A Metabolomics Approach Uncovers Differences between Traditional and Commercial Dairy Products in Buryatia (Russian Federation)
Abstract
:1. Introduction
2. Results and Discussion
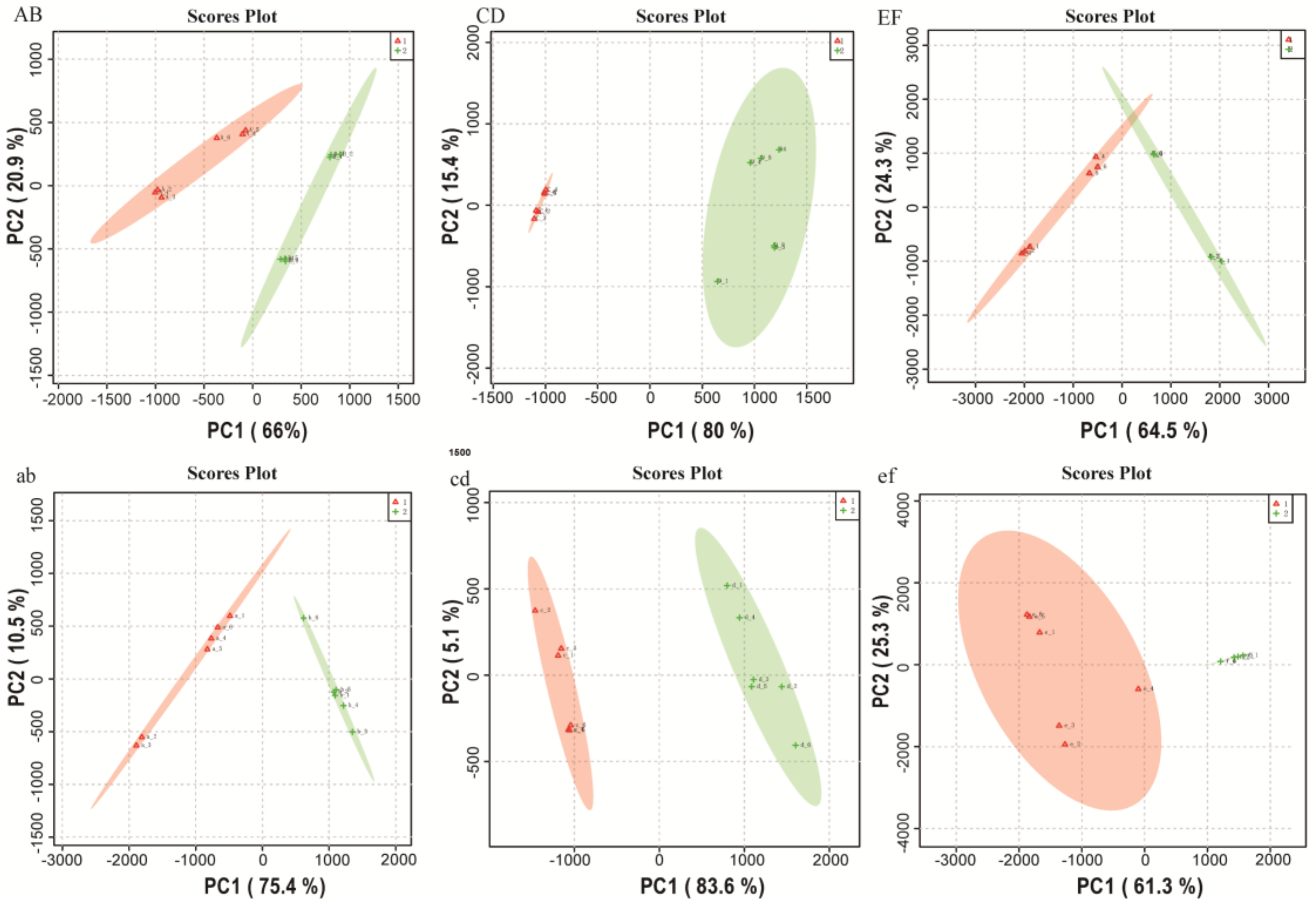
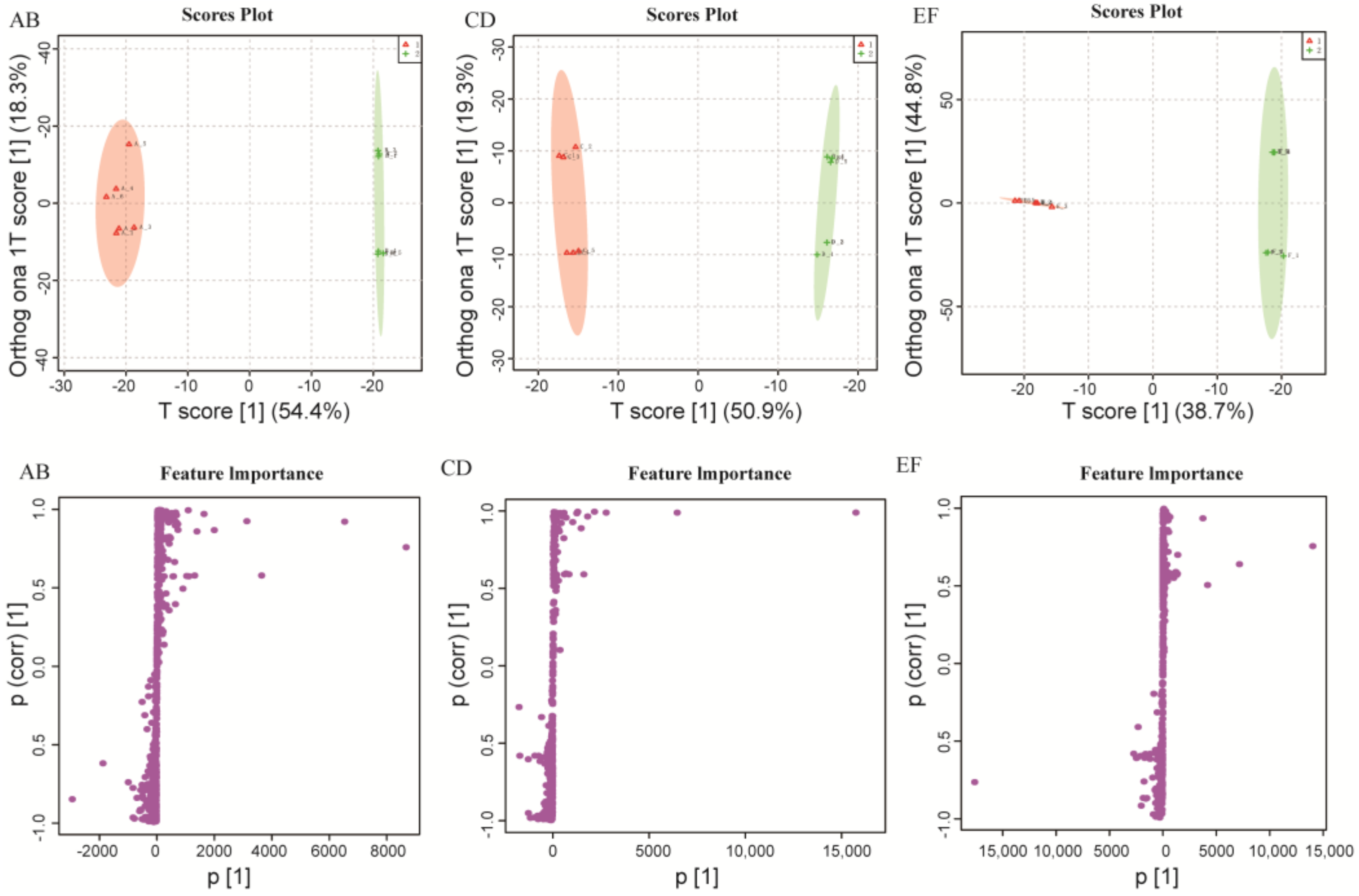
2.1. Metabolomics Data Analysis
2.2. Comparison of Metabolite Profiles Between the Different Groups
3. Discussion
3.1. Effects of Starter Diversity on the Metabolites of Dairy Products
3.2. Amino Acids
3.3. Peptides
3.4. Other Metabolites
4. Materials and Methods
4.1. Sample Collection
4.2. Sample Preparation for UPLC-Q-TOF MSE Analysis
4.3. UPLC-Q-TOF MSE Analysis
4.4. UPLC-Q-TOF MSE Data Processing and Multivariate Statistical Analyses
4.5. Identification of Key Metabolites
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Le Boucher, C.; Courant, F.; Jeanson, S.; Chereau, S.; Maillard, M.B.; Royer, A.L.; Thierry, A.; Dervilly-Pinel, G.; Le Bizec, B.; Lortal, S. First mass spectrometry metabolic fingerprinting of bacterial metabolism in a model cheese. Food Chem. 2013, 141, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, H.M.; Zha, M.S.; Qing, Y.T.; Bai, N.; Ren, Y.; Xi, X.X.; Liu, W.J.; Menghe, B.L.G.; Zhang, H.P. Molecular identification and quantification of lactic acid bacteria in traditional fermented dairy foods of Russia. J. Dairy Sci. 2015, 98, 5143–5154. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martín, P.; Flórez, A.B.; Hernández-Barranco, A.; Mayo, B. Interaction between dairy yeasts and lactic acid bacteria strains during milk fermentation. Food Control 2008, 19, 62–70. [Google Scholar] [CrossRef]
- Gadaga, T.H.; Mutukumira, A.N.; Narvhus, J.A. The growth and interaction of yeasts and lactic acid bacteria isolated from Zimbabwean naturally fermented milk in UHT milk. Int. J. Food Microbiol. 2001, 68, 21–32. [Google Scholar] [CrossRef]
- Hugenholtz, J. Citrate metabolism in lactic acid bacteria. FEMS Microbiol. Rev. 1993, 12, 165–178. [Google Scholar] [CrossRef]
- Panesar, P.S. Fermented Dairy Products: Starter Cultures and Potential Nutritional Benefits. Food Nutr. Sci. 2011, 2, 47–51. [Google Scholar] [CrossRef]
