A Reconstruction Method for the Estimation of Temperatures of Multiple Sources Applied for Nanoparticle-Mediated Hyperthermia
Abstract
:1. Introduction
2. Materials and Methods
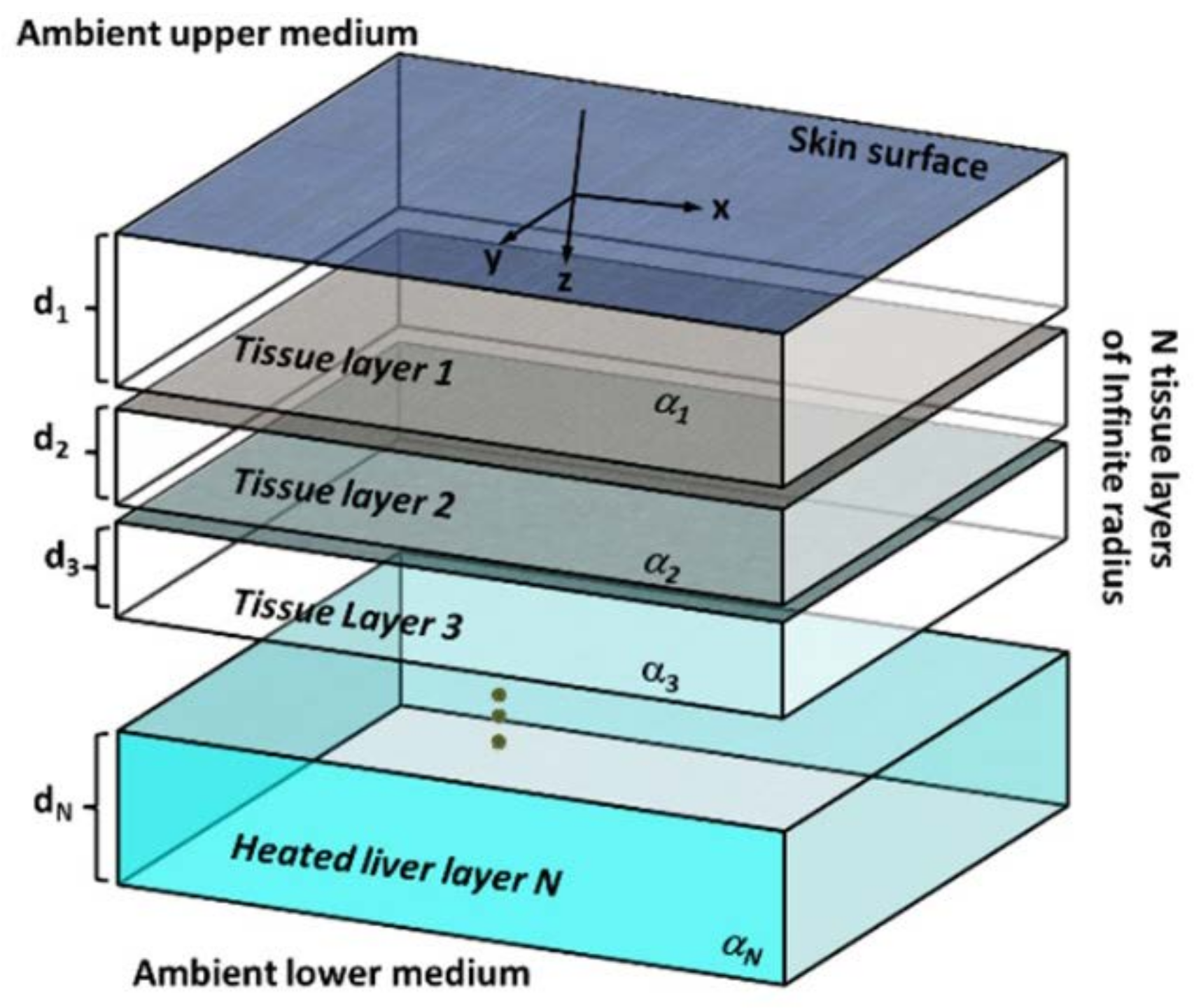
