression in Gastric Mucosa
2.2. The Melatonin Effect on Bax Protein Expression in Gastric Mucosa
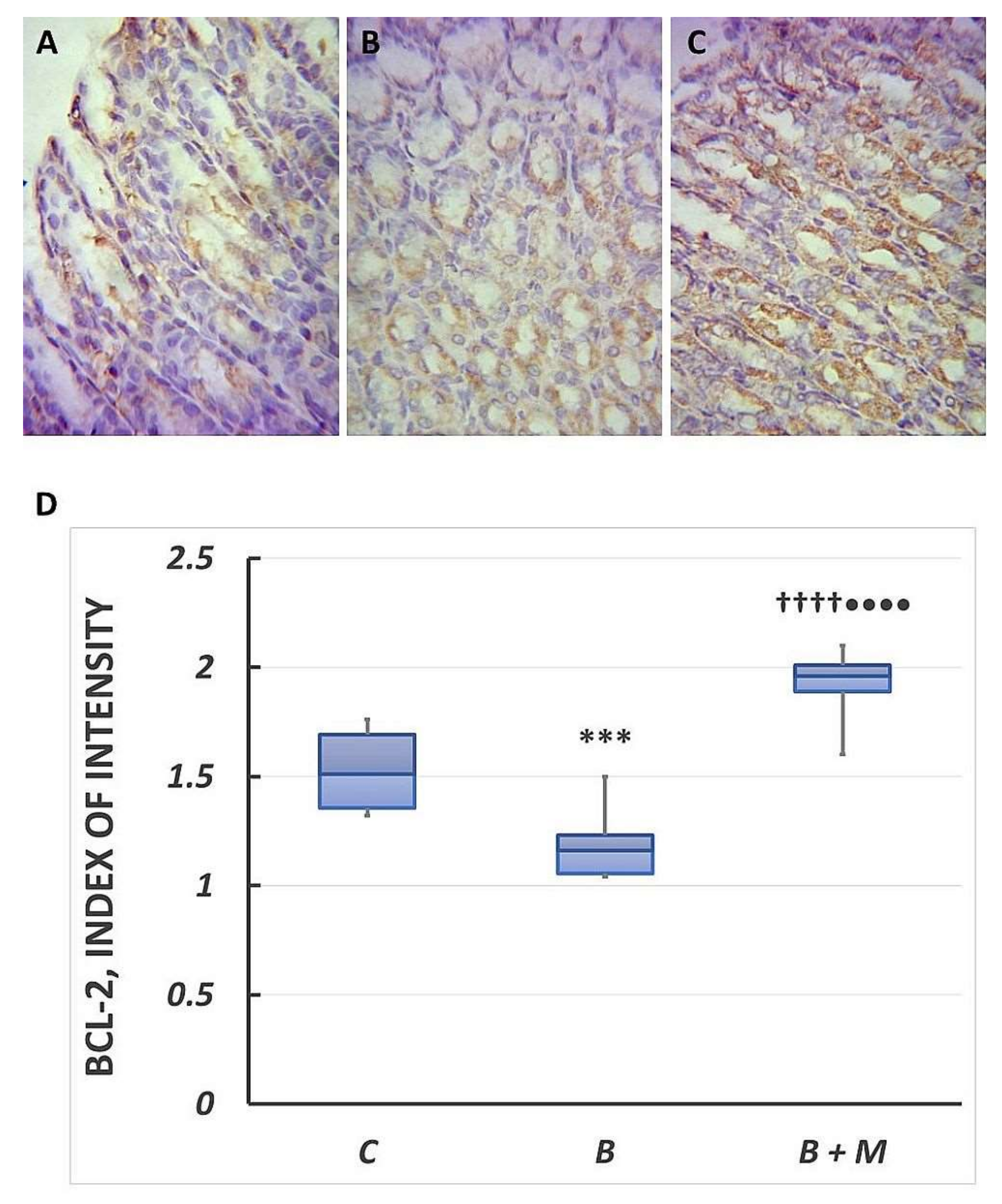
2.3. The Melatonin Effect on Bcl-2 Protein Expression in Gastric Mucosa
2.4. Changes in Bax/Bcl-2 Ratio in Gastric Mucosa Following Burn Trauma
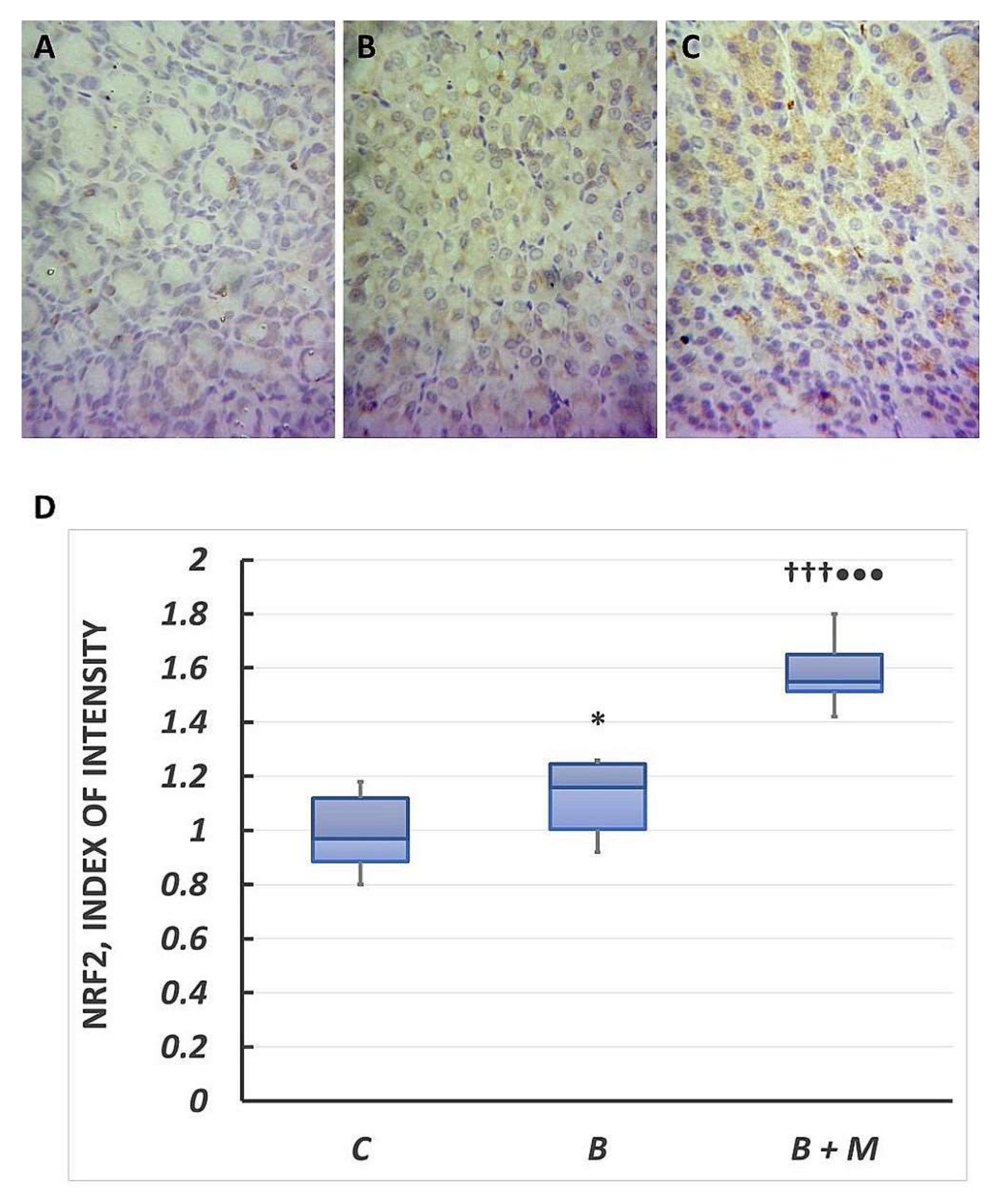
2.5. The Melatonin Effect on Nrf2 Expression in Gastric Mucosa
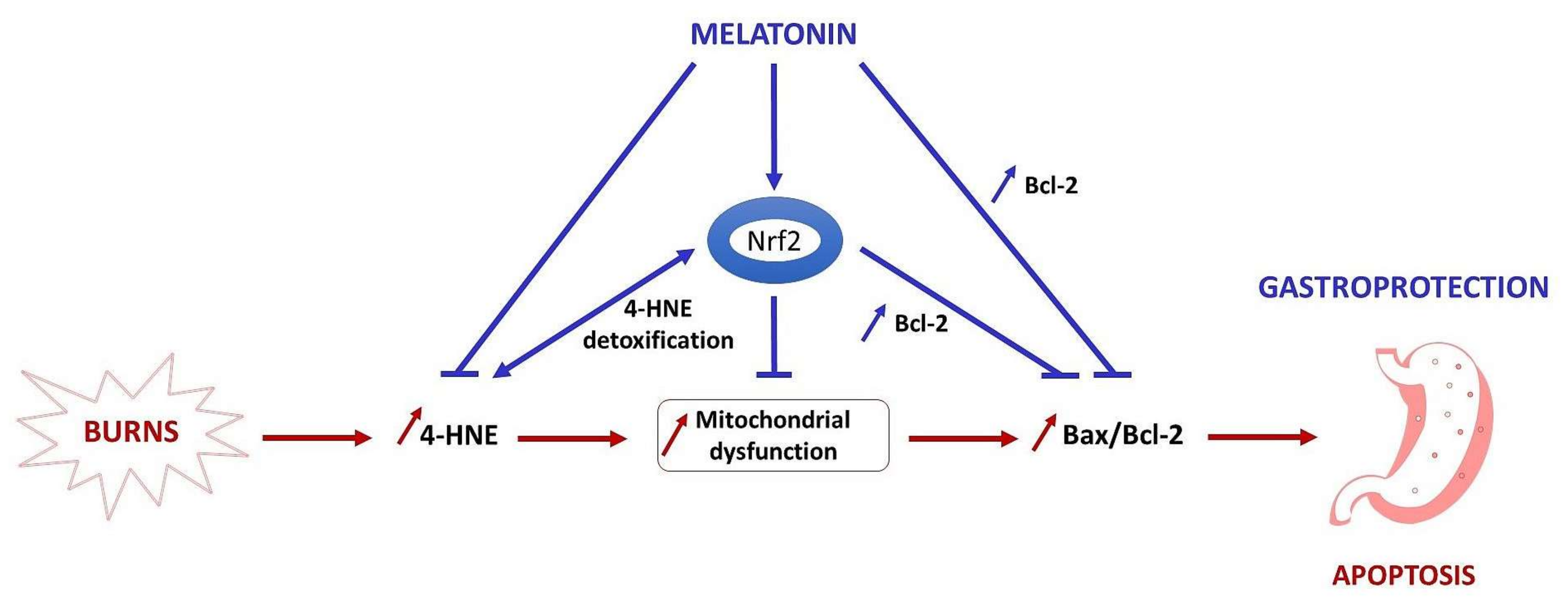
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Thermal Injury and Melatonin Treatment
4.3. Paraffin Processing of Tissue
4.4. Immunohistochemistry
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jeschke, M.G. The hepatic response to thermal injury: Is the liver important for postburn outcomes? Mol. Med. 2009, 15, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.D.; Murillo-Cabezas, F.; Calvo, J.R.; Lardone, P.J.; Tan, D.X.; Guerrero, J.M.; Reiter, R.J. Melatonin as pharmacologic support in burn patients: A proposed solution to thermal injury-related lymphocytopenia and oxidative damage. Crit. Care Med. 2007, 35, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Kwiecień, S.; Brzozowski, T.; Konturek, S.J. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J. Physiol. Pharmacol. 2002, 53, 39–50. [Google Scholar] [PubMed]
- Magierowski, M.; Hubalewska-Mazgaj, M.; Magierowska, K.; Wojcik, D.; Sliwowski, Z.; Kwiecien, S.; Brzozowski, T. Nitric oxide, afferent sensory nerves, and antioxidative enzymes in the mechanism of protection mediated by tricarbonyldichlororuthenium(II) dimer and sodium hydrosulfide against aspirin-induced gastric damage. J. Gastroenterol. 2018, 53, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Dai, Z.; Wu, G.; Wu, Z. 4-Hydroxy-2-nonenal induces apoptosis by activating ERK1/2 signaling and depleting intracellular glutathione in intestinal epithelial cells. Sci. Rep. 2016, 6, 32929. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Gao, Y.; Zhang, J.; Chen, Y.; Jiang, Y.; Ji, J.; Zhang, J.; Chen, X.; Yang, Q.; Su, L.; et al. Mitochondrial aldehyde dehydrogenase 2 protects gastric mucosa cells against DNA damage caused by oxidative stress. Free Radic. Biol. Med. 2016, 93, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, B.; Allocati, N.; Graziano, V.; Di Ilio, C.; De Laurenzi, V. Role of Apoptosis in disease. Aging 2012, 4, 330–349. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, S.J.; Shutter, J.R.; Veis, D.J.; Merry, D.E.; Oltvai, Z.N. Bcl-2/Bax: A rheostat that regulates an anti-oxidant pathway and cell death. Semin. Cancer Biol. 1993, 4, 327–332. [Google Scholar] [PubMed]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.S.; Yu, S.; Kong, A.N. Activation of NRF2-antioxidant signaling attenuates NFκB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 2008, 76, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of NRF2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.K.; Jaiswal, A.K. NRF2 protein up-regulates antiapoptotic protein BCL-2 and prevents cellular apoptosis. J. Biol. Chem. 2012, 287, 9873–9886. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Q.; Fichna, J.; Bashashati, M.; Li, Y.Y.; Storr, M. Distribution, function and physiological role of melatonin in the lower gut. World J. Gastroenterol. 2011, 17, 3888–3898. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Radogna, F.; Cristofanon, S.; Paternoster, L.; D’Alessio, M.; De Nicola, M.; Cerella, C.; Dicato, M.; Diederich, M.; Ghibelli, L. Melatonin antagonizes the intrinsic pathway of apoptosis via mitochondrial targeting of Bcl-2. J. Pineal Res. 2008, 44, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Chuffa, L.G.; Alves, M.S.; Martinez, M.; Camargo, I.C.; Pinheiro, P.F.; Domeniconi, R.F.; Júnior, L.A.; Martinez, F.E. Apoptosis is triggered by melatonin in an in vivo model of ovarian carcinoma. Endocr. Relat. Cancer 2016, 23, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Sener-Muratoglu, G.; Paskaloglu, K.; Arbak, S.; Hurdag, C.; Ayanoglu-Dulger, G. Protective effect of famotidine, omeprazole, and melatonin against acetylsalicylic acid-induced gastric damage in rats. Dig. Dis. Sci. 2001, 46, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, J.; Motilva, V.; Martín, M.J.; de la Lastra, C.A. Mechanisms involved in gastric protection of melatonin against oxidant stress by ischemia-reperfusion in rats. Life Sci. 2001, 68, 1405–1415. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; León, J.; Carazo, A.; Khaldy, H. Mitochondrial regulation by melatonin and its metabolites. Adv. Exp. Med. Biol. 2003, 527, 549–557. [Google Scholar] [PubMed]
- Bekyarova, G.; Atanasova, M.; Tzaneva, M.; Dimitrova, A. Melatonin modulates inflammatory response and suppresses burn-induced apoptotic injury. J. Mind Med. Sci. 2017, 4, 59–66. [Google Scholar] [CrossRef]
- Pruitt, B.A., Jr.; Goodwin, C.W., Jr. Stress ulcer disease in the burned patient. World J. Surg. 1981, 5, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Markell, K.W.; Renz, E.M.; White, C.E.; Albrecht, M.E.; Blackbourne, L.H.; Park, M.S.; Barillo, D.A.; Chung, K.K.; Kozar, R.A.; Minei, J.P.; et al. Abdominal complications after severe burns. J. Am. Coll. Surg. 2009, 208, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.; Green, D.R. Mitochondrial regulation of cell death. Cold Spring Harb. Perspect. Biol. 2013, 5, a008706. [Google Scholar] [CrossRef] [PubMed]
- Huiqin, Z.; Huiyong, Y. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biol. 2015, 4, 193–199. [Google Scholar]
- Bekyarova, G.; Tzaneva, M.; Hristova, M. Melatonin modulates the expression of BCL-2 family proteins in liver after thermal injury in rats. Adv. Biosci. Biotechnol. 2013, 4, 41–47. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Herndon, D.N.; Finnerty, C.C.; Bolder, U.; Thompson, J.C.; Mueller, U.; Wolf, S.E.; Przkora, R. The effect of growth hormone on gut mucosal homeostasis and cellular mediators after severe trauma. J. Surg. Res. 2005, 127, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Song, H.P.; Zhang, L.; Dang, Y.M.; Yan, H.; Chu, Z.G.; Huang, Y.S. The phosphatidylinositol 3-kinase-AKT pathway protects Cardiomyocytes from Ischaemic and hypoxic apoptosis via mitochondrial function. Clin. Exp. Pharmacol. Physiol. 