- Oguro, Y.; Nishiwaki, T.; Shinada, R.; Kobayashi, K.; Kurahashi, A. Metabolite profile of koji amazake and its lactic acid fermentation product by Lactobacillus sakei UONUMA. J. Biosci. Bioeng. 2017, 124, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Le Boucher, C.; Courant, F.; Royer, A.-L.; Jeanson, S.; Lortal, S.; Dervilly-Pinel, G.; Thierry, A.; Le Bizec, B. LC–HRMS fingerprinting as an efficient approach to highlight fine differences in cheese metabolome during ripening. Metabolomics 2015, 11, 1117–1130. [Google Scholar] [CrossRef]
- Mozzi, F.; Ortiz, M.E.; Bleckwedel, J.; De Vuyst, L.; Pescuma, M. Metabolomics as a tool for the comprehensive understanding of fermented and functional foods with lactic acid bacteria. Food Res. Int. 2013, 54, 1152–1161. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C. Systems biology: Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar] [CrossRef] [PubMed]
- Reaves, M.L.; Rabinowitz, J.D. Metabolomics in systems microbiology. Curr. Opin. Biotechnol. 2011, 22, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Lindon, J.C.; Holmes, E.; Nicholson, J.K. Metabonomics techniques and applications to pharmaceutical research & development. Pharm. Res. 2006, 23, 1075–1088. [Google Scholar] [PubMed]
- Klein, M.S.; Almstetter, M.F.; Schlamberger, G.; Nurnberger, N.; Dettmer, K.; Oefner, P.J.; Meyer, H.H.; Wiedemann, S.; Gronwald, W. Nuclear magnetic resonance and mass spectrometry-based milk metabolomics in dairy cows during early and late lactation. J. Dairy Sci. 2010, 93, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Yang, H.J.; Kim, M.J.; Han, E.S.; Kim, H.J.; Kwon, D.Y. Metabolomic analysis of meju during fermentation by ultra performance liquid chromatography-quadrupole-time of flight mass spectrometry (UPLC-Q-TOF MS). Food Chem. 2011, 127, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Sundekilde, U.K.; Larsen, L.B.; Bertram, H.C. NMR-Based Milk Metabolomics. Metabolites 2013, 3, 204–222. [Google Scholar] [CrossRef] [PubMed]
- Boudonck, K.J.; Mitchell, M.W.; Wulff, J.; Ryals, J.A. Characterization of the biochemical variability of bovine milk using metabolomics. Metabolomics 2009, 5, 375–386. [Google Scholar] [CrossRef]
- Piras, C.; Marincola, F.C.; Savorani, F.; Engelsen, S.B.; Cosentino, S.; Viale, S.; Pisano, M.B. A NMR metabolomics study of the ripening process of the Fiore Sardo cheese produced with autochthonous adjunct cultures. Food Chem. 2013, 141, 2137–2147. