Design, Synthesis, and Biological Evaluation of Axitinib Derivatives
Abstract
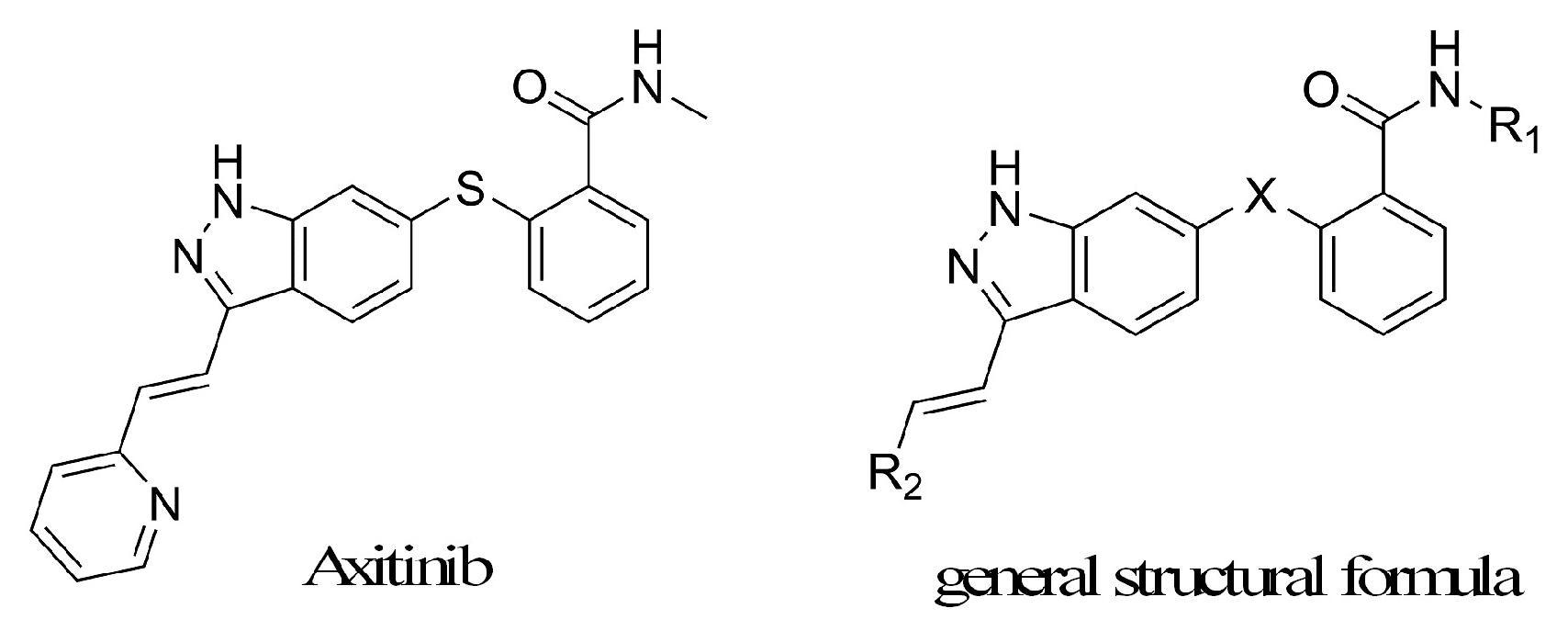
:1. Introduction
2. Results and Discussion
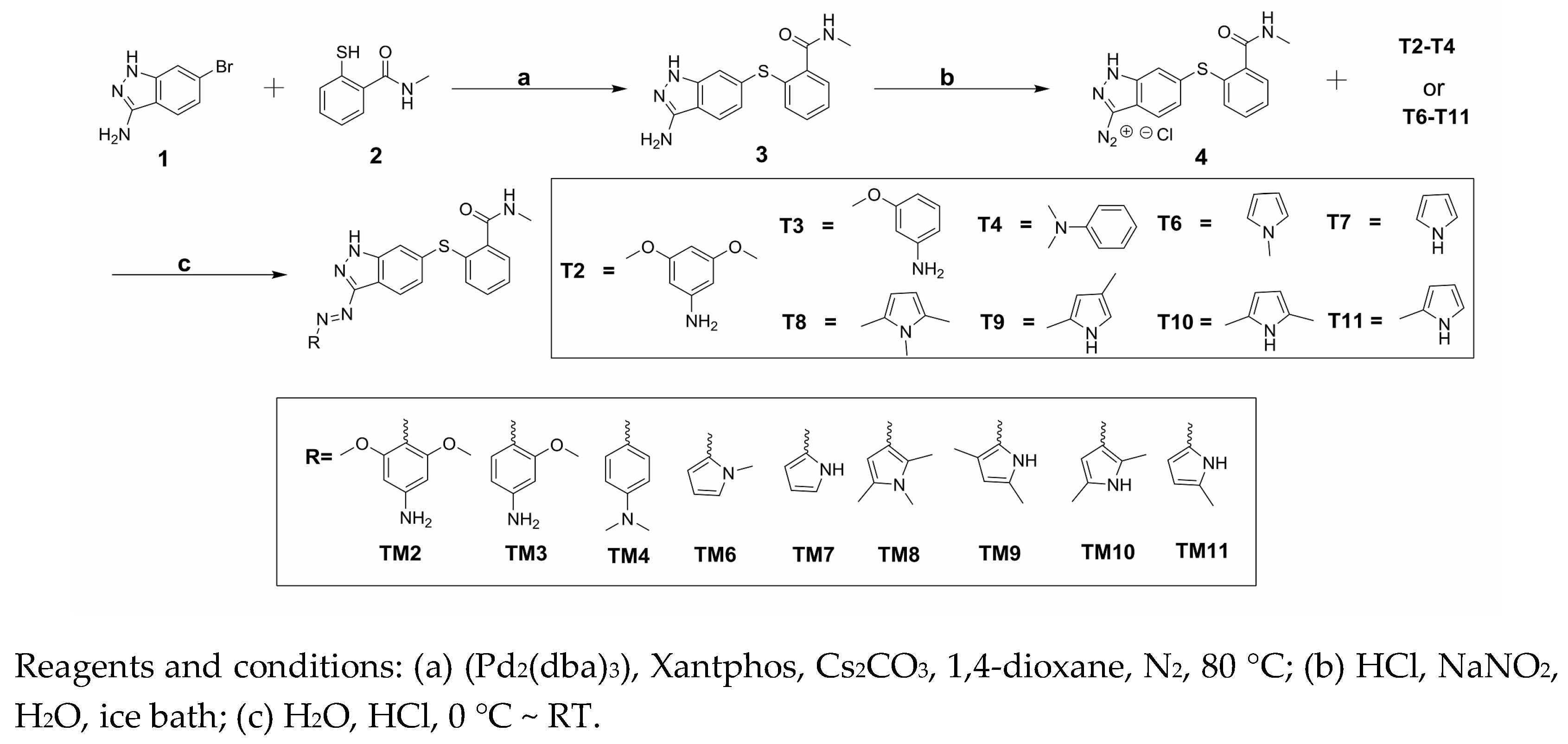
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. VEGFR-2 Kinase Assay
2.2.2. Molecular Docking Study
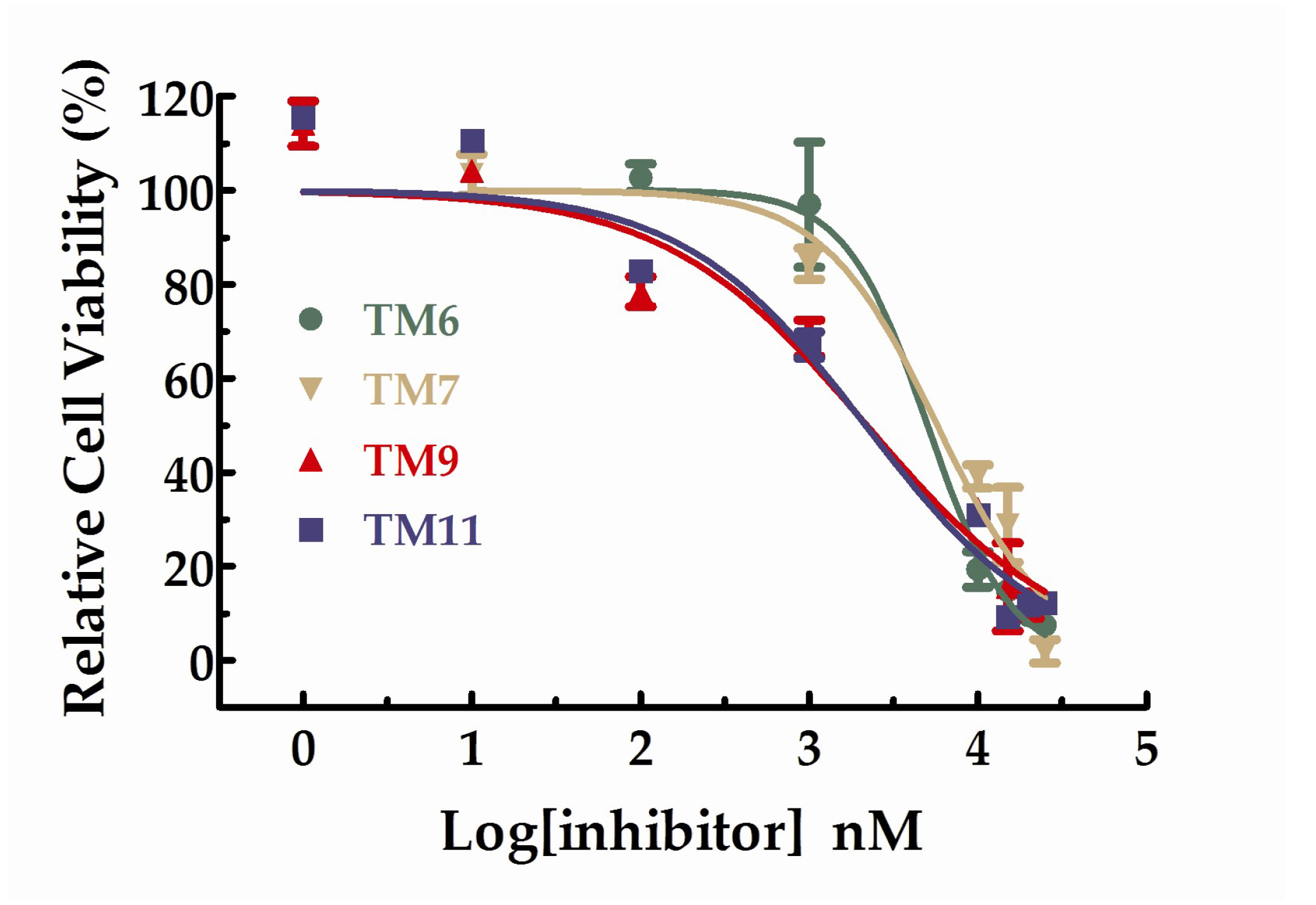
2.2.3. HUVEC Cell Anti-Proliferation Assay
3. Materials and Methods
3.1. Chemistry
General Procedure 1
3.2. VEGFR2 Kinase Assay
3.3. Molecular Docking Study
3.4. HUVEC Cell Proliferation Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brown, J.M.; Giaccia, A.J. The unique physiology of solid tumors: Opportunities (and problems) for cancer therapy. Cancer Res. 1998, 58, 1408–1416. [Google Scholar] [PubMed]
- Harris, P.A.; Boloor, A.; Cheung, M.; Kumar, R.; Crosby, R.M.; Davis-Ward, R.G.; Epperly, A.H.; Hinkle, K.W.; Hunter, R.N.; Johnson, J.H.; et al. Discovery of 5-[[4-[(2,3-dimethyl-2H-indazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-methyl-benzenesulfonamide (Pazopanib), a novel and potent vascular endothelial growth factor receptor inhibitor. J. Med. Chem. 2008, 51, 4632–4640. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Li, B.; Winer, J.; Armanini, M.; Gillett, N.; Phillips, H.S.; Ferrara, N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 1993, 362, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Hu-Lowe, D.D.; Zou, H.Y.; Grazzini, M.L.; Hallin, M.E.; Wickman, G.R.; Amundson, K.; Chen, J.H.; Rewolinski, D.A.; Yamazaki, S.; Wu, E.Y.; et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin. Cancer Res. 2008, 14, 7272–7283. [Google Scholar] [CrossRef] [PubMed]
