Melatonin Balance the Autophagy and Apoptosis by Regulating UCP2 in the LPS-Induced Cardiomyopathy
Abstract
:1. Introduction
2. Material and Methods
2.1. Animal Model and Treatment
2.2. Cardiac Echo Examination
2.3. Cell Culture and Treatment
2.4. Detection mRNA and Protein Expression
2.5. Myocardial and Mitochondrial Injury Detection
2.6. ELISA Assay
2.7. Cell Proliferation and Apoptosis
2.8. Statistical Analysis
3. Results
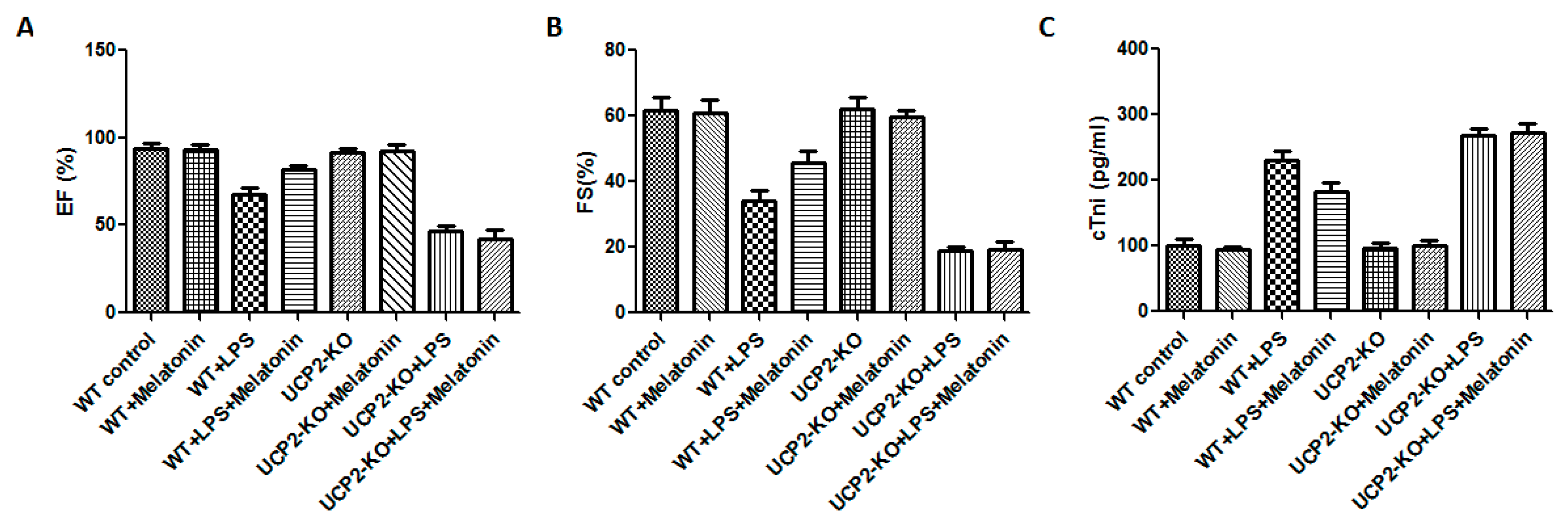
3.1. Changes in Cardiac Function and Myocardial Damage after LPS Exposure
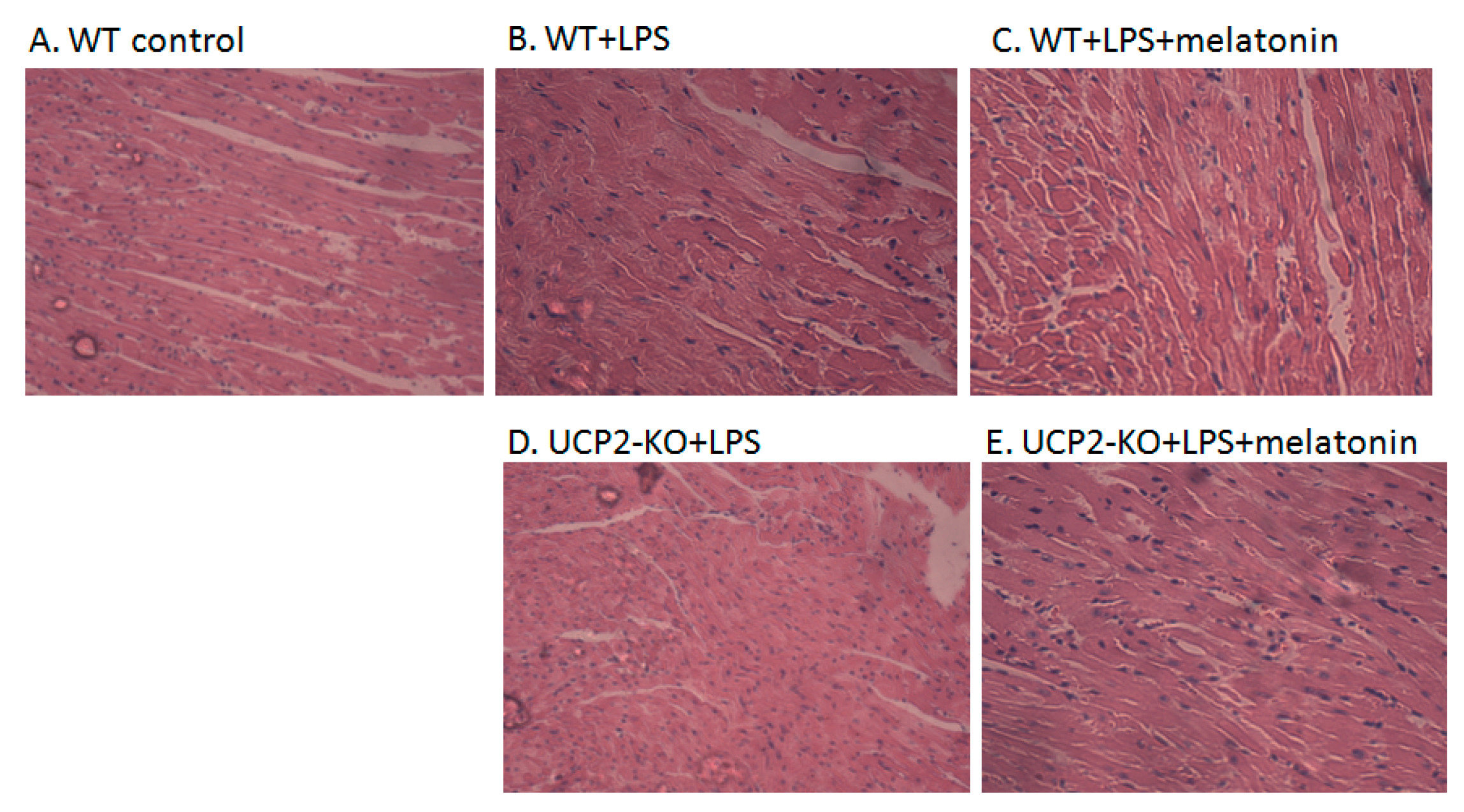
3.2. Alterations in Morphological Characteristics of the Heart Tissue and AC-16 Cell
3.3. UCP2 Expression In Vitro and In Vivo
3.4. Mitochondrial Injury of the Myocardial Cells
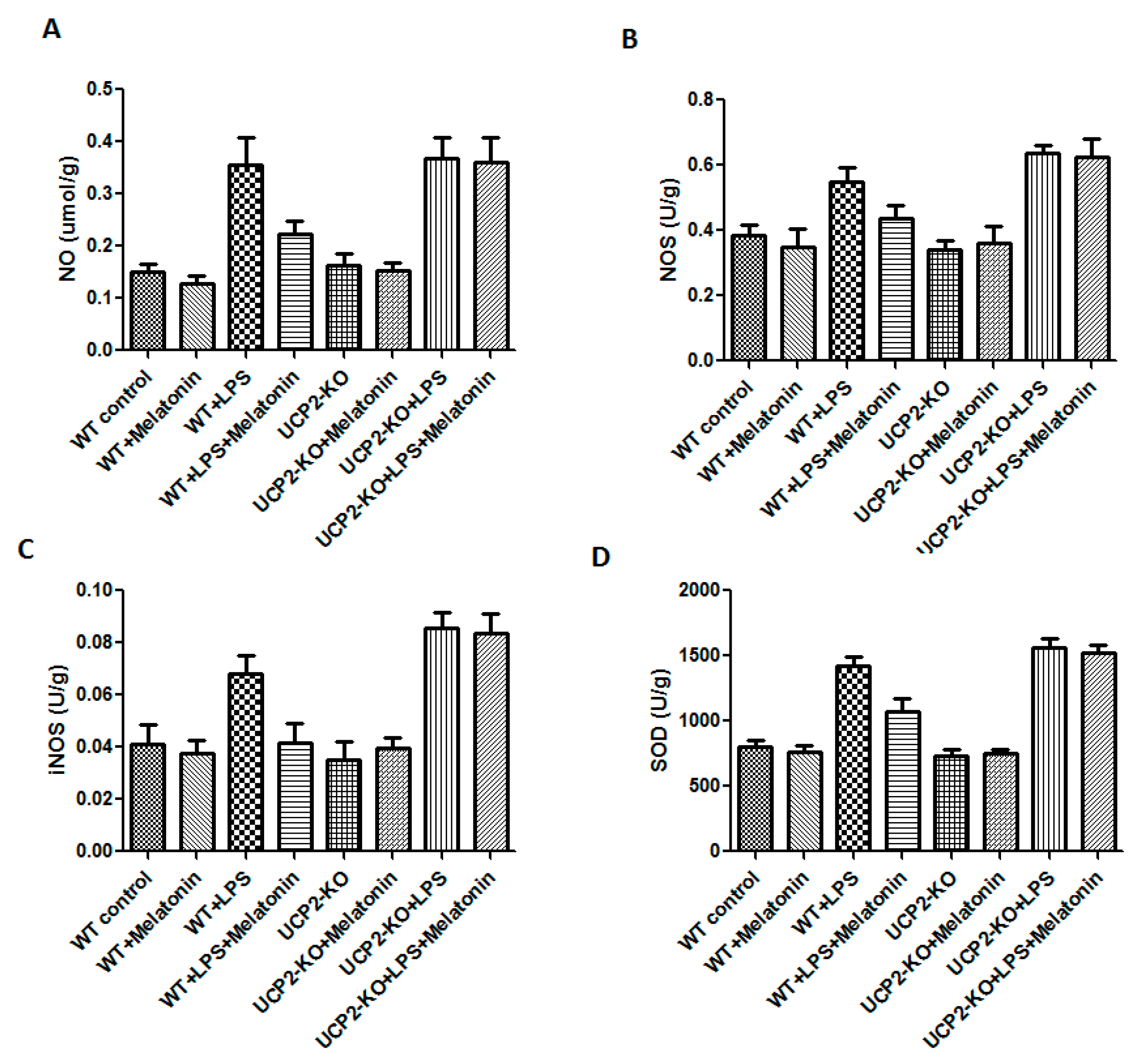
3.5. Oxidative Injury in the Heart Tissue
3.6. Effect on the Calcium Loading and Reactive Oxygen Species Production in AC-16 Cell
3.7. Effect on Cardiomyocyte Viability and Apoptosis
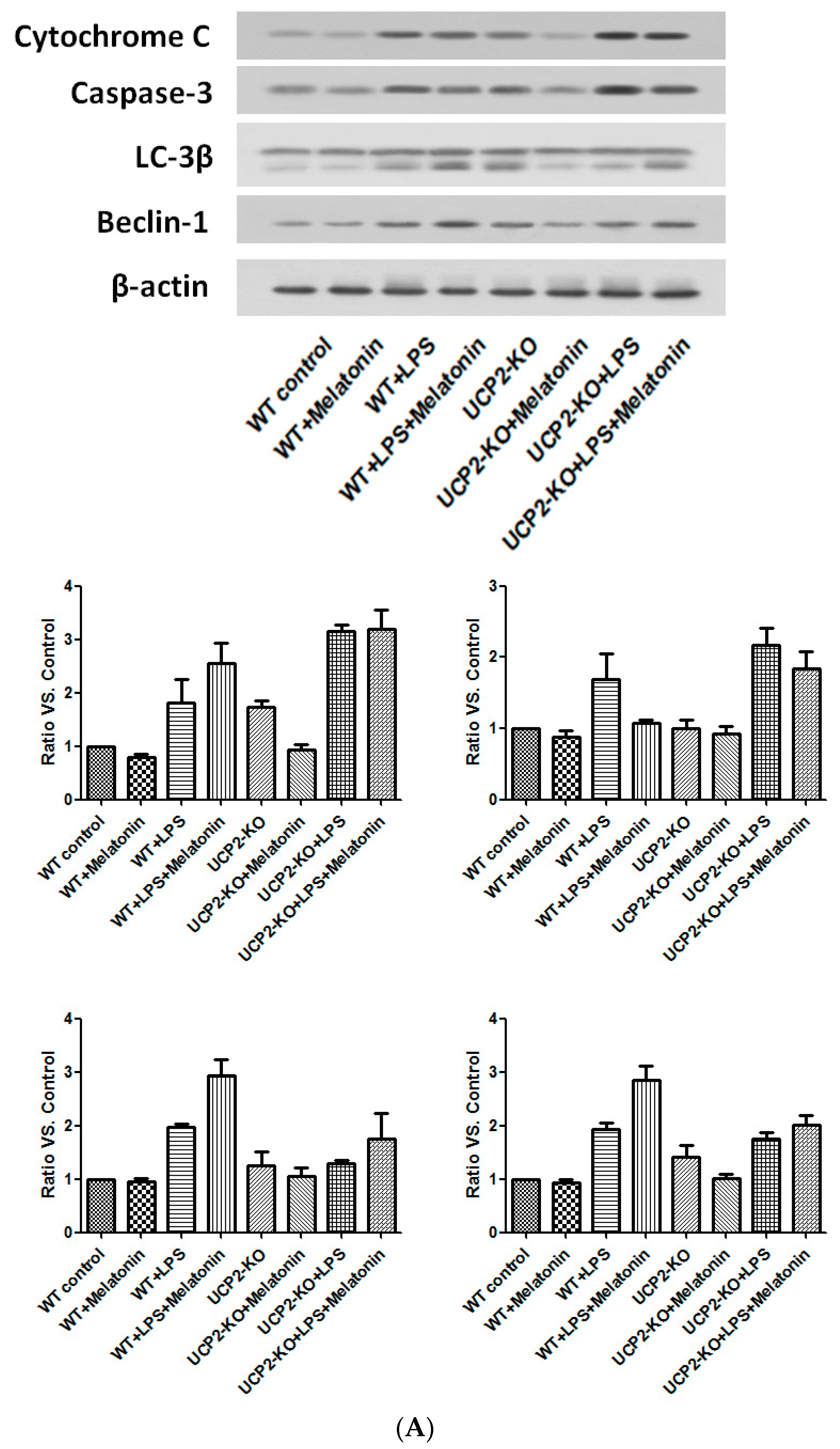
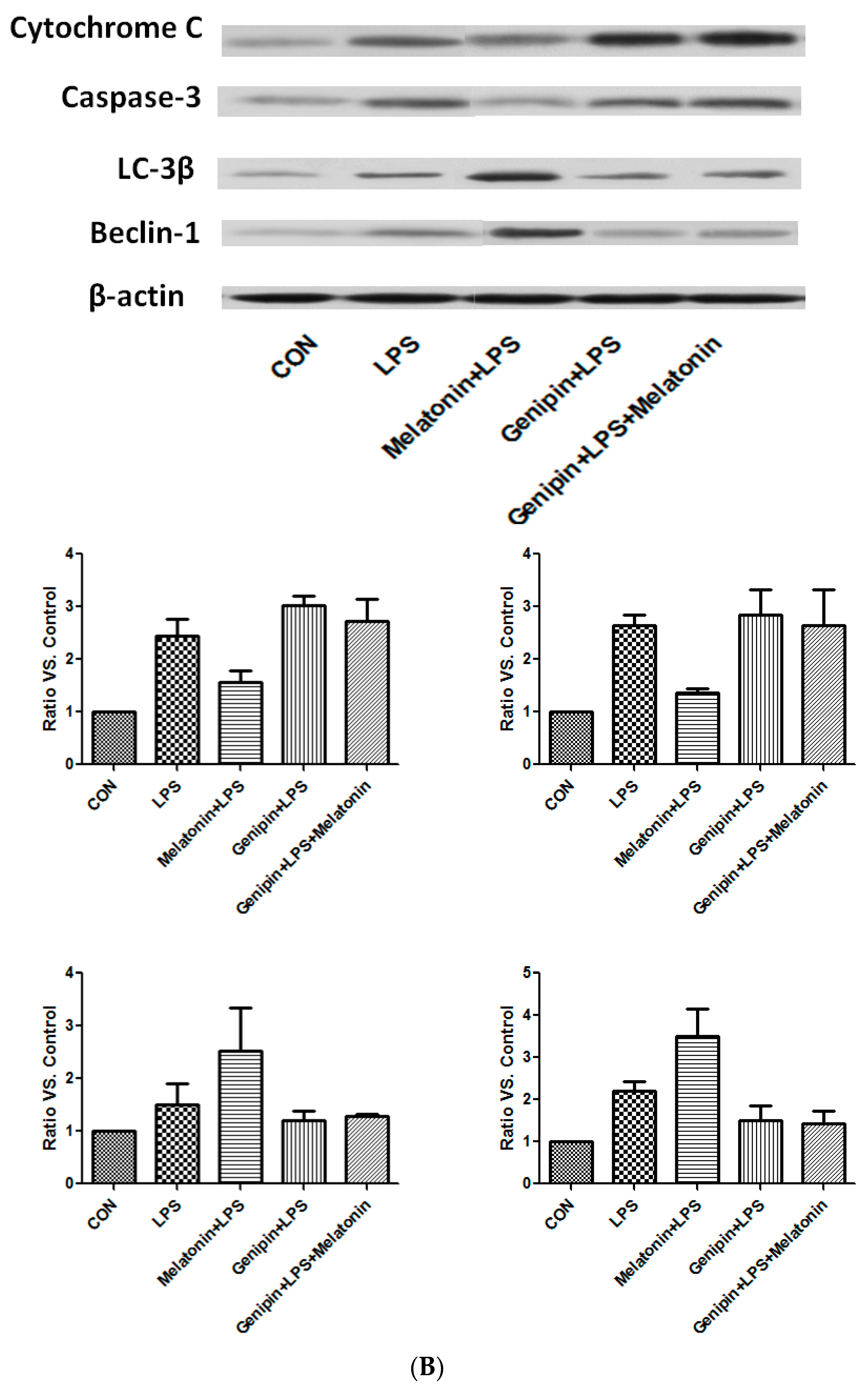
3.8. Effect on the Proteins Associated with Apoptosis and Autophagy
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Rudiger, A.; Singer, M. The heart in sepsis: From basic mechanisms to clinical management. Curr. Vasc. Pharmacol. 2013, 11, 187–195. [Google Scholar] [PubMed]
- Flynn, A.; Chokkalingam Mani, B.; Mather, P.J. Sepsis-induced cardiomyopathy: A review of pathophysiologic mechanisms. Heart Fail. Rev. 2010, 15, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Rudiger, A.; Singer, M. Mechanisms of sepsis-induced cardiac dysfunction. Crit. Care Med. 2007, 35, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Flierl, M.A.; Rittirsch, D.; Huber-Lang, M.S.; Sarma, J.V.; Ward, P.A. Molecular events in the cardiomyopathy of sepsis. Mol. Med. 2008, 14, 327–336. [Google Scholar] [PubMed]
- Liu, Y.C.; Yu, M.M.; Shou, S.T.; Chai, Y.F. Sepsis-induced cardiomyopathy: Mechanisms and treatments. Front. Immunol. 2017, 8, 1021. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.X.; Barger, J.L.; Boyer, B.B.; Brand, M.D.; Pan, G.; Adams, S.H. Impact of endotoxin on ucp homolog mrna abundance, thermoregulation, and mitochondrial proton leak kinetics. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E433–E446. [Google Scholar] [CrossRef] [PubMed]