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.O.; Kim, M.S.; Liu, K.H.; Auh, J.H.; Kim, Y.S.; Kwon, D.Y.; Choi, H.K. Classification of fermented soybean paste during fermentation by 1H nuclear magnetic resonance spectroscopy and principal component analysis. Biosci. Biotechnol. Biochem. 2009, 73, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Namgung, H.J.; Park, H.J.; Cho, I.H.; Choi, H.K.; Kwon, D.Y.; Shim, S.M.; Kim, Y.S. Metabolite profiling of doenjang, fermented soybean paste, during fermentation. J. Sci. Food Agric. 2010, 90, 1926–1935. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Cho, I.H.; Lee, S.; Choi, H.K.; Kwon, D.Y.; Kim, Y.S. Metabolite profiling of Cheonggukjang, a fermented soybean paste, during fermentation by gas chromatography-mass spectrometry and principal component analysis. Food Chem. 2010, 122, 1313–1319. [Google Scholar] [CrossRef]
- Ochi, H.; Naito, H.; Iwatsuki, K.; Bamba, T.; Fukusaki, E. Metabolomics-based component profiling of hard and semi-hard natural cheeses with gas chromatography/time-of-flight-mass spectrometry, and its application to sensory predictive modeling. J. Biosci. Bioeng. 2012, 113, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Han, R.W.; Zheng, N.; Yu, Z.N.; Wang, J.; Xu, X.M.; Qu, X.Y.; Li, S.L.; Zhang, Y.D.; Wang, J.Q. Simultaneous determination of 38 veterinary antibiotic residues in raw milk by UPLC-MS/MS. Food Chem. 2015, 181, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Metabolomics: Applications to food science and nutrition research. Trends Food Sci. Technol. 2008, 19, 482–493. [Google Scholar] [CrossRef]
- Settachaimongkon, S.; Nout, M.J.; Antunes Fernandes, E.C.; Hettinga, K.A.; Vervoort, J.M.; van Hooijdonk, T.C.; Zwietering, M.H.; Smid, E.J.; van Valenberg, H.J. Influence of different proteolytic strains of Streptococcus thermophilus in co-culture with Lactobacillus delbrueckii subsp. bulgaricus on the metabolite profile of set-yoghurt. Int. J. Food Microbiol. 2014, 177, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Settachaimongkon, S.; van Valenberg, H.J.; Gazi, I.; Nout, M.J.; van Hooijdonk, T.C.; Zwietering, M.H.; Smid, E.J. Influence of Lactobacillus plantarum WCFS1 on post-acidification, metabolite formation and survival of starter bacteria in set-yoghurt. Food Microbiol. 2016, 59, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.G.; Shim, S.M.; Kwon, D.Y.; Choi, H.K.; Lee, C.H.; Kim, Y.S. Metabolite profiling of Cheonggukjang, a fermented soybean paste, inoculated with various Bacillus strains during fermentation. Biosci. Biotechnol. Biochem. 