2.1. Time-Harmonic Analytical Model
2.2. Thermal Phantom and Setup
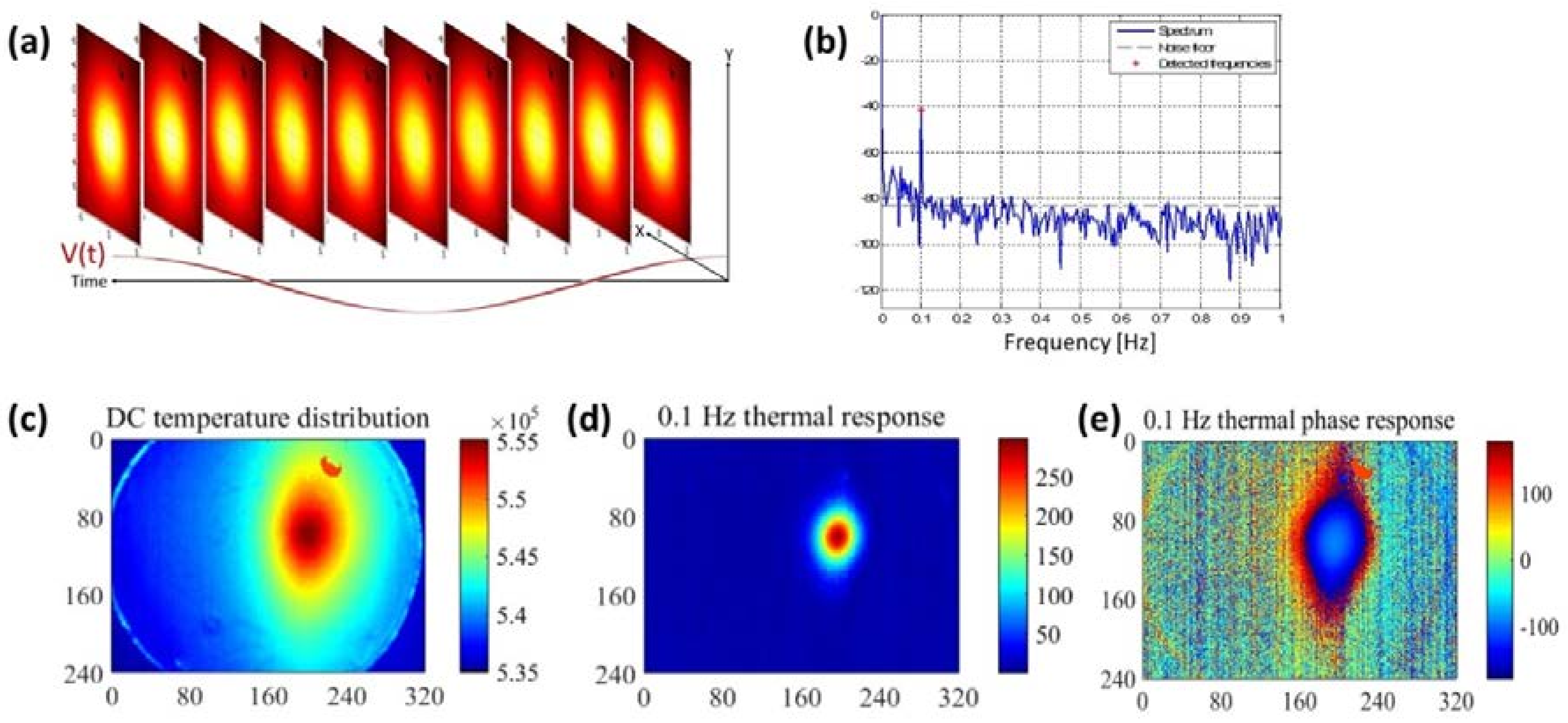
2.3. Thermal Image Processing
3. Results
3.1. Validation of the Thermal Model