- Ricquier, D.; Miroux, B.; Cassard-Doulcier, A.M.; Levi-Meyrueis, C.; Gelly, C.; Raimbault, S.; Bouillaud, F. Contribution to the identification and analysis of the mitochondrial uncoupling proteins. J. Bioenerg. Biomembr. 1999, 31, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.E.; Gerrits, M.F. Mitochondrial uncoupling proteins as potential targets for pharmacological agents. Curr. Opin. Pharmacol. 2004, 4, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Song, Y.B.; Wang, H.L.; Wang, Y.; Lv, J.J.; Che, D.; Zeng, Q.Y. Effect of ucp2-sirna on inflammatory response of cardiomyocytes induced by septic serum. Zhongguo Dang Dai Er Ke Za Zhi 2014, 16, 851–855. [Google Scholar] [PubMed]
- Hoshovs’ka Iu, V.; Shymans’ka, T.V.; Sahach, V.F. Effect of UCP2 activity inhibitor genipin on heart function of aging rats. Fiziol. Zhurnal 2009, 55, 28–34. [Google Scholar]
- Safari, F.; Bayat, G.; Shekarforoush, S.; Hekmatimoghaddam, S.; Anvari, Z.; Moghadam, M.F.; Hajizadeh, S. Expressional profile of cardiac uncoupling protein-2 following myocardial ischemia reperfusion in losartan- and ramiprilat-treated rats. J. Renin Angiotensin Aldost. Syst. 2014, 15, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Nie, H.G.; Zhang, X.D.; Tian, Y.; Yu, B. Down-regulated energy metabolism genes associated with mitochondria oxidative phosphorylation and fatty acid metabolism in viral cardiomyopathy mouse heart. Mol. Biol. Rep. 2011, 38, 4007–4013. [Google Scholar] [CrossRef] [PubMed]
- Acuna-Castroviejo, D.; Rahim, I.; Acuna-Fernandez, C.; Fernandez-Ortiz, M.; Solera-Marin, J.; Sayed, R.K.A.; Diaz-Casado, M.E.; Rusanova, I.; Lopez, L.C.; Escames, G. Melatonin, clock genes and mitochondria in sepsis. Cell. Mol. Life Sci. 2017, 74, 3965–3987. [Google Scholar] [CrossRef] [PubMed]
- Galley, H.F.; Lowes, D.A.; Allen, L.; Cameron, G.; Aucott, L.S.; Webster, N.R. Melatonin as a potential therapy for sepsis: A phase i dose escalation study and an ex vivo whole blood model under conditions of sepsis. J. Pineal Res. 2014, 56, 427–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Aranda, A.; Fernandez-Vazquez, G.; Campos, D.; Tassi, M.; Velasco-Perez, L.; Tan, D.X.; Reiter, R.J.; Agil, A. Melatonin induces browning of inguinal white adipose tissue in zucker diabetic fatty rats. J. Pineal Res. 2013, 55, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Yu, J.; Wong, S.H.; Zhang, L.; Liu, X.; Wong, W.T.; Leung, C.C.; Choi, G.; Wang, M.H.; Gin, T.; et al. Autophagy in sepsis: Degradation into exhaustion? Autophagy 2016, 12, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gao, P.; Zhang, J. Crosstalk between autophagy and apoptosis: Potential and emerging therapeutic targets for cardiac diseases. Int. J. Mol. Sci. 2016, 17, 332. [Google Scholar] [CrossRef] [PubMed]