2010, 37, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.Y.; Liu, X.J.; Xu, J.D.; Deng, L.J.; Wang, G. Propofol inhibits burn injury-induced hyperpermeability through an apoptotic signal pathway in microvascular endothelial cells. Braz. J. Med. Biol. Res. 2015, 48, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Maity, P.; Bindu, S.; Choubey, V.; Alam, A.; Mitra, K.; Goyal, M.; Dey, S.; Guha, M.; Pal, C.; Bandyopadhyay, U. Lansoprazole protects and heals gastric mucosa from non-steroidal anti-inflammatory drug (NSAID)-induced gastropathy by inhibiting mitochondrial as well as Fas-mediated death pathways with concurrent induction of mucosal cell renewal. J. Biol. Chem. 2008, 283, 14391–14401. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jang, K.J.; Kim, C.H.; Lee, Y.H.; Lee, S.J.; Kim, B.H.; Yoon, H.M. Ganoderma lucidum Pharmacopuncture for the Treatment of Acute Gastric Ulcers in Rats. J. Pharmacopunct. 2014, 17, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Z.S.; Wan, X.J.; Wang, W. Expression and function of apoptosis-related genes BCL-2/Bax and Fas/Fas L in the course of stress ulcer. Zhonghua Yi Xue Za Zhi 2003, 83, 504–509. [Google Scholar] [PubMed]
- Niki, E. Lipid peroxidation: Physiological levels and dual biological effects. Free Radic. Biol. Med. 2009, 47, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, H.; Xu, C.J.; Yuan, J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 1998, 94, 491–501. [Google Scholar] [CrossRef]
- Hristova, M.; Bekyarova, G.; Atanasova, M.; Tzaneva, M. Melatonin attenuates oxidative stress and modulates inflammatory response after experimental burn trauma. J. Mind Med. Sci. 2018, in press. [Google Scholar]
- Huber, C.; Zanner, R.; Pohlinger, A.; Mahr, S.; Neu, B.; Prinz, C. Tumor necrosis factor-α effects on rat gastric enterochromaffin-like cells. Digestion 2002, 65, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Anand, T.; Pandareesh, M.D.; Bhat, P.V.; Venkataramana, M. Anti-apoptotic mechanism of Bacoside rich extract against reactive nitrogen species induced activation of iNOS/Bax/caspase 3 mediated apoptosis in L132 cell line. Cytotechnology 2014, 66, 823–838. [Google Scholar] [CrossRef] [PubMed]