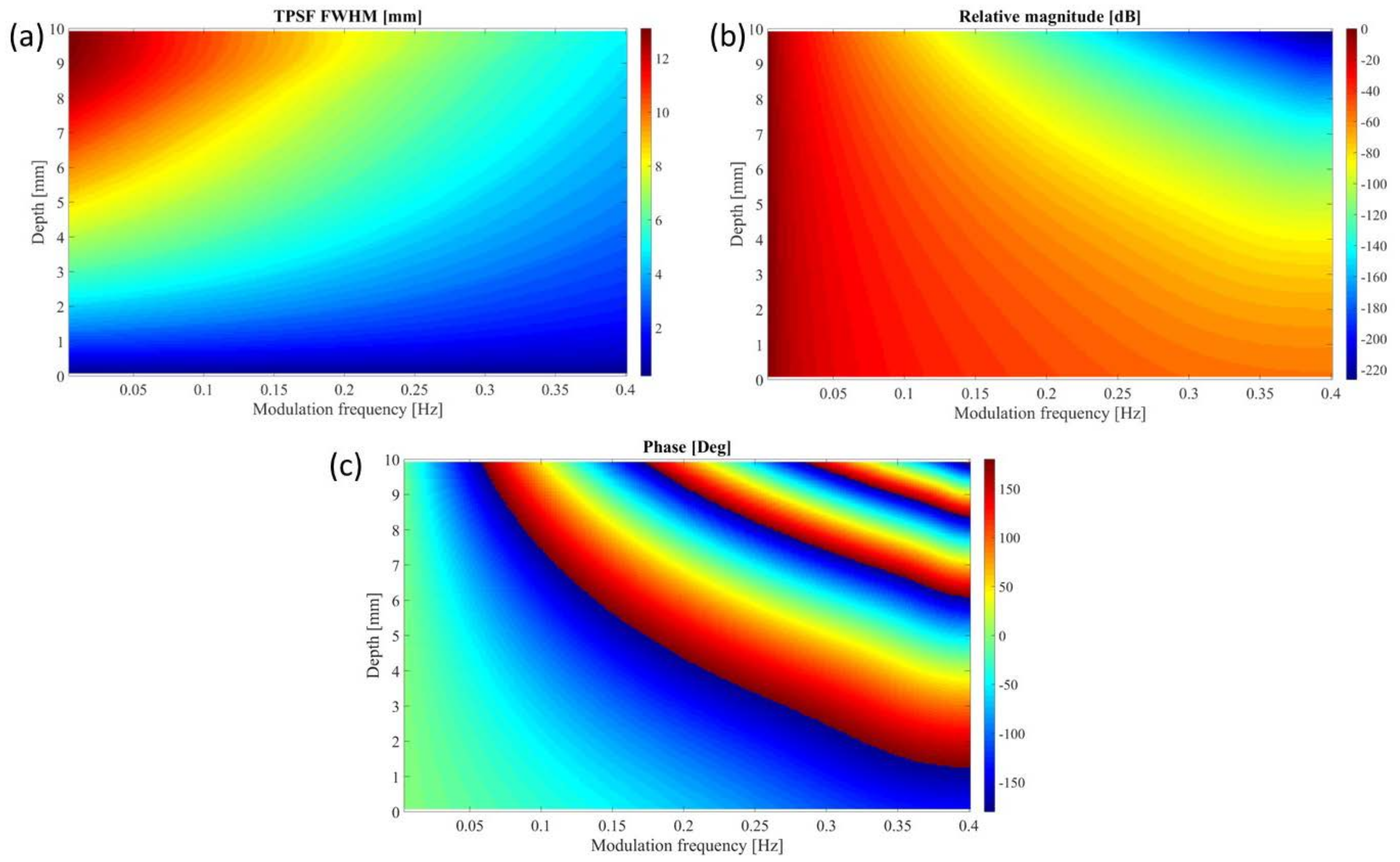
3.2. Theoretical PSF Analysis
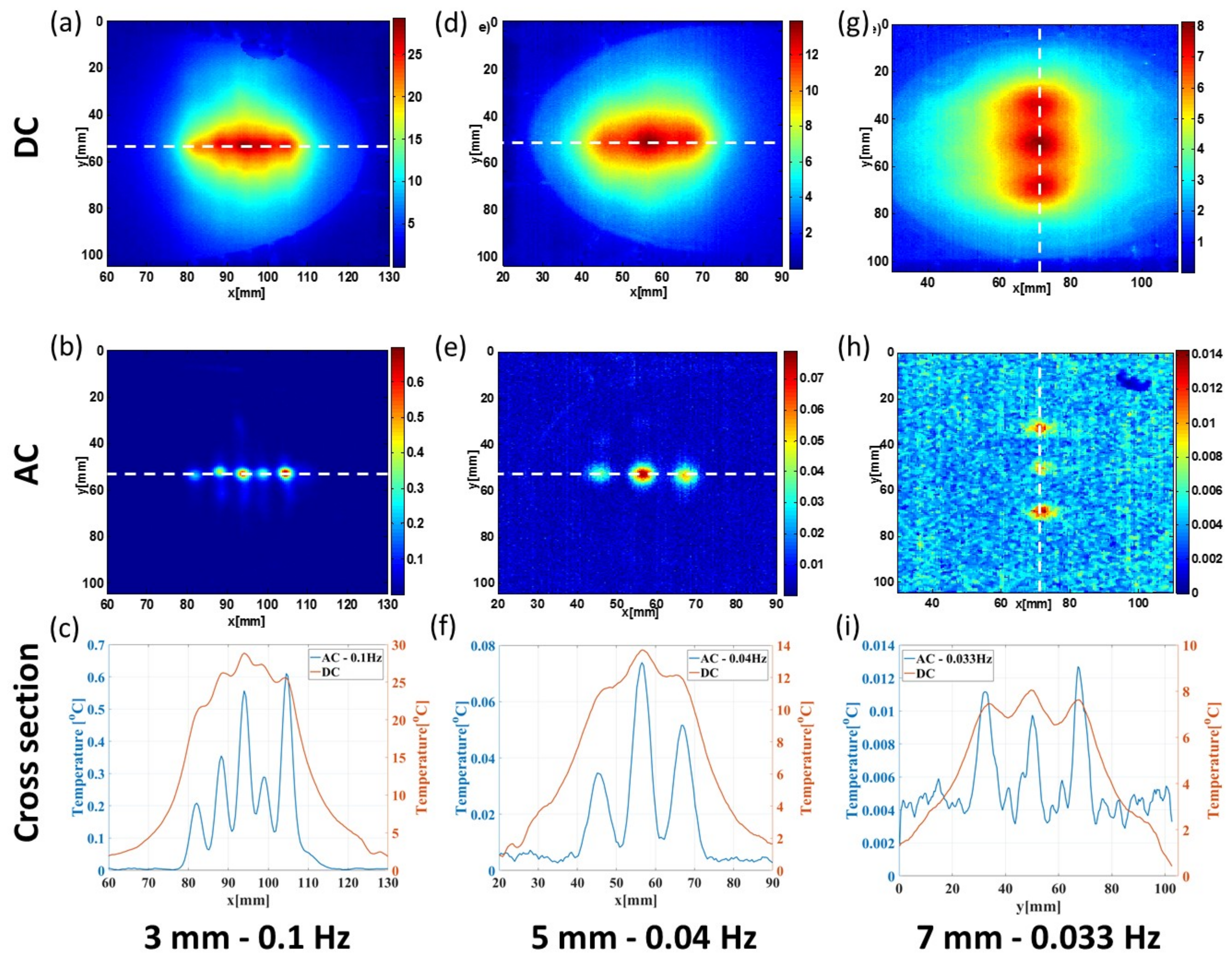
3.3. Transverse Thermal Resolution Analysis
3.4. SNR Analysis
3.5. Thermal Visibility Analysis
3.6. Reconstruction of the Heat Source Distribution
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- WHO Cancer Burden 2017. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/ (accessed on 1 March 2018).