- Bravo-San Pedro, J.M.; Kroemer, G.; Galluzzi, L. Autophagy and mitophagy in cardiovascular disease. Circ. Res. 2017, 120, 1812–1824. [Google Scholar] [CrossRef] [PubMed]
- Goldenthal, M.J. Mitochondrial involvement in myocyte death and heart failure. Heart Fail. Rev. 2016, 21, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Turdi, S.; Han, X.; Huff, A.F.; Roe, N.D.; Hu, N.; Gao, F.; Ren, J. Cardiac-specific overexpression of catalase attenuates lipopolysaccharide-induced myocardial contractile dysfunction: Role of autophagy. Free Radic. Biol. Med. 2012, 53, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kang, S.M.; Lee, K.M.; Lee, J.E. The protective effect of melatonin on neural stem cell against lps-induced inflammation. Biomed. Res. Int. 2015, 2015, 854359. [Google Scholar] [CrossRef] [PubMed]
- Kiers, D.; van der Heijden, W.A.; van Ede, L.; Gerretsen, J.; de Mast, Q.; van der Ven, A.J.; El Messaoudi, S.; Rongen, G.A.; Gomes, M.; Kox, M.; et al. A randomised trial on the effect of anti-platelet therapy on the systemic inflammatory response in human endotoxaemia. Thromb. Haemost. 2017, 117, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.C.; Kim, D.Y.; Bae, J.S. Sulforaphane reduces hmgb1-mediated septic responses and improves survival rate in septic mice. Am. J. Chin. Med. 2017, 45, 1253–1271. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Lyu, J.; Liu, S.; Huang, J.; Liu, C.; Xiang, D.; Xie, M.; Zeng, Q. Silencing of uncoupling protein 2 by small interfering rna aggravates mitochondrial dysfunction in cardiomyocytes under septic conditions. Int. J. Mol. Med. 2015, 35, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.S.; Julian, M.W.; Huff, J.E.; Bauer, J.A.; Xia, Y.; Crouser, E.D. Calcineurin regulates myocardial function during acute endotoxemia. Am. J. Respir. Crit. Care Med. 2006, 173, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.C.; Escames, G.; Tapias, V.; Utrilla, P.; Leon, J.; Acuna-Castroviejo, D. Identification of an inducible nitric oxide synthase in diaphragm mitochondria from septic mice: Its relation with mitochondrial dysfunction and prevention by melatonin. Int. J. Biochem. Cell Biol. 2006, 38, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Escames, G.; Lopez, L.C.; Ortiz, F.; Lopez, A.; Garcia, J.A.; Ros, E.; Acuna-Castroviejo, D. Attenuation of cardiac mitochondrial dysfunction by melatonin in septic mice. FEBS J. 2007, 274, 2135–2147. [Google Scholar] [CrossRef] [PubMed]