- Rössler, J.; Monnet, Y.; Farace, F.; Opolon, P.; Daudigeos-Dubus, E.; Bourredjem, A.; Vassal, G.; Geoerger, B. The selective VEGFR1-3 inhibitor axitinib (AG-013736) shows antitumor activity in human neuroblastoma xenografts. Int. J. Cancer 2011, 128, 2748–2758. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Plimack, E.R.; Puzanov, I.; Fishman, M.N.; McDermott, D.F.; Cho, D.C.; Vaishampayan, U.; George, S.; Olencki, TE.; Tarazi, J.C.; et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: A non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol. 2018, 19, 405–415. [Google Scholar] [CrossRef]
- Bellesoeur, A.; Carton, E.; Alexandre, J.; Goldwasser, F.; Huillard, O. Axitinib in the treatment of renal cell carcinoma: Design, development, and place in therapy. Drug Des. Dev. Ther. 2017, 11, 2801–2811. [Google Scholar] [CrossRef] [PubMed]
- Solowiej, J.; Chen, J.H.; Zou, H.Y.; Grant, S.K.; Murray, B.W. Substrate-specific conformational regulation of the receptor tyrosine kinase VEGFR2 catalytic domain. ACS Chem. Biol. 2013, 17, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Pharma, A. Indazole Compounds and Pharmaceutical Compositions for Inhibiting Protein Kinases, and Methods for Their Use. Patent WO0102369, 11 January 2001. [Google Scholar]
- Dabbagh, H.A.; Teimouri, A.; Chermahini, A.N. Green and efficient diazotization and diazo coupling reactions on clays. Dyes Pigments 2007, 73, 239–244. [Google Scholar] [CrossRef]
- Morales-Guio, C.G.; Yuranov, I.; Kiwi-Minsker, L. Highly Selective Catalytic Reduction of Nitro- to Azoarenes under Ambient Conditions. Top. Catal. 2014, 57, 1526–1532. [Google Scholar] [CrossRef]