2010, 74, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Chattoraj, D.K.; Chattopadhyay, P. Studies on changes in microstructure and proteolysis in cow and soy milk curd during fermentation using lactic cultures for improving protein bioavailability. J. Food Sci. Technol. 2013, 50, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Pisano, M.B.; Scano, P.; Murgia, A.; Cosentino, S.; Caboni, P. Metabolomics and microbiological profile of Italian mozzarella cheese produced with buffalo and cow milk. Food Chem. 2016, 192, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Hagi, T.; Kobayashi, M.; Nomura, M. Metabolome analysis of milk fermented by gamma-aminobutyric acid-producing Lactococcus lactis. J. Dairy Sci. 2016, 99, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, P.; Piccolo, A. 1H HRMAS-NMR metabolomic to assess quality and traceability of mozzarella cheese from Campania buffalo milk. Food Chem. 2012, 132, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all dairy products are available from the authors. |
| RT (min) | m/z | Identities | MF | MP | Fold-Change | p-Value |
|---|---|---|---|---|---|---|
| Group AB/Positive | ||||||
| 0.8962 | 85.0224 | 4-Aminobutyraldehyde | C4H9NO | AA | 0.42 | 2.38 × 10−5 |
| 0.7689 | 248.0213 | 4,4′-Sulfonyldianiline | C12H12N2O2S | AA | 248,090 | 6.23 × 10−5 |
| 0.8486 | 163.0584 | N-Acetyl-l-cysteine | C5H9NO3S | - | 0.52 | 7.50 × 10−5 |
| 0.8739 | 145.0474 | l-allysine | C6H11NO3 | - | 0.38 | 0.01876 |
| 0.9598 | 289.0916 | Asp-Arg | C10H19N5O5 | PP | 0.0051876 | 3.63 × 10−7 |
| 0.9691 | 127.0359 | N-Cyclohexylformamide | C7H13NO | - | 0.07 | 0.00059 |
| 0.9945 | 254.1610 | Hexadecenoic acid | C16H30O2 | LL | 0.02 | 1.31 × 10−8 |
| 1.5347 | 120.7750 | Phenylacetaldehyde | C8H8O | AA | 1.36 | 2.46 × 10−5 |
| 1.6726 | 100.0709 | 4-Methylpentanal | C6H12O | LL | 12.84 | 1.76 × 10−8 |
| 2.6366 | 146.0578 | l-Lysine | C6H14N2O2 | AA/SM/LL/MT/CV/TL | 3.15 | 4.14 × 10−5 |
| 2.6422 | 188.0694 | homo-cis-Aconitate | C7H8O6 | AA | 3.97 | 2.96 × 10−5 |
| 3.7878 | 227.1748 | Deoxycytidine | C9H13N3O4 | NT | 240,070 | 6.62 × 10−7 |
| 3.9296 | 246.1697 | Methionyl proline | C10H18N2O3S | TL | 191,130 | 0.00021 |
| 5.2484 | 141.0520 | Histidinol | C6H11N3O | AA/SM | 4.64 × 10−7 | 1.35 × 10−6 |