- Escames, G.; Lopez, L.C.; Tapias, V.; Utrilla, P.; Reiter, R.J.; Hitos, A.B.; Leon, J.; Rodriguez, M.I.; Acuna-Castroviejo, D. Melatonin counteracts inducible mitochondrial nitric oxide synthase-dependent mitochondrial dysfunction in skeletal muscle of septic mice. J. Pineal Res. 2006, 40, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, D.; Wang, X.; Chen, X.; Long, Y.; Chai, W.; Zhou, X.; Rui, X.; Zhang, Q.; Wang, H.; et al. Melatonin improved rat cardiac mitochondria and survival rate in septic heart injury. J. Pineal Res. 2013, 55, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.T.; Garesse, R.; Kaguni, L.S. Animal models of mitochondrial DNA transactions in disease and ageing. Exp. Gerontol. 2010, 45, 489–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donadelli, M.; Dando, I.; Fiorini, C.; Palmieri, M. UCP2, a mitochondrial protein regulated at multiple levels. Cell. Mol. Life Sci. 2014, 71, 1171–1190. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.M.; Yang, Q.H.; Zhu, C.Q. UCP2 in early diagnosis and prognosis of sepsis. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 549–553. [Google Scholar] [PubMed]
- Wang, X.; Liu, D.; Chai, W.; Long, Y.; Su, L.; Yang, R. The role of uncoupling protein 2 during myocardial dysfunction in a canine model of endotoxin shock. Shock 2015, 43, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Roshon, M.J.; Kline, J.A.; Thornton, L.R.; Watts, J.A. Cardiac UCP2 expression and myocardial oxidative metabolism during acute septic shock in the rat. Shock 2003, 19, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Liu, Y.; Du, L.; Wang, Y.; Cheng, X.; Xiao, W.; Wang, X.; Jin, H.; Yang, X.; Liu, S.; et al. Targeted expression of uncoupling protein 2 to mouse liver increases the susceptibility to lipopolysaccharide/galactosamine-induced acute liver injury. Hepatology 2009, 50, 1204–1216. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, J.; Hu, M.; Yang, Y.; Guo, L.; Xu, J.; Lei, C.; Jiao, Y.; Xu, J. Uncoupling protein 2 increases susceptibility to lipopolysaccharide-induced acute lung injury in mice. Mediat. Inflamm. 2016, 2016, 9154230. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Qin, L.; Reiter, R.J. Melatonin: A mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int. J. Mol. Sci. 2016, 17, 2124. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Alarcon, M.; Ruiz-Ojeda, F.J.; Blanca-Herrera, R.M.; MM, A.S.; Acuna-Castroviejo, D.; Fernandez-Vazquez, G.; Agil, A. Melatonin and metabolic regulation: A review. Food Funct. 2014, 5, 2806–2832. [Google Scholar] [CrossRef] [PubMed]