- Gunduz, N.; Fisher, B.; Saffer, E.A. Effect of surgical removal on the growth and kinetics of residual tumor. Cancer Res. 1979, 39, 3861–3865. [Google Scholar] [PubMed]
- Gan, G.N.; Weickhardt, A.J.; Scheier, B.; Doebele, R.C.; Gaspar, L.E.; Kavanagh, B.D.; Camidge, D.R. Stereotactic radiation therapy can safely and durably control sites of extra-central nervous system oligoprogressive disease in anaplastic lymphoma kinase-positive lung cancer patients receiving crizotinib. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Jaffray, D.A.; Gospodarowicz, M.K. Radiation therapy for cancer. In Cancer: Disease Control Priorities; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2015; p. 239. [Google Scholar]
- DeVita, V.T., Jr.; Chu, E. Physicians’ Cancer Chemotherapy Drug Manual 2016; Jones & Bartlett Learning: Burlington, NJ, USA, 2016. [Google Scholar]
- Gustavsson, B.; Carlsson, G.; Machover, D.; Petrelli, N.; Roth, A.; Schmoll, H.-J.; Tveit, K.-M.; Gibson, F. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin. Color. Cancer 2015, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Zaidi, S.F. Treating cancer with heat: Hyperthermia as promising strategy to enhance apoptosis. J. Pak. Med. Assoc. 2013, 63, 504–508. [Google Scholar] [PubMed]
- Baronzio, G.; Parmar, G.; Ballerini, M.; Szasz, A.; Baronzio, M.; Cassutti, V. A brief overview of hyperthermia in cancer treatment. J. Integr. Oncol. 2014, 3, 1–10. [Google Scholar] [CrossRef]
- Storm, F.K.; Harrison, W.H.; Elliott, R.S.; Morton, D.L. Normal tissue and solid tumor effects of hyperthermia in animal models and clinical trials. Cancer Res. 1979, 39, 2245–2251. [Google Scholar] [PubMed]
- Repasky, E.A.; Evans, S.S.; Dewhirst, M.W. Temperature matters! And why it should matter to tumor immunologists. Cancer Immunol. Res. 2013, 1, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Ivkov, R.; DeNardo, S.J.; Daum, W.; Foreman, A.R.; Goldstein, R.C.; Nemkov, V.S.; DeNardo, G.L. Application of high amplitude alternating magnetic fields for heat induction of nanoparticles localized in cancer. Clin. Cancer Res. 2005, 11, 7093–7103. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T. Cancer hyperthermia using magnetic nanoparticles. Biotechnol. J. 2011, 6, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Shoval, A.; Tepper, M.; Tikochkiy, J.; Gur, L.B.; Markovich, G.; Keisari, Y.; Gannot, I. Magnetic nanoparticles-based acoustical detection and hyperthermic treatment of cancer, in vitro and in vivo studies. J. Nanophotonics 2016, 10, 036007. [Google Scholar] [CrossRef]
- Calixto, G.M.F.; Bernegossi, J.; de Freitas, L.M.; Fontana, C.R.; Chorilli, M. Nanotechnology-based drug delivery systems for photodynamic therapy of cancer: A review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef] [PubMed]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Triesscheijn, M.; Baas, P.; Schellens, J.H.M.; Stewart, F.A. Photodynamic therapy in oncology. Oncologist 2006, 11, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Kirisits, C.; Rivard, M.J.; Baltas, D.; Ballester, F.; De Brabandere, M.; van der Laarse, R.; Niatsetski, Y.; Papagiannis, P.; Hellebust, T.P.; Perez-Calatayud, J. Review of clinical brachytherapy uncertainties: Analysis guidelines of GEC-ESTRO and the AAPM. Radiother. Oncol. 