| 8.7712 | 230.1133 | Ergothioneine | C9H15N3O2S | AA | 9.16 | 1.83 × 10−9 |
| 5.2484 | 126.0279 | Thymine | C5H6N2O2 | NT | 0.0001341 | 8.27 × 10−9 |
| 6.0582 | 120.0774 | Purine | C5H4N4 | NT/CH | 286,000 | 2.64 × 10−7 |
| 8.2841 | 251.1497 | Deoxyadenosine | C10H13N5O3 | NT | 197,920 | 4.11 × 10−7 |
| 9.4986 | 231.1697 | Gly-arg | C8H17N5O3 | PP | 4.87 × 10−6 | 1.42 × 10−6 |
| 14.6231 | 274.2742 | Lys-Lys | C12H26N4O3 | PP | 123.70 | 0.00381 |
| 15.2054 | 149.0212 | l-Methionine | C5H11NO2S | AA/TL/SM | 1.62 × 10−6 | 1.29 × 10−5 |
| Group AB/Negative | ||||||
| 1.5263 | 252.0899 | His-Pro | C11H16N4O3 | PP | 1.91 × 10−7 | 0.00020 |
| 1.6961 | 229.1568 | Pro-Asn | C9H15N3O4 | PP | 4,255,800 | 2.97 × 10−7 |
| 2.3017 | 119.0502 | Threonine | C4H9NO3 | AA | 2.59 × 10−7 | 2.52 × 10−9 |
| 2.6692 | 236.0936 | Ala-Phe-Ala | C12H16N2O3 | PP | 3.33 × 10−7 | 3.00 × 10−7 |
| 1.0490 | 218.0679 | N-Acetylserotonin | C12H14N2O2 | AA | 3.40 × 10−7 | 5.55 × 10−6 |
| 2.0917 | 109.0293 | Aminophenol | C6H7NO | AA | 3.78 × 10−7 | 5.44 × 10−8 |
| 4.6603 | 277.1568 | Arg-Cys | C9H19N5O3S | PP | 1,862,300 | 0.00020 |
| 1.5262 | 188.0940 | Ethyl glutarate | C9H16O4 | - | 5.78 × 10−7 | 6.58 × 10−6 |
| 2.5673 | 140.1082 | Ethyl furoate | C7H8O3 | - | 5.92 × 10−7 | 5.11 × 10−6 |
| 2.2441 | 158.1203 | Pentyl butyrate | C9H18O2 | - | 1.40 × 10−6 | 3.29 × 10−5 |
| 0.7095 | 95.9522 | Sodium propionate | C3H5NaO2 | - | 578,660 | 4.89 × 10−5 |
| 1.8233 | 144.0477 | Octanoic acid | C8H16O2 | - | 504,840 | 0.00070 |
| 1.7620 | 175.0628 | l-Citrulline | C6H13N3O3 | AA/SM | 371,400 | 0.00043 |
| 1.7515 | 115.0419 | l-Proline | C5H9NO2 | AA/SM/MT/TL | 360,600 | 8.55 × 10−5 |
| 1.0344 | 305.0895 | Thr-Trp | C15H19N3O4 | PP | 0.000104 | 6.20 × 10−6 |
| 1.5826 | 242.0134 | Thymidine | C10H14N2O5 | NT | 0.000872 | 9.14 × 10−9 |
| 1.7400 | 180.0685 | d-Fructose | C6H12O6 | CH | 604.44 | 1.32 × 10−6 |
| 1.5606 | 162.0562 | Trimethylamine | C3H9N | EG/CH | 0.002953 | 4.10 × 10−7 |
| 0.7924 | 197.0226 | N-Hydroxy-l-tyrosine | C9H11NO4 | SM | <0.003601 | 1.43 × 10−5 |
| 2.7630 | 204.0687 | Ala-Asp | C7H12N2O5 | PP | <0.004426 | 3.90 × 10−5 |
| 1.0189 | 190.0735 | Asp-Gly | C6H10N2O5 | PP | 0.01 | 1.80 × 10−7 |
| 1.7732 | 252.0892 | Ala-Tyr | C12H16N2O4 | PP | 140.78 | 0.00041 |
| 2.5732 | 263.1406 | Asn-Met | C9H17N3O4S | PP | 132.26 | 8.29 × 10−7 |
| 0.7738 | 149.0453 | l-Methionine | C5H11NO2S | AA/TL/SM | 0.011586 | 1.33 × 10−5 |