- Hansen, M.J.; Lerch, M.M.; Szymanski, W.; Feringa, B.L. Direct and Versatile Synthesis of Red-Shifted Azobenzenes. Angew. Chem. Int. Ed. 2016, 55, 13514–13518. [Google Scholar] [CrossRef] [PubMed]
- Chekal, B.P.; Guinness, S.M.; Lillie, B.M.; McLaughlim, R.W.; Palmer, C.W.; Post, R.J.; Sieser, J.E.; Singer, R.A.; Sluggett, G.W.; Vaidyanathan, R.; et al. Development of an Efficient Pd-Catalyzed Coupling Process for Axitinib. Org. Process. Res. Dev. 2013, 18, 266–274. [Google Scholar] [CrossRef]
- Kowanetz, M.; Ferrara, N. Vascular endothelial growth factor signaling pathways: Therapeutic perspective. Clin. Cancer Res. 2006, 12, 5018–5022. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Huang, Z.; Bai, Z.; Xie, R.; Sun, L.; Lin, K. Development and strategies of VEGFR-2/KDR inhibitors. Future Med. Chem. 2012, 4, 1839–1852. [Google Scholar] [CrossRef] [PubMed]
- Mathis, G.; HTRF®. Technology. J. Biomol. Screen. 1999, 4, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Degorce, F.; Card, A.; Soh, S.; Trinquet, E.; Knapik, G.P.; Xie, B. HTRF: A technology tailored for drug discovery—A review of theoretical aspects and recent applications. Curr. Chem. Genom. 2009, 3, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, H.; Guo, N.; Niu, G.; Ma, Y.; Kiesewetter, D.; Chen, X. Site-specific labeling of scVEGF with fluorine-18 for positronemission tomography imaging. Theranostics 2012, 2, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Zhang, Y.; Georgescu, S.P.; Johnson, K.L.; Kong, D.; Galper, J.B. Human