- Agil, A.; El-Hammadi, M.; Jimenez-Aranda, A.; Tassi, M.; Abdo, W.; Fernandez-Vazquez, G.; Reiter, R.J. Melatonin reduces hepatic mitochondrial dysfunction in diabetic obese rats. J. Pineal Res. 2015, 59, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Vazquez, G.; Reiter, R.J.; Agil, A. Melatonin increases brown adipose tissue mass and function in zucker diabetic fatty rats: Implications for obesity control. J. Pineal Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Pecqueur, C.; Alves-Guerra, M.C.; Gelly, C.; Levi-Meyrueis, C.; Couplan, E.; Collins, S.; Ricquier, D.; Bouillaud, F.; Miroux, B. Uncoupling protein 2, in vivo distribution, induction upon oxidative stress, and evidence for translational regulation. J. Biol. Chem. 2001, 276, 8705–8712. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.S.; Lee, S.; Park, M.A.; Siempos, I.I.; Haslip, M.; Lee, P.J.; Yun, M.; Kim, C.K.; Howrylak, J.; Ryter, S.W.; et al. UCP2-induced fatty acid synthase promotes NLRP3 inflammasome activation during sepsis. J. Clin. Investig. 2015, 125, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Teshima, Y.; Akao, M.; Jones, S.P.; Marban, E. Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes. Circ. Res. 2003, 93, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, J.; Gao, F.; Tian, Y.; Song, R.; Zhu, S.J. The role of uncoupling protein 2 in the apoptosis induced by free fatty acid in rat cardiomyocytes. J. Cardiovasc. Pharmacol. 2010, 55, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Akhmedov, A.T.; Rybin, V.; Marin-Garcia, J. Mitochondrial oxidative metabolism and uncoupling proteins in the failing heart. Heart Fail. Rev. 2015, 20, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Robson-Doucette, C.A.; Wheeler, M.B. Uncoupling protein 2 regulates reactive oxygen species formation in islets and influences susceptibility to diabetogenic action of streptozotocin. J. Endocrinol. 2009, 203, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Arsenijevic, D.; Onuma, H.; Pecqueur, C.; Raimbault, S.; Manning, B.S.; Miroux, B.; Couplan, E.; Alves-Guerra, M.C.; Goubern, M.; Surwit, R.; et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat. Genet. 