| 1.0465 | 146.0453 | L-Lysine | C6H14N2O2 | AA/SM/LL/MT/CV/TL | 0.02 | 2.29 × 10−5 |
| 0.9995 | 129.0195 | Isoquinoline | C9H7N | AA/SM | 0.03 | 1.56 × 10−9 |
| 0.9995 | 220.0733 | Met-Ala | C8H16N2O3S | PP | 0.03 | 0.00019 |
| 1.4599 | 181.0513 | Tyrosine | C9H11NO3 | AA | 0.04 | 2.68 × 10−7 |
| 1.4716 | 258.0065 | His-Cys | C9H14N4O3S | PP | 0.06 | 7.40 × 10−7 |
| 0.7334 | 105.0183 | l-Serine | C3H7NO3 | AA/SM | 0.07 | 4.63 × 10−5 |
| 1.0926 | 117.0190 | l-Valine | C5H11NO2 | AA | 0.07 | 1.43 × 10−6 |
| 1.4746 | 163.0403 | N-Acetyl-l-cysteine | C5H9NO3S | - | 0.09 | 1.96 × 10−5 |
| 1.4847 | 119.0499 | Threonine | C4H9NO3 | AA | 0.10 | 2.38 × 10−5 |
| 1.4851 | 135.0452 | l-Homocysteine | C4H9NO2S | AA/SM | 0.11 | 6.19 × 10−5 |
| 1.3484 | 218.1040 | Thr-Val | C9H18N2O4 | PP | 0.13 | 3.68 × 10−5 |
| 1.7620 | 208.0990 | Cys-Ser | C6H12N2O4S | PP | 7.35 | 0.00038 |
| 1.7771 | 147.0336 | O-Acetyl-l-serine | C5H9NO4 | AA | 6.19 | 0.00068 |
| Group CD/Positive | ||||||
| 0.9984 | 343.1224 | Coenzyme B | C11H22NO7PS | - | 2.54 × 10−6 | 3.37 × 10−11 |
| 1.0121 | 149.2115 | Methionine | C5H11NO2S | AA/SM | 0.57 | 0.00035 |
| 3.7640 | 227.1748 | Pretyrosine | C10H13NO5 | AA | 89,506 | 1.12 × 10−7 |
| 0.7825 | 360.1494 | Aldosterone | C21H28O5 | LL | 87,489 | 1.23 × 10−8 |
| 8.2828 | 229.1543 | Pro-Asn | C9H15N3O4 | PP | 5852.70 | 2.76 × 10−7 |
| 8.7424 | 245.1130 | β-Alanyl-l-arginine | C9H19N5O3 | - | 693.81 | 245.11300 |
| 0.8106 | 219.0260 | O-Succinylhomoserine | C8H13NO6 | AA | 0.001788 | 219.02600 |
| 0.7955 | 203.0515 | Lys-Gly | C8H17N3O3 | PP | 0.013665 | 1.92 × 10−9 |
| 1.0017 | 145.0471 | 2-Oxoglutaramate | C5H7NO4 | AA | 0.03 | 9.12 × 10−9 |
| 0.9794 | 85.0226 | Piperidine | C5H11N | AA | 0.34 | 1.46 × 10−6 |
| Group CD/Negative | ||||||
| 0.8057 | 59.0112 | Trimethylamine | C3H9N | EG | 0.05 | 1.41 × 10−7 |
| 0.6974 | 79.9565 | Pyrimidine | C4H4N2 | NT/AA/CH | 34,677 | 0.00034 |
| 2.4645 | 103.0543 | N,N-Dimethylglycine | C4H9NO2 | AA | 0.000495 | 3.01 × 10−5 |
| 0.8080 | 119.0356 | l-Homoserine | C4H9NO3 | AA | 0.03 | 6.21 × 10−11 |
| 3.0080 | 119.0507 | Threonine | C4H9NO3 | AA | 45,026 | 0.00058 |
| 4.8198 | 121.0595 | l-Cysteine | C3H7NO2S | AA | 4.29 | 5.07 × 10−6 |
| 0.8044 | 125.0252 | 5-Methylcytosine | C5H7N3O | AA/SM | 0.07 | 1.95 × 10−6 |
| 1.0160 | 129.0206 | 4-Oxoproline | C5H7NO3 | AA | 0.30 | 1.53 × 10−6 |