- Hristova, M.; Bekyarova, G.; Tzaneva, M. Heme oxygenase 1 expression and oxidative stress—Related markers in gastric mucosa in skin burns and protection with melatonin. Trakia J. Sci. 2016, 4, 307–313. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of NRF2. Mol. Cell. Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Wang, H.; Zhu, L.; Wang, X.; Cong, Z.; Sun, K.; Fan, Y. The involvement of NRF2-ARE pathway in regulation of apoptosis in human glioblastoma cell U251. Neurol. Res. 2013, 35, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Hristova, M.; Bekyarova, G.; Tzaneva, M. Heme oxygenase-1 upregulated by melatonin: Potential protection against burn-induced oxidative gastric mucosal injury. J. IMAB 2015, 21, 779–783. [Google Scholar] [CrossRef]
- Maity, P.; Bindu, S.; Dey, S.; Goyal, M.; Alam, A.; Pal, C.; Reiter, R.; Bandyopadhyay, U. Melatonin reduces indomethacin-induced gastric mucosal cell apoptosis by preventing mitochondrial oxidative stress and the activation of mitochondrial pathway of apoptosis. J. Pineal Res. 2009, 46, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Zhang, H.; Liu, B.; Wu, Z.; Zhao, W.; Wang, Z. Exogenous melatonin modulates apoptosis in the mouse brain induced by high-LET carbon ion irradiation. J. Pineal Res. 2012, 52, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Beneficial neurobiological effect of melatonin under condition of increased oxidative stress. Curr. Med. Chem. 2002, 4, 45–58. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Terron, M.P.; Flores, L.J.; Czarnocki, Z. Melatonin and its metabolites: New findings regarding their production and their radical scavenging actions. Acta Biochim. Pol. 2007, 54, 1–9. [Google Scholar] [PubMed]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Antolín, I.; Rodríguez, C.; Saínz, R.M.; Mayo, J.C.; Uría, H.; Kotler, M.L.; Rodríguez-Colunga, M.J.; Tolivia, D.; Menéndez-Peláez, A. Neurohormone melatonin prevents cell damage: Effect on gene expression for antioxidant enzymes. FASEB J. 1996, 10, 882–890. [Google Scholar] [CrossRef] [PubMed]