2000, 26, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yi, B.; Liao, Z.; Tang, L.; Yin, D.; Zeng, S.; Yao, J.; He, M. 14–3-3gamma protein attenuates lipopolysaccharide-induced cardiomyocytes injury through the bcl-2 family/mitochondria pathway. Int. Immunopharmacol. 2014, 21, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Crompton, M.; Costi, A.; Hayat, L. Evidence for the presence of a reversible Ca2+-dependent pore activated by oxidative stress in heart mitochondria. Biochem. J. 1987, 245, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Le Minh, K.; Kuhla, A.; Abshagen, K.; Minor, T.; Stegemann, J.; Ibrahim, S.; Eipel, C.; Vollmar, B. Uncoupling protein-2 deficiency provides protection in a murine model of endotoxemic acute liver failure. Crit. Care Med. 2009, 37, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.J.; Wang, C.J.; He, Y.; Zhou, Y.L.; Peng, X.D.; Liu, S.K. Resveratrol alleviates diabetic cardiomyopathy in rats by improving mitochondrial function through PGC-1alpha deacetylation. Acta Pharmacol. Sin. 2017, 39, 59. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.Y.; Wei, J.; Yan, R.; Lin, L.; Li, H. Effect of uncoupling protein 2 on high-glucose induced mitochondrial damage and apoptosis of cardiomyocytes. Zhonghua Yi Xue Za Zhi 2016, 96, 2493–2497. [Google Scholar] [PubMed]
- Chen, G.G.; Yan, J.B.; Wang, X.M.; Zheng, M.Z.; Jiang, J.P.; Zhou, X.M.; Cai, B.; Shen, Y.L. Mechanism of uncoupling protein 2mediated myocardial injury in hypothermic preserved rat hearts. Mol. Med. Rep. 2016, 14, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Liu, J.; Yan, X.Y.; Zhang, Y.; Zhang, J.J.; Zhang, L.C.; Sun, L.K. Cytoprotective effect of the UCP2-sirt3 signaling pathway by decreasing mitochondrial oxidative stress on cerebral ischemia-reperfusion injury. Int. J. Mol. Sci. 2017, 18, 1599. [Google Scholar] [CrossRef]
- Kretzschmar, C.; Roolf, C.; Timmer, K.; Sekora, A.; Knubel, G.; Escobar, H.M.; Jaster, R.; Muller, S.; Fuellen, G.; Kohling, R.; et al. Uncoupling protein 2 deficiency results in higher neutrophil counts and lower β-cell counts during aging in mice. Exp. Hematol. 2016, 44, 1085–1091.e2. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Polito, C.C.; Haines, J.K.; Shafizadeh, S.F.; Fiorini, R.N.; Zhou, X.; Schmidt, M.G.; Chavin, K.D. Decrease of intracellular atp content downregulated UCP2 expression in mouse hepatocytes. Biochem. Biophys. Res. Commun. 2003, 308, 573–580. [Google Scholar] [CrossRef]