umbilical vein endothelial cells and humandermal microvascular endothelial cells offer new insights into therelationship between lipid metabolism and angiogenesis. Stem. Cell Rev. Rep. 2006, 2, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Shi, W.; Sun, L.; Li, H.; Jiang, Z.; Zhang, L. Pristimerin, a triterpenoid, inhibits tumorangiogenesis by targeting VEGFR2 activation. Molecules 2012, 17, 6854–6868. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Jin, H.; Chen, Z.; Xie, W.; Wang, Y.; Wang, Y.; Wang, M.; Zhang, J.; Acheampong, D.O. A novel bispecific diabody targeting both vascular endothelial growth factor receptor 2 and epidermal growth factor receptor for enhanced antitumor activity. Biotechnol. Prog. 2016, 32, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J. The role of VEGF and EGFR inhibition: Implications for combining anti-VEGF and anti-EGFR agents. Mol. Cancer Res. 2007, 5, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Pemovska, T.; Johnson, E.; Kontro, M.; Repasky, G.A.; Chen, J.; Wells, P.; Cronin, C.N.; McTigue, M.; Kallioniemi, O.; Porkka, K.; et al. Axitinib effectively inhibits BCR-ABL1(T315I) with a distinct binding conformation. Nature 2015, 519, 102–105. [Google Scholar] [CrossRef] [PubMed]
- McTigue, M.; Murray, B.W.; Chen, J.H.; Deng, Y.L.; Solowiej, J.; Kania, R.S. Molecular conformations, interactions, and properties associated with drug efficiency and clinical performance among VEGFR TK inhibitors. Proc. Natl. Acad. Sci. USA 2012, 109, 18281–18289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Zhang, D.; Wang, L.; Lu, T.; Jiao, Y. Discovery of Novel Potent VEGFR-2 Inhibitors Exerting Significant Antiproliferative Activity against Cancer Cell Lines. J. Med. Chem. 2017, 61, 140–157. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| Compound | IC50 (nM) ± SD (n = 3) |
