Nutraceutical Potential of Phenolics from ′Brava′ and ′Mansa′ Extra-Virgin Olive Oils on the Inhibition of Enzymes Associated to Neurodegenerative Disorders in Comparison with Those of ′Picual′ and ′Cornicabra′
Abstract
:1. Introduction
2. Results
2.1. Phenolic Profile
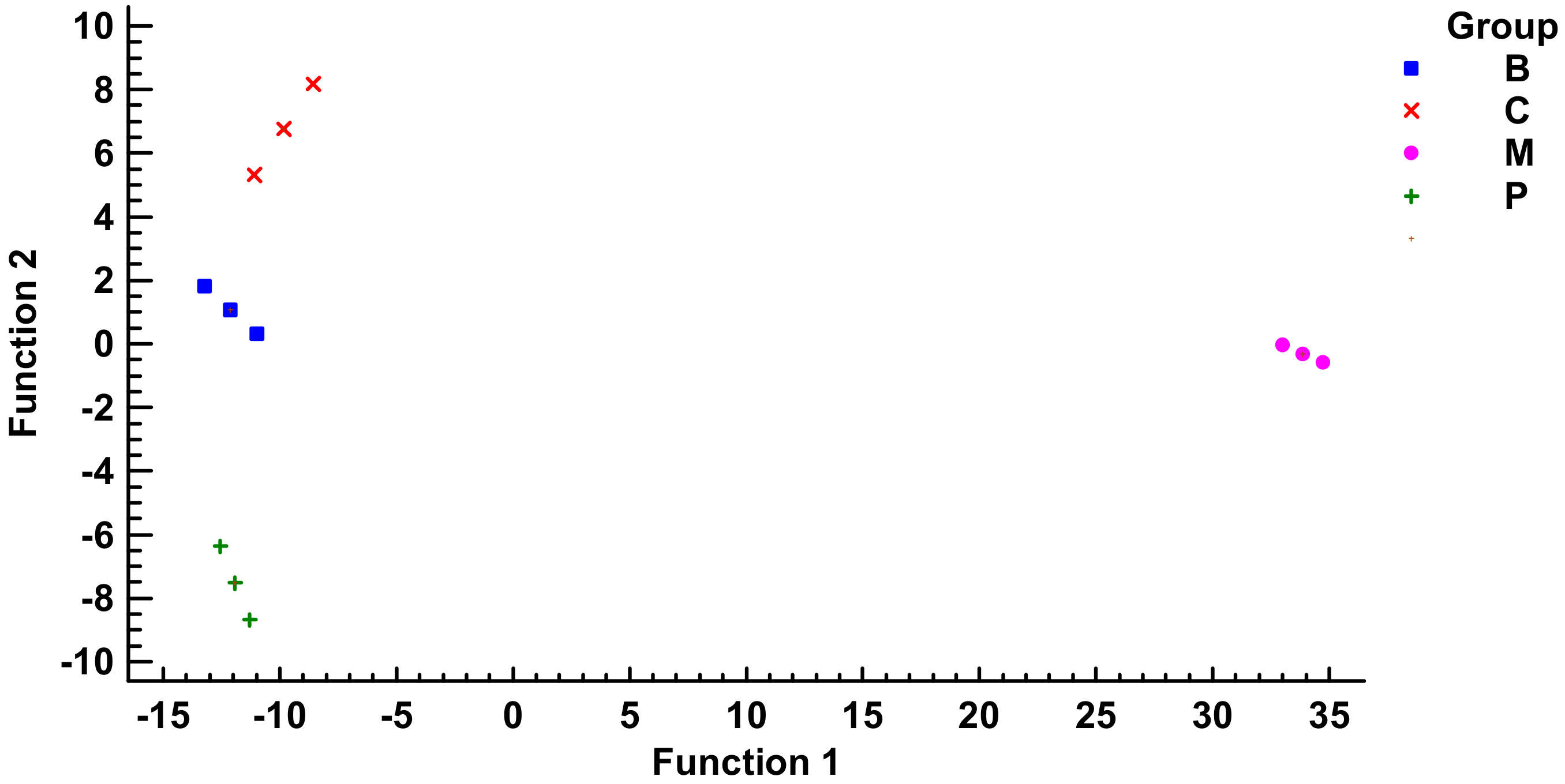
2.2. Neuroprotective Potential
3. Discussion
4. Materials and Methods
4.1. Chemicals and Standards
4.2. Extra-Virgin Olive Oil Samples
4.3. Phenolic Compounds Analysis
4.4. In Vitro Enzyme Inhibition. Potential Neuroprotective Activity
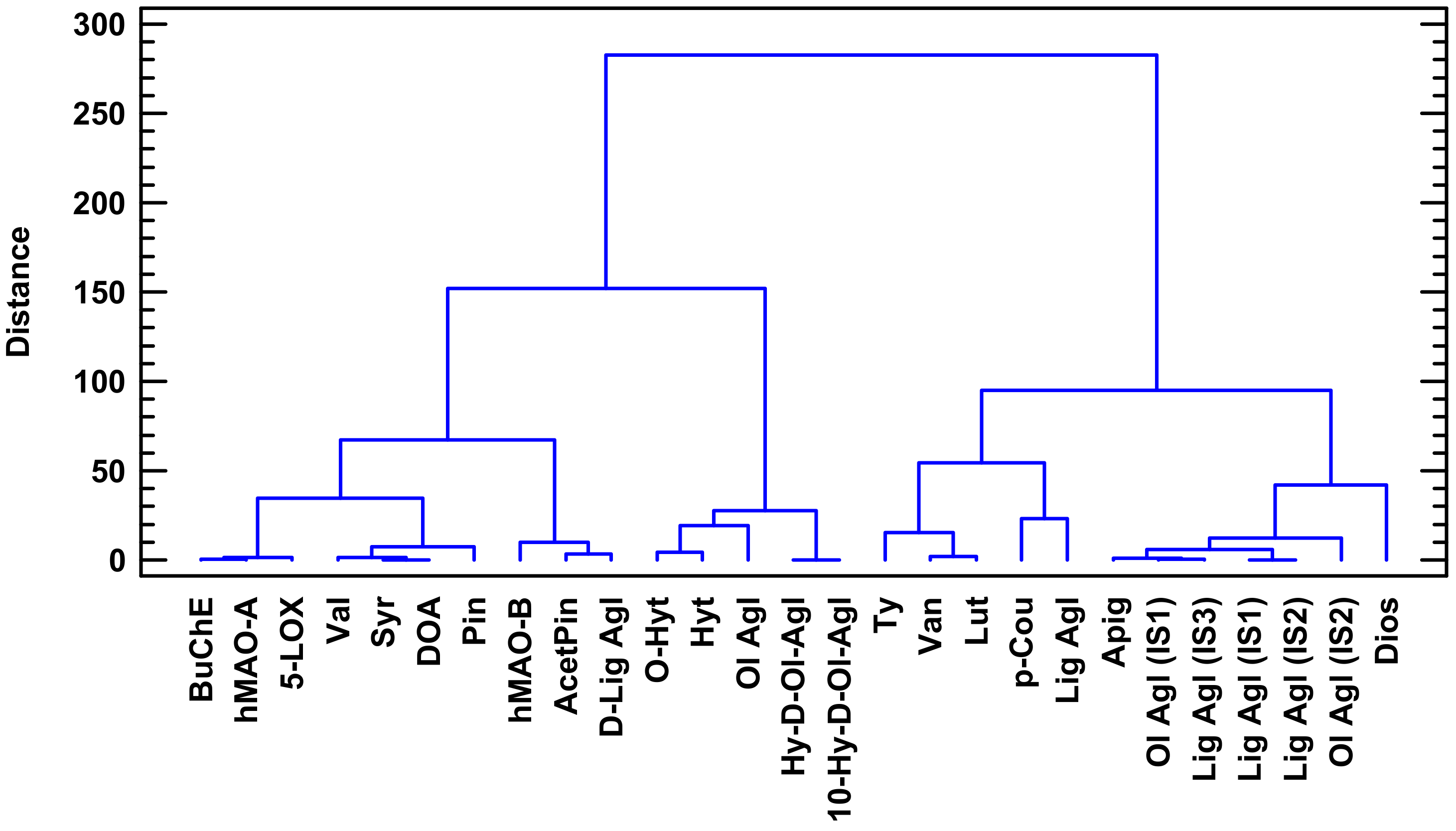
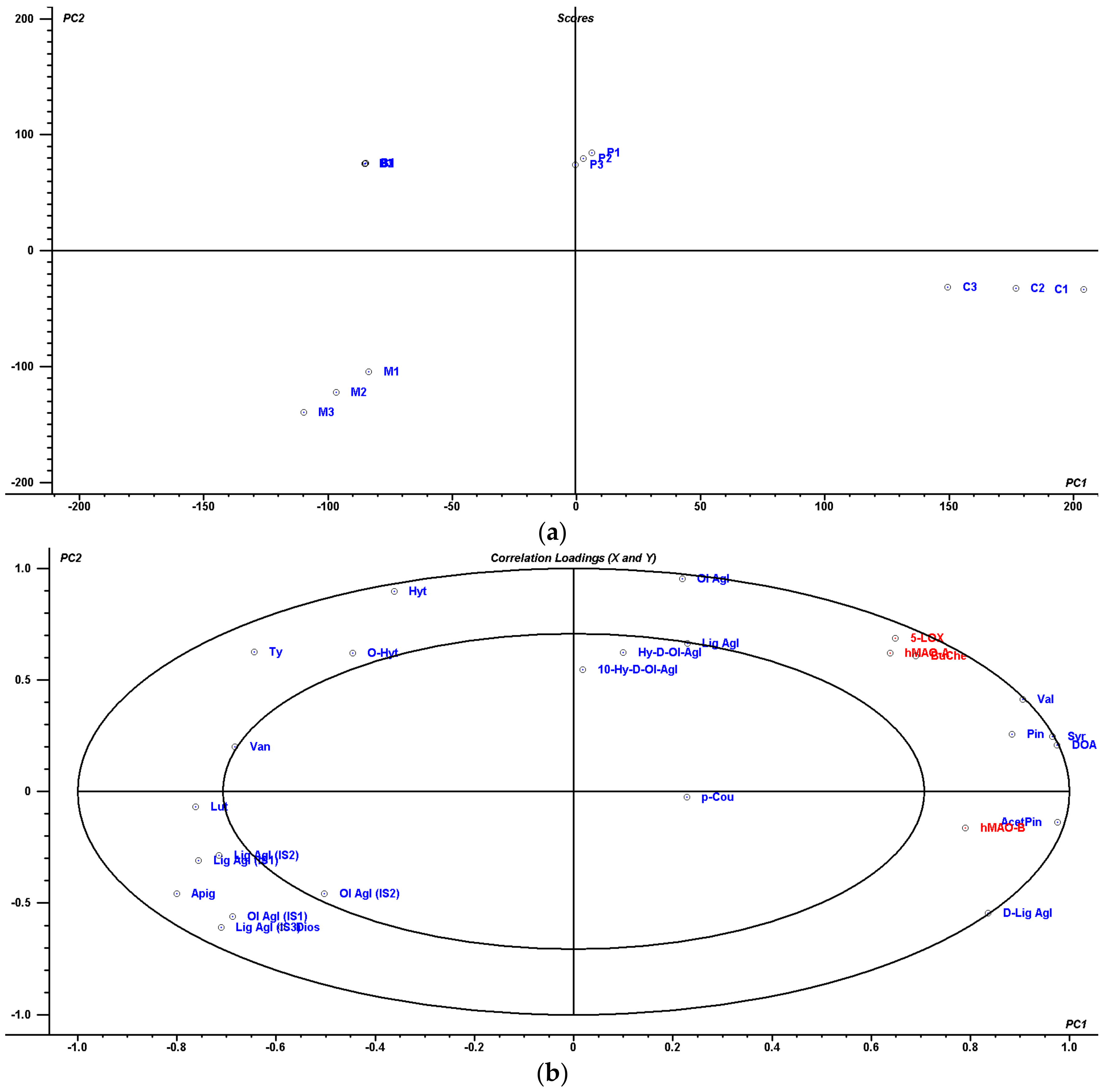
4.5. Multivariate Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bernardo, J.; Ferreres, F.; Gil-Izquierdo, A.; Valentão, P.; Andrade, P.B. Medicinal species as MTDLs: Turnera diffusa Willd. Ex Schult inhibits CNS-enzymes and delays glutamate excitotoxicity in SH-SY5Y cells via oxidative damage. Food Chem. Toxicol. 2017, 106, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.B.; Grosso, C.; Valentão, P.; Bernardo, J. Flavonoids in Neurodegeneration: Limitations and Strategies to Cross CNS Barriers. Curr. Med. Chem. 2016, 23, 4151–4174. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Rupasinghe, H.P.V. Polyphenols: Multipotent Therapeutic Agents in Neurodegenerative Diseases. Oxid. Med. Cell Longev. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Hernández, J.; Zaldívar-Machorro, V.J.; Villanueva-Porras, D.; Vega-Ávila, E.; Chavarría, A. A Potential Alternative against Neurodegenerative Diseases: Phytodrugs. Oxid. Med. Cell Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D. Dietary Polyphenols as Modulators of Brain Functions: Biological Actions and Molecular Mechanisms Underpinning Their Beneficial Effects. Oxid. Med. Cell Longev. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [PubMed]