- Brand, M.D. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp. Gerontol. 2000, 35, 811–820. [Google Scholar] [CrossRef]
- Rose, G.; Crocco, P.; De Rango, F.; Montesanto, A.; Passarino, G. Further support to the uncoupling-to-survive theory: The genetic variation of human UCP genes is associated with longevity. PLoS ONE 2011, 6, e29650. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Tan, J.; Miao, Y.; Li, M.; Zhang, Q. Crosstalk of autophagy and apoptosis: Involvement of the dual role of autophagy under er stress. J. Cell. Physiol. 2017, 232, 2977–2984. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Shen, Z.; Yang, L.; Yin, M.; Zheng, W.; Wu, B.; Liu, T.; Song, H. Protective effects of heme oxygenase-1-transduced bone marrow-derived mesenchymal stem cells on reducedsize liver transplantation: Role of autophagy regulated by the erk/mtor signaling pathway. Int. J. Mol. Med. 2017, 40, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Gunst, J.; Derese, I.; Aertgeerts, A.; Ververs, E.J.; Wauters, A.; Van den Berghe, G.; Vanhorebeek, I. Insufficient autophagy contributes to mitochondrial dysfunction, organ failure, and adverse outcome in an animal model of critical illness. Crit. Care Med. 2013, 41, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Pei, F.; Yang, F.; Li, L.; Amin, A.D.; Liu, S.; Buchan, J.R.; Cho, W.C. Role of autophagy and apoptosis in non-small-cell lung cancer. Int. J. Mol. Sci. 2017, 18, 367. [Google Scholar] [CrossRef] [PubMed]
- Kemp, M.G. Crosstalk between apoptosis and autophagy: Environmental genotoxins, infection, and innate immunity. J. Cell Death 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Marques-Aleixo, I.; Santos-Alves, E.; Balca, M.M.; Rizo-Roca, D.; Moreira, P.I.; Oliveira, P.J.; Magalhaes, J.; Ascensao, A. Physical exercise improves brain cortex and cerebellum mitochondrial bioenergetics and alters apoptotic, dynamic and auto(mito)phagy markers. Neuroscience 2015, 301, 480–495. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Zhang, F.; Duan, P.; Zhu, N.; Zhang, J.; Ye, F.; Shan, D.; Chen, H.; Lu, X.; Zhu, C.; et al. Mitochondrial uncoupling protein 2 in human cumulus cells is associated with regulating autophagy and apoptosis, maintaining gap junction integrity and progesterone synthesis. Mol. Cell. Endocrinol. 