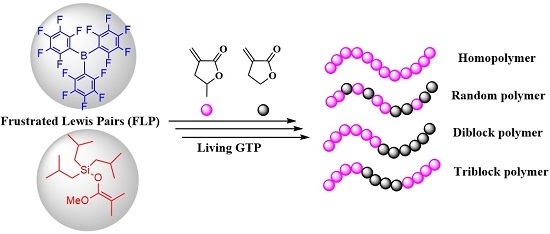
Silyl Ketene Acetals/B(C6F5)3 Lewis Pair-Catalyzed Living Group Transfer Polymerization of Renewable Cyclic Acrylic Monomers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Slow MMA Polymerization by R3SiH/B(C6F5)3 and SKA/B(C6F5)3
2.2. Controlled MMBL Polymerization by R3SiH/B(C6F5)3 and SKA/B(C6F5)3
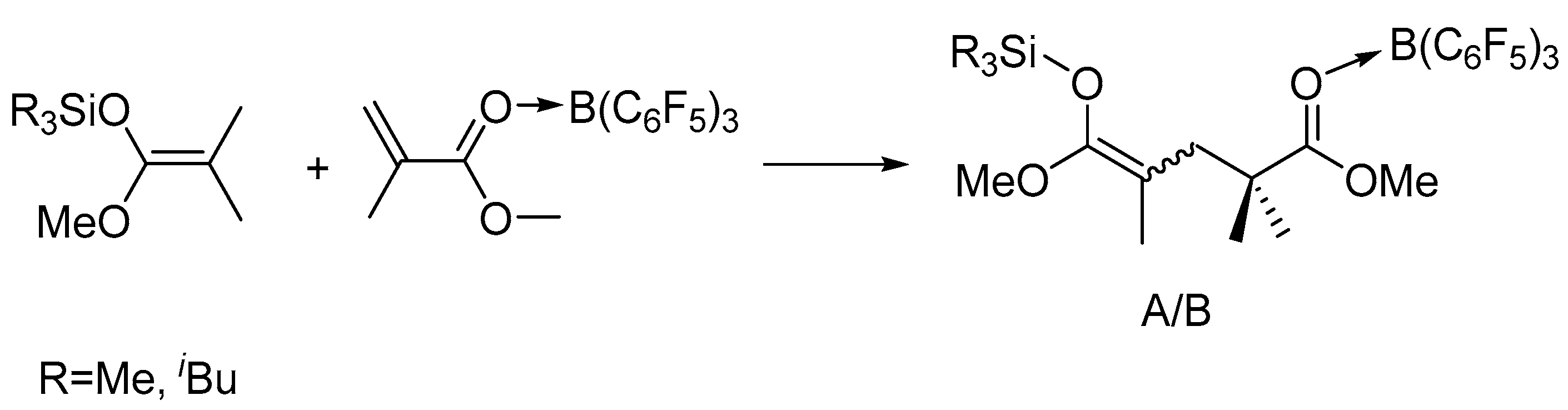
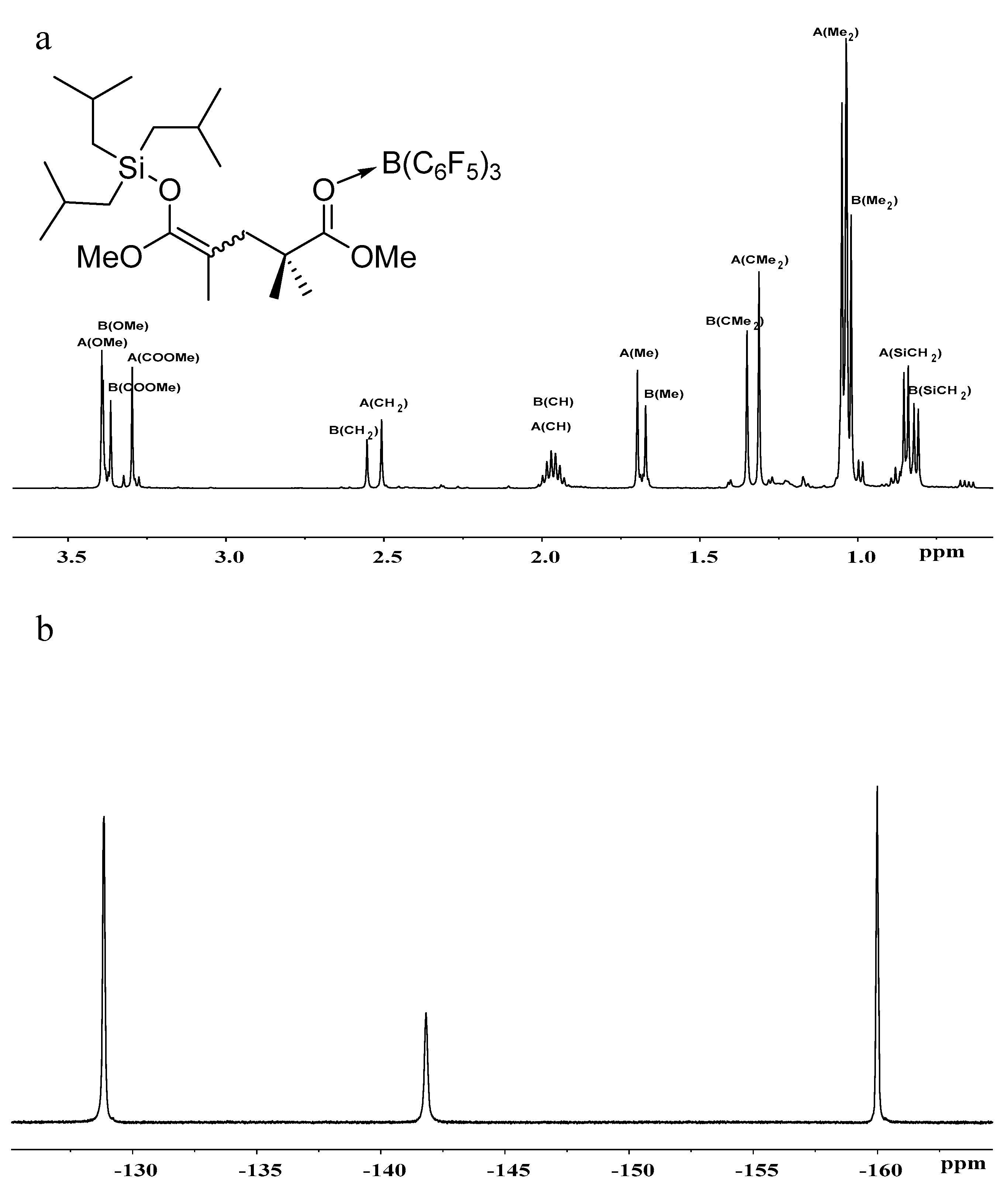
2.3. Characterization of Key Intermediates
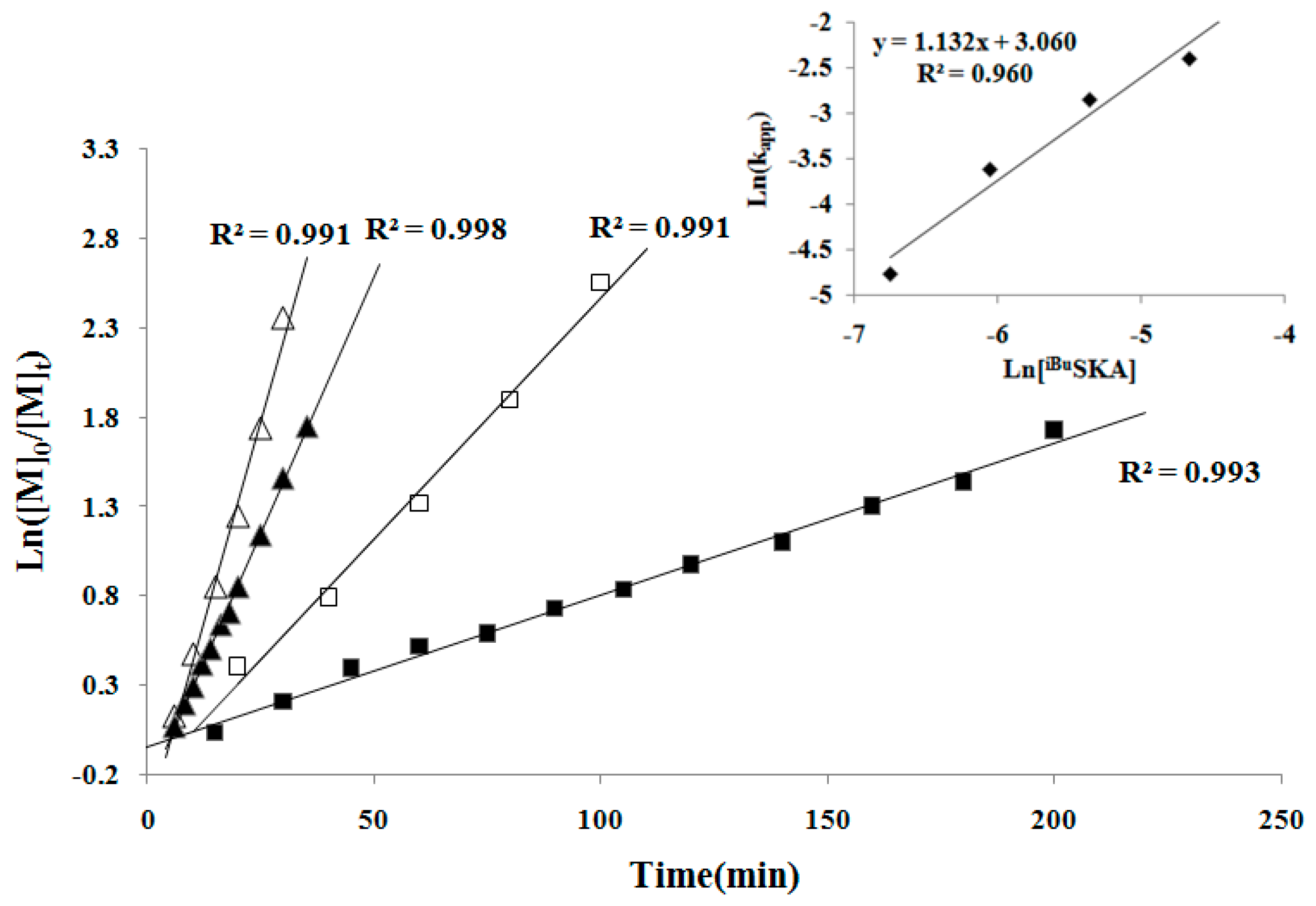