- Kulshreshtha, A.; Piplani, P. Current pharmacotherapy and putative disease-modifying therapy for Alzheimer′s disease. Neurol. Sci. 2016, 37, 1403–1435. [Google Scholar] [CrossRef] [PubMed]
- Custódio, L.; Patarra, J.; Alberício, F.; da Rosa Neng, N.; Nogueira, J.M.F.; Romano, A. Phenolic composition, antioxidant potential and in vitro inhibitory activity of leaves and acorns of Quercus suber on key enzymes relevant for hyperglycemia and Alzheimer′s disease. Ind. Crop. Prod. 2015, 64, 45–51. [Google Scholar] [CrossRef]
- Finberg, J.P.M.; Rabey, J.M. Inhibitors of MAO-A and MAO-B in Psychiatry and Neurology. Front. Pharmacol. 2016, 7, 340. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Roy, A.; Choi, J.S. In vitro monoamine oxidase A and B inhibitory activity and molecular docking simulations of fucoxanthin. Fish. Sci. 2017, 83, 123–132. [Google Scholar] [CrossRef]
- Czapski, G.A.; Czubowicz, K.; Strosznajder, J.B.; Strosznajder, R.P. The lipoxygenases: Their regulation and implication in Alzheimer′s disease. Neurochem. Res. 2016, 41, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Leyen, V.K.; Arai, K.; Jin, G.; Kenyon, V.; Gerstner, B.; Rosenberg, P.A.; Holman, T.R.; Lo, E.H. Novel Lipoxygenase inhibitors as neuroprotective reagents. J. Neurosci. Res. 2008, 86, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, L.; Keast, R. Oleocanthal, a phenolic derived from virgin olive oil: A review of the beneficial effects on inflammatory disease. Int. J. Mol. Sci. 2014, 5, 12323–12334. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Rigano, D.; Formisano, C.; Bruno, M.; Loizzo, M.R.; Menichini, F.; Senatore, F. Metabolite profile and in vitro activities of Phagnalon saxatile (L.) Cass. relevant to treatment of Alzheimer′s disease. J. Enzyme Inhib. Med. Chem. 2010, 25, 97–104. [Google Scholar] [CrossRef] [PubMed]
- El-Hassan, A.; El-Sayed, M.; Hamed, A.I.; Rhee, I.K.; Ahmen, A.A.; Zeller, K.P.; Verpoorte, R. Bioactive constituents of Leptadenia arborea. Fitoterapia 2003, 74, 184–187. [Google Scholar] [CrossRef]
- Szwajgier, D.; Borowiec, K. Phenolic acids from malt are efficient acetylcholinesterase and butyrylcholinesterase inhibitors. J. Inst. Brew. 2012, 118, 40–48. [Google Scholar] [CrossRef]
- Tang, H.Y.; Bai, M.M.; Tian, J.M.; Pescitelli, G.; Ivšić, T.; Huang, X.H.; Lee, H.; Son, Y.N.; Kim, J.H.; Kim, Y.H.; et al. Chemical components from the seeds of Catalpa bungei and their inhibitions of soluble epoxide hydrolase, cholinesterase and nuclear factor kappa B activities. RSC Adv. 2016, 6, 40706–40716. [Google Scholar] [CrossRef]
- Bekir, J.; Mars, M.; Souchard, J.P.; Bouajila, J. Assessment of antioxidant, anti-inflammatory, anti-cholinesterase and cytotoxic activities of pomegranate (Punica granatum) leaves. Food Chem. Toxicol. 2013, 55, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Voss, C.; Sepulveda-Boza, S.; Zilliken, F.W. New isoflavonoids as inhibitors of porcine 5-lipoxygenase. Biochem. Pharmacol. 1992, 44, 157–162. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, J.S.; Kim, H.P.; Lee, J.H.; Kang, S.S. Phenolic constituents from the flower buds of Lonicera japonica and their 5-lipoxygenase inhibitory activities. Food Chem. 2010, 120, 134–139. [Google Scholar] [CrossRef]
- Bandaruk, Y.; Mukai, R.; Kawamura, T.; Nemoto, H.; Terao, J. Evaluation of the inhibitory effects of quercetin-related flavonoids and tea catechins on the monoamine oxidase reaction in mouse brain mitochondria. J. Agric. Food Chem. 2012, 60, 10270–10277. [Google Scholar] [CrossRef] [PubMed]
- Chaurasiya, N.D.; Ibrahim, M.A.; Muhammad, I.; Walker, L.A.; Tekwani, B.L. Monoamine Oxidase Inhibitory Constituents of Propolis: Kinetics and Mechanism of Inhibition of Recombinant Human MAO-A and MAO-B. Molecules 2014, 19, 18936–18952. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, F.; Ortiz, M.; Valenzuela, R.; Videla, L.A. Hydroxytyrosol and Cytoprotection: A Projection for Clinical Interventions. Int. J. Mol. Sci. 2017, 18, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, L.; Cicerale, S. The Health Benefiting Mechanisms of Virgin Olive Oil Phenolic Compounds. Molecules 2016, 21, 1734–1746. [Google Scholar] [CrossRef] [PubMed]
- Reboredo-Rodríguez, P.; Figueiredo-González, M.; González-Barreiro, C.; Simal-Gándara, J.; Salvador, M.D.; Cancho-Grande, B.; Fregapane, G. State-of-the-art on functional virgin olive oils enriched with bioactive compounds and their properties. Int. J. Mol. Sci. 2017, 18, 668–695. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Stefani, M. Nutraceutical properties of olive oil polyphenols. An itinerary from cultured cells through animal models to humans. Int. J. Mol. Sci. 2016, 17, 843–871. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morató, J.; Xicota, L.; Fitó, M.; Farré, M.; Dierssen, M.; de la Torre, R. Potential role of olive oil phenolic compounds in the prevention of neurodegenerative diseases. Molecules 2015, 20, 4655–4680. [Google Scholar] [CrossRef] [PubMed]
- Soto-Alarcón, S.A.; Valenzuela, R.; Valenzuela, A.; Videla, L.A. Liver Protective Effects of Extra Virgin Olive Oil: Interaction between Its Chemical Composition and the Cell-signaling Pathways Involved in Protection. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Keservani, R.K.; Kesharwani, R.K.; Sharma, A.K.; Gautam, S.P.; Verma, S.K. Nutraceutical Formulations and Challenges. Dev. New Funct. Food Nutraceutical Prod. 2017, 9, 161–177. [Google Scholar]
- Casamenti, F.; Stefani, M. Olive polyphenols: New promising agents to combat aging-associated neurodegeneration. Expert Rev. Neurother. 2017, 17, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Khalatbary, A.R. Olive oil phenols and neuroprotection. Nutr. Neurosci. 2013, 16, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo-González, M.; Reboredo-Rodríguez, P.; González-Barreiro, C.; Simal-Gándara, J.; Valentão, P.; Carrasco-Pancorbo, A.; Andrade, P.B.; Cancho-Grande, B. Evaluation of the neuroprotective and antidiabetic potential of phenol-rich extracts from virgin olive oils by in vitro assays. Food Res. Int. 2018, 106, 558–567. [Google Scholar] [CrossRef]