2017, 443, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cai, T.; Xu, J.; Jiang, L.; Wu, J.; Sun, Q.; Zen, K.; Yang, J. UCP2 attenuates apoptosis of tubular epithelial cells in renal ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 2017, 313, F926–F937. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Wang, Y.; Wang, X.; Jiang, Y. Uncoupling protein 2 regulates palmitic acid-induced hepatoma cell autophagy. Biomed. Res. Int. 2014, 2014, 810401. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Beclin 1 interactome controls the crosstalk between apoptosis, autophagy and inflammasome activation: Impact on the aging process. Ageing Res. Rev. 2013, 12, 520–534. [Google Scholar] [CrossRef] [PubMed]
- Marquez, R.T.; Xu, L. Bcl-2:Beclin 1 complex: Multiple, mechanisms regulating autophagy/apoptosis toggle switch. Am. J. Cancer Res. 2012, 2, 214–221. [Google Scholar] [PubMed]
- Scherz-Shouval, R.; Weidberg, H.; Gonen, C.; Wilder, S.; Elazar, Z.; Oren, M. P53-dependent regulation of autophagy protein LC3 supports cancer cell survival under prolonged starvation. Proc. Natl. Acad. Sci. USA 2010, 107, 18511–18516. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, S.; Plesca, D.; Almasan, A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol. Biol. 2008, 414, 13–21. [Google Scholar] [PubMed]
- Zhu, Y.; Zhao, L.; Liu, L.; Gao, P.; Tian, W.; Wang, X.; Jin, H.; Xu, H.; Chen, Q. Beclin 1 cleavage by caspase-3 inactivates autophagy and promotes apoptosis. Protein Cell 2010, 1, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.X.; Yu, J.; Liang, Y.X.; Zeng, J.S.; Huang, R.X.; Liao, S.J. Beclin 1 knockdown retards re-endothelialization and exacerbates neointimal formation via a crosstalk between autophagy and apoptosis. Atherosclerosis 2014, 237, 146–154. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, P.; Zhang, H.; Su, L.; Wang, X.; Liu, D. Melatonin Balance the Autophagy and Apoptosis by Regulating UCP2 in the LPS-Induced Cardiomyopathy. Molecules 2018, 23, 675. https://doi.org/10.3390/molecules23030675
Pan P, Zhang H, Su L, Wang X, Liu D. Melatonin Balance the Autophagy and Apoptosis by Regulating UCP2 in the LPS-Induced Cardiomyopathy. Molecules. 2018; 23(3):675. https://doi.org/10.3390/molecules23030675
Chicago/Turabian StylePan, Pan, Hongmin Zhang, Longxiang Su, Xiaoting Wang, and Dawei Liu. 2018. "Melatonin Balance the Autophagy and Apoptosis by Regulating UCP2 in the LPS-Induced Cardiomyopathy" Molecules 23, no. 3: 675. https://doi.org/10.3390/molecules23030675