2014, 110, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Thomadsen, B.R.; Erickson, B.A.; Eifel, P.J.; Hsu, I.C.; Patel, R.R.; Petereit, D.G.; Fraass, B.A.; Rivard, M.J. A review of safety, quality management, and practice guidelines for high-dose-rate brachytherapy: Executive summary. Pract. Radiat. Oncol. 2014, 4, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Dayan, A.; Ben-David, M.; Gannot, I. A new thermography-based approach to early detection of cancer utilizing magnetic nanoparticles theory simulation and in vitro validation. Nanomed. Nanotechnol. Boil. Med. 2010, 6, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Tepper, M.; Gannot, I. Monitoring tumor state from thermal images in animal and human models. Med. Phys. 2015, 42, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Tepper, M.; Shoval, A.; Gannot, I. The effect of geometry on tumor thermal profile and its use in tumor functional state estimation. J. Biophotonics 2015, 8, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Tepper, M.; Shoval, A.; Hoffer, O.; Confino, H.; Schmidt, M.; Kelson, I.; Keisari, Y.; Gannot, I. Thermographic investigation of tumor size, and its correlation to tumor relative temperature, in mice with transplantable solid breast carcinoma. J. Biomed. Opt. 2013, 18, 111410. [Google Scholar] [CrossRef] [PubMed]
- Tsalach, A.; Steinberg, I.; Gannot, I. Tumor localization using magnetic nanoparticle-induced acoustic signals. IEEE Trans. Biomed. Eng. 2014, 61, 2313–2323. [Google Scholar] [CrossRef] [PubMed]
- Opsal, J. Fundamentals of thermal wave physics. In Review of Progress in Quantitative Nondestructive Evaluation; Springer: Boston, MA, USA, 1987; pp. 217–225. [Google Scholar]
- Mandelis, A. Diffusion-Wave Fields: Mathematical Methods and Green Functions; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Duck, F.A. Physical Properties of Tissues; Academic Press: London, UK, 1990. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |




| Thermal Conductivity | Medium Density | Heat Capacitance | Thermal Diffusivity | |
|---|---|---|---|---|
| Polyester Resin | 0.155 | 1090 | 1670 | 8.52 × 10−8 |
| Agarose Gel 0.75% | 0.382 | 999 | 4178 | 9.15 × 10−8 |
| Soft Tissue | 0.499 | 1020 | 3600 | 1.5 × 10−7 |
| Source @ 3 mm | Source @ 5 mm | Source @ 7 mm | |
|---|---|---|---|
| Resolution w/o Modulation | 29.12 mm | 33.06 mm | 58.93 mm |
| Resolution with Modulation | 1.80 mm | 3.58 mm | 3.89 mm |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinberg, I.; Tamir, G.; Gannot, I. A Reconstruction Method for the Estimation of Temperatures of Multiple Sources Applied for Nanoparticle-Mediated Hyperthermia. Molecules 2018, 23, 670. https://doi.org/10.3390/molecules23030670
Steinberg I, Tamir G, Gannot I. A Reconstruction Method for the Estimation of Temperatures of Multiple Sources Applied for Nanoparticle-Mediated Hyperthermia. Molecules. 2018; 23(3):670. https://doi.org/10.3390/molecules23030670
Chicago/Turabian StyleSteinberg, Idan, Gil Tamir, and Israel Gannot. 2018. "A Reconstruction Method for the Estimation of Temperatures of Multiple Sources Applied for Nanoparticle-Mediated Hyperthermia" Molecules 23, no. 3: 670. https://doi.org/10.3390/molecules23030670