- Menichini, F.; Tundis, R.; Bonesi, M.; Loizzo, M.R.; Conforti, F.; Statti, G.; De Cindio, B.; Houghton, P.J.; Menichini, F. The influence of fruit ripening on the phytochemical content and biological activity of Capsicum chinense Jacq. cv. Habanero. Food Chem. 2009, 114, 553–560. [Google Scholar] [CrossRef]
- Giacobini, E. Cholinesterases: New roles in brain function and in Alzheimer′s disease. Neurochem. Res. 2004, 28, 515–522. [Google Scholar] [CrossRef]
- Taveira, M.; Sousa, C.; Valentão, P.; Ferreres, F.; Teixeira, J.P.; Andrade, P.B. Neuroprotective effect of steroidal alkaloids on glutamate-induced toxicity by preserving mitochondrial membrane potential and reducing oxidative stress. J. Steroid Biochem. Mol. Biol. 2014, 140, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, J.; Grosso, C.; Valentão, P.; Andrade, P.B.; Ferreres, F.; Durand, T.; Guy, A.; Galano, J.M.; Torrecillas, A.; Gil-Izquierdo, Á. Inhibition of α-glucosidase and α-amylase by Spanish extra virgin olive oils: The involvement of bioactive compounds other than oleuropein and hydroxytyrosol. Food Chem. 2017, 235, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Valli, E.; Bendini, A.; GallinaToschi, T.; Simal-Gandara, J. Characterization of virgin olive oils produced with autochthonous Galician varieties. Food Chem. 2016, 212, 162–171. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Implementing Regulation (EU) No 1348/2013 of 16 December 2013 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Union 2013, L338, 31–67. [Google Scholar]
- Bajoub, A.; Fernández-Gutierrez, A.; Carrasco-Pancorbo, A. Evaluating the potential of phenolic profiles as discriminant features among extra virgin olives oils form Moroccan controlled designations of origin. Food Res. Int. 2016, 84, 41–51. [Google Scholar] [CrossRef]