| 0.7957 | 131.0359 | l-Leucine | C6H13NO2 | AA | 18.69 | 8.05 × 10−5 |
| 1.7654 | 147.0343 | O-Acetyl-l-serine | C5H9NO4 | AA | 12.01 | 0.00037 |
| 0.7748 | 161.0462 | O-Acetyl-l-homoserine | C6H11NO4 | AA | 0.35 | 1.09 × 10−5 |
| 1.7504 | 162.0563 | Gly-Ser | C5H10N2O4 | PP | 31,159 | 7.46 × 10−5 |
| 2.9981 | 165.0566 | l-Phenylalanine | C9H11NO2 | AA/TL | 1,200,100 | 0.00061 |
| 1.0140 | 177.0224 | N-Formyl-l-methionine | C6H11NO3S | AA | 13.71 | 7.75 × 10−5 |
| 1.5544 | 181.0513 | Tyrosine | C9H11NO3 | AA | 0.44 | 0.00051 |
| 4.4615 | 193.0874 | Phenylacetylglycine | C10H11NO3 | AA | 1062.60 | 0.00031 |
| 0.7555 | 202.9701 | Cystathionine | C7H14N2O4S | AA | 57,753 | 0.00044 |
| 1.6778 | 208.0980 | Cys-Ser | C6H12N2O4S | PP | 3.77 | 5.36 × 10−5 |
| 1.6254 | 222.0797 | Gly-Phe | C11H14N2O3 | PP | 5.03 | 0.00029 |
| 5.0840 | 226.0190 | Carnosine | C9H14N4O3 | AA | 14.70 | 226.01900 |
| 0.8045 | 236.0386 | Ser-Met | C8H16N2O4S | PP | 53,541 | 0.00051 |
| 2.9149 | 242.0137 | Thymidine | C10H14N2O5 | NT | 0.12 | 8.74 × 10−5 |
| 1.7009 | 252.0893 | Pro-His | C11H16N4O3 | PP | 111.38 | 7.03 × 10−9 |
| 2.5898 | 263.1405 | Met-Asn | C9H17N3O4S | PP | 568.34 | 0.00035 |
| 0.7992 | 269.0884 | His-Asn | C10H15N5O4 | PP | 0.06 | 3.72 × 10−5 |
| 2.8149 | 283.0847 | Gln-His | C11H17N5O4 | PP | 151,190 | 0.00049 |
| 1.6477 | 293.1519 | Lys-Phe | C15H23N3O3 | PP | 73.11 | 0.00022 |
| 0.8082 | 341.1089 | His-Trp | C17H19N5O3 | PP | 0.001072 | 3.93 × 10−12 |
| Group EF/Positive | ||||||
| 0.7924 | 133.0260 | Aspartic acid | C4H7NO4 | - | 109.94 | 0.00018 |
| 0.8467 | 163.0592 | N-Acetyl-l-cysteine | C5H9NO3S | - | 2.94 | 0.00824 |
| 3.9198 | 187.0960 | N-Heptanoylglycine | C9H17NO3 | - | 6.41 | 1.18 × 10−8 |
| 0.9499 | 193.0341 | Phenylacetylglycine | C10H11NO3 | - | 106.36 | 9.63 × 10−5 |
| 0.9514 | 210.0607 | d-Glucaric acid | C6H10O8 | - | 608.64 | 0.00012 |
| 1.9229 | 232.1542 | Asp-Val | C9H16N2O5 | PP | 4.48 | 2.74 × 10−8 |
| 0.9217 | 235.0874 | Cys-Asn | C7H13N3O4S | PP | 1.76 × 10−5 | 0.00808 |
| 3.9316 | 246.1701 | Leu-Asp | C10H18N2O5 | PP | 6.99 | 4.74 × 10−9 |
| 0.7986 | 248.0219 | Asp-Asp | C8H12N2O7 | PP | 214,930 | 0.00021 |
| 0.9859 | 254.1610 | His-Val | C11H18N4O3 | PP | 0.08 | 0.00063 |
| 14.5615 | 274.2738 | Lys-Lys | C12H26N4O3 | PP | 6.13 × 10−7 | 0.00424 |
| 0.8153 | 293.0983 | Gln-Phe | C14H19N3O4 | PP | 75376 | 0.001205 |