|---|---|
| Axitinib | 7.3 ± 1.07 |
| TM2 | 3000 ± 1.11 |
| TM3 | 195 ± 1.11 |
| TM4 | 135 ± 1.25 |
| TM6 | 2290 ± 1.17 |
| TM7 | 123 ± 1.08 |
| TM8 | 158 ± 1.14 |
| TM9 | 201 ± 1.26 |
| TM10 | 44 ± 1.15 |
| TM11 | 330 ± 1.14 |
| Compound | Relative Cell Viability (%) ± SD (n = 3) | |
|---|---|---|
| 1 µM | 10 µM | |
| Axitinib | 79.97 ± 6.64 | 53.22 ± 9.11 |
| TM2 | 106.56 ± 1.29 | 94.40 ± 5.01 |
| TM3 | 125.46 ± 8.92 | 84.14 ± 6.83 |
| TM4 | 94.12 ± 1.89 | 82.76 ± 5.32 |
| TM6 | 96.95 ± 10.44 | 19.33 ± 3.82 |
| TM7 | 84.36 ± 4.35 | 39.13 ± 2.49 |
| TM8 | 92.34 ± 9.05 | 61.26 ± 10.94 |
| TM9 | 68.54 ± 0.29 | 32.20 ± 1.99 |
| TM10 | 99.29 ± 0.78 | 66.22 ± 4.43 |
| TM11 | 67.03 ± 0.19 | 30.97 ± 0.61 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, N.; Liang, J.; Peng, S.; Sun, Q.; Dai, Q.; Dong, M. Design, Synthesis, and Biological Evaluation of Axitinib Derivatives. Molecules 2018, 23, 747. https://doi.org/10.3390/molecules23040747
Wei N, Liang J, Peng S, Sun Q, Dai Q, Dong M. Design, Synthesis, and Biological Evaluation of Axitinib Derivatives. Molecules. 2018; 23(4):747. https://doi.org/10.3390/molecules23040747
Chicago/Turabian StyleWei, Na, Jianqing Liang, Shengming Peng, Qiang Sun, Qiuyun Dai, and Mingxin Dong. 2018. "Design, Synthesis, and Biological Evaluation of Axitinib Derivatives" Molecules 23, no. 4: 747. https://doi.org/10.3390/molecules23040747