- Vinholes, J.; Grosso, C.; Andrade, P.B.; Gil-Izquierdo, A.; Valentão, P.; de Pinho, P.G.; Ferreres, F. In vitro studies to assess the antidiabetic, anti-cholinesterase and antioxidant potential of Spergularia rubra. Food Chem. 2011, 129, 454–462. [Google Scholar] [CrossRef]
- Pereira, R.B.; Taveira, M.; Valentão, P.; Sousa, C.; Andrade, P.B. Fatty acids from edible sea hares: Anti-inflammatory capacity in LPS-stimulated RAW 264.7 cells involves iNOS modulation. RSC Adv. 2015, 5, 8981–8987. [Google Scholar] [CrossRef]
- Ward, J.H., Jr. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

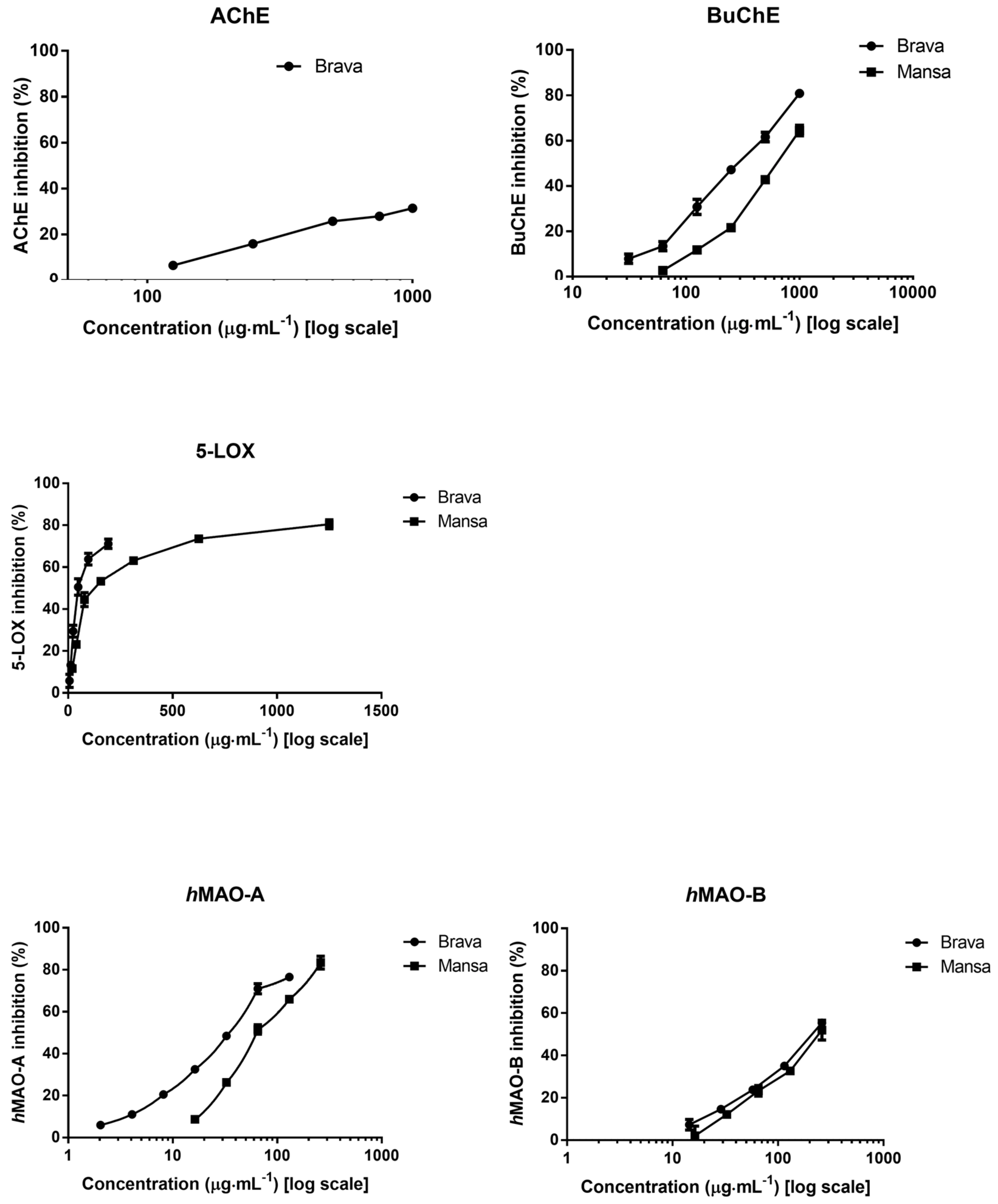
| EVOOs | Positive Controls | ||||
|---|---|---|---|---|---|
| ′Brava′ μg Dry Extract·mL−1 | ′Mansa′ μg Dry Extract·mL−1 | Galanthamine μg·mL−1 | Quercetin μg·mL−1 | Clorgyline μg·mL−1 | |
| Neuroprotection | |||||
| BuChE 1 | 298 ± 6 a | 668 ± 26 b | 7 ± 0.5 | ||
| AChE 2,3 | 483 ± 30 a | -- | 2 ± 0.3 | ||
| 5-LOX 1 | 50 ± 8 a | 124 ± 17 b | 3 ± 0.2 | ||
| hMAO-A 1 | 35 ± 2 a | 64 ± 4 b | 0.03 ± ˂0.01 | ||
| hMAO-B 1 | 223 ± 10 a | 235 ± 16 a | 23 ± 0.3 | ||
| Phenolic Compounds | BuChE | 5-LOX | hMAO-A | hMAO-B | ||
|---|---|---|---|---|---|---|
| Secoiridois | Oleuropein derivatives | DOA/3,4-DHPEA-EDA | −0.8202 | −0.7893 | −0.7757 | −0.7637 |
| Ol Agl/3,4 DHPEA-EA | −0.6791 | −0.7845 | −0.6947 | |||
| Total | −0.9069 | −0.9263 | −0.8765 | −0.5123 | ||
| Ligstroside derivatives | Lig Agl/p-HPEA-EA | −0.7231 | −0.5837 | −0.6705 | −0.7731 | |
| Total | −0.7583 | |||||