| 0.8302 | 360.1497 | Trp-Arg | C17H24N6O3 | PP | 11.74 | 3.78 × 10−5 |
| Group EF/Negative | ||||||
| 0.7839 | 89.0235 | l-Alanine | C3H7NO2 | AA/SM/TL | 1.06 × 10−5 | 0.00079 |
| 0.7404 | 95.9509 | Methanesulfonic acid | CH4O3S | EG | 1387.50 | 1.19 × 10−5 |
| 0.6579 | 107.9440 | Benzoquinone | C6H4O2 | SM | 4959.20 | 4.51 × 10−6 |
| 0.9486 | 111.0112 | Cytosine | C4H5N3O | NT | 3.65 | 6.50 × 10−5 |
| 1.6075 | 115.0384 | l-Proline | C5H9NO2 | AA/SM | 5.53 | 1.06 × 10−5 |
| 1.5504 | 117.0549 | l-Valine | C5H11NO2 | AA/TL/SM | 20.24 | 3.07 × 10−7 |
| 1.6300 | 119.0499 | l-Homoserine | C4H9NO3 | AA | 2.02 × 10−5 | 0.00039 |
| 0.9670 | 129.0186 | 4-Oxoproline | C5H7NO3 | AA | 6128 | 7.20 × 10−8 |
| 0.7902 | 146.9834 | Glutamic acid | C5H9NO4 | - | 1.34 × 10−5 | 8.08 × 10−5 |
| 0.6532 | 152.9172 | 3-Sulfino-l-alanine | C3H7NO4S | - | 106,100 | 0.00022 |
| 1.7560 | 160.0406 | d-Alanyl-d-alanine | C6H12N2O3 | - | 4.43 × 10−5 | 0.00197 |
| 0.7889 | 161.0465 | O-Acetyl-l-homoserine | C6H11NO4 | - | 0.21 | 0.00013 |
| 0.9159 | 211.0028 | Phosphocreatine | C4H10N3O5P | - | 51.19 | 0.00298 |
| 1.3969 | 218.1037 | Thr-Val | C9H18N2O4 | PP | 0.26 | 0.00024 |
| 1.7541 | 239.9973 | l-Cystine | C6H12N2O4S2 | - | <0.01 | 0.00303 |
| 0.7222 | 291.0835 | Ser-Trp | C14H17N3O4 | PP | 89.85 | 5.05 × 10−5 |
| 10.5696 | 293.1765 | Lys-Phe | C15H23N3O3 | PP | 0.001526 | 0.00025 |
| 0.7730 | 426.9988 | Adenosine diphosphate | C10H15N5O10P2 | - | 6227.70 | 7.00 × 10−5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.; Yu, J.; Mi, Z.; Mo, L.; Jin, H.; Yao, C.; Ren, D.; Menghe, B. A Metabolomics Approach Uncovers Differences between Traditional and Commercial Dairy Products in Buryatia (Russian Federation). Molecules 2018, 23, 735. https://doi.org/10.3390/molecules23040735
Pan L, Yu J, Mi Z, Mo L, Jin H, Yao C, Ren D, Menghe B. A Metabolomics Approach Uncovers Differences between Traditional and Commercial Dairy Products in Buryatia (Russian Federation). Molecules. 2018; 23(4):735. https://doi.org/10.3390/molecules23040735
Chicago/Turabian StylePan, Lin, Jie Yu, Zhihui Mi, Lanxin Mo, Hao Jin, Caiqing Yao, Dongyan Ren, and Bilige Menghe. 2018. "A Metabolomics Approach Uncovers Differences between Traditional and Commercial Dairy Products in Buryatia (Russian Federation)" Molecules 23, no. 4: 735. https://doi.org/10.3390/molecules23040735