- León, J.; Acuña-Castroviejo, D.; Escames, G.; Tan, D.X.; Reiter, R.J. Melatonin mitigates mitochondrial malfunction. J. Pineal Res. 2005, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.A.; Sayeed, I.; Siemen, D.; Wolf, G.; Horn, T.F. Direct inhibition of the mitochondrial permeability transition pore: A possible mechanism responsible for anti-apoptotic effects of melatonin. FASEB J. 2004, 18, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.; Macías, M.; Escames, G.; Reiter, R.J.; Agapito, M.T.; Ortiz, G.G.; Acuña-Castroviejo, D. Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J. Pineal Res. 2000, 28, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Espino, J.; Rodríguez, A.B.; Pariente, J.A. The inhibition of TNF-α-induced leucocyte apoptosis by melatonin involves membrane receptor MT1/MT2 interaction. J. Pineal Res. 2013, 54, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Acuña Castroviejo, D.; Escames, G.; Carazo, A.; León, J.; Khaldy, H.; Reiter, R.J. Melatonin, mitochondrial homeostasis and mitochondrial-related diseases. Curr. Top. Med. Chem. 2002, 2, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Tzaneva, M. Electron microscopic immunohistochemical investigation of Chromogranin A in endocrine cells in human oxyntic gastric mucosa. Acta Histochem. 2001, 103, 179–194. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hristova, M.; Tzaneva, M.; Bekyarova, G.; Chivchibashi, D.; Stefanova, N.; Kiselova-Kaneva, Y. Molecular Mechanisms of Melatonin Protection from Gastric Mucosal Apoptotic Injury in Experimental Burns. Molecules 2018, 23, 749. https://doi.org/10.3390/molecules23040749
Hristova M, Tzaneva M, Bekyarova G, Chivchibashi D, Stefanova N, Kiselova-Kaneva Y. Molecular Mechanisms of Melatonin Protection from Gastric Mucosal Apoptotic Injury in Experimental Burns. Molecules. 2018; 23(4):749. https://doi.org/10.3390/molecules23040749
Chicago/Turabian StyleHristova, Minka, Maria Tzaneva, Ganka Bekyarova, Dariya Chivchibashi, Nadezhda Stefanova, and Yoana Kiselova-Kaneva. 2018. "Molecular Mechanisms of Melatonin Protection from Gastric Mucosal Apoptotic Injury in Experimental Burns" Molecules 23, no. 4: 749. https://doi.org/10.3390/molecules23040749