| Total | −0.5804 | −0.5351 | −0.9377 | |||
| Lignans | Syr | −0.8362 | −0.8168 | −0.7996 | −0.7489 | |
| Pin | −0.6418 | −0.7162 | −0.6069 | |||
| Total | −0.7006 | −0.7220 | −0.6611 | −0.6570 | ||
| Phenolic acids | p-cou | −0.6388 | ||||
| Val | −0.8833 | −0.8870 | −0.8544 | −0.6545 | ||
| Total |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo-González, M.; Reboredo-Rodríguez, P.; González-Barreiro, C.; Carrasco-Pancorbo, A.; Simal-Gándara, J.; Cancho-Grande, B. Nutraceutical Potential of Phenolics from ′Brava′ and ′Mansa′ Extra-Virgin Olive Oils on the Inhibition of Enzymes Associated to Neurodegenerative Disorders in Comparison with Those of ′Picual′ and ′Cornicabra′. Molecules 2018, 23, 722. https://doi.org/10.3390/molecules23040722
Figueiredo-González M, Reboredo-Rodríguez P, González-Barreiro C, Carrasco-Pancorbo A, Simal-Gándara J, Cancho-Grande B. Nutraceutical Potential of Phenolics from ′Brava′ and ′Mansa′ Extra-Virgin Olive Oils on the Inhibition of Enzymes Associated to Neurodegenerative Disorders in Comparison with Those of ′Picual′ and ′Cornicabra′. Molecules. 2018; 23(4):722. https://doi.org/10.3390/molecules23040722
Chicago/Turabian StyleFigueiredo-González, María, Patricia Reboredo-Rodríguez, Carmen González-Barreiro, Alegría Carrasco-Pancorbo, Jesús Simal-Gándara, and Beatriz Cancho-Grande. 2018. "Nutraceutical Potential of Phenolics from ′Brava′ and ′Mansa′ Extra-Virgin Olive Oils on the Inhibition of Enzymes Associated to Neurodegenerative Disorders in Comparison with Those of ′Picual′ and ′Cornicabra′" Molecules 23, no. 4: 722. https://doi.org/10.3390/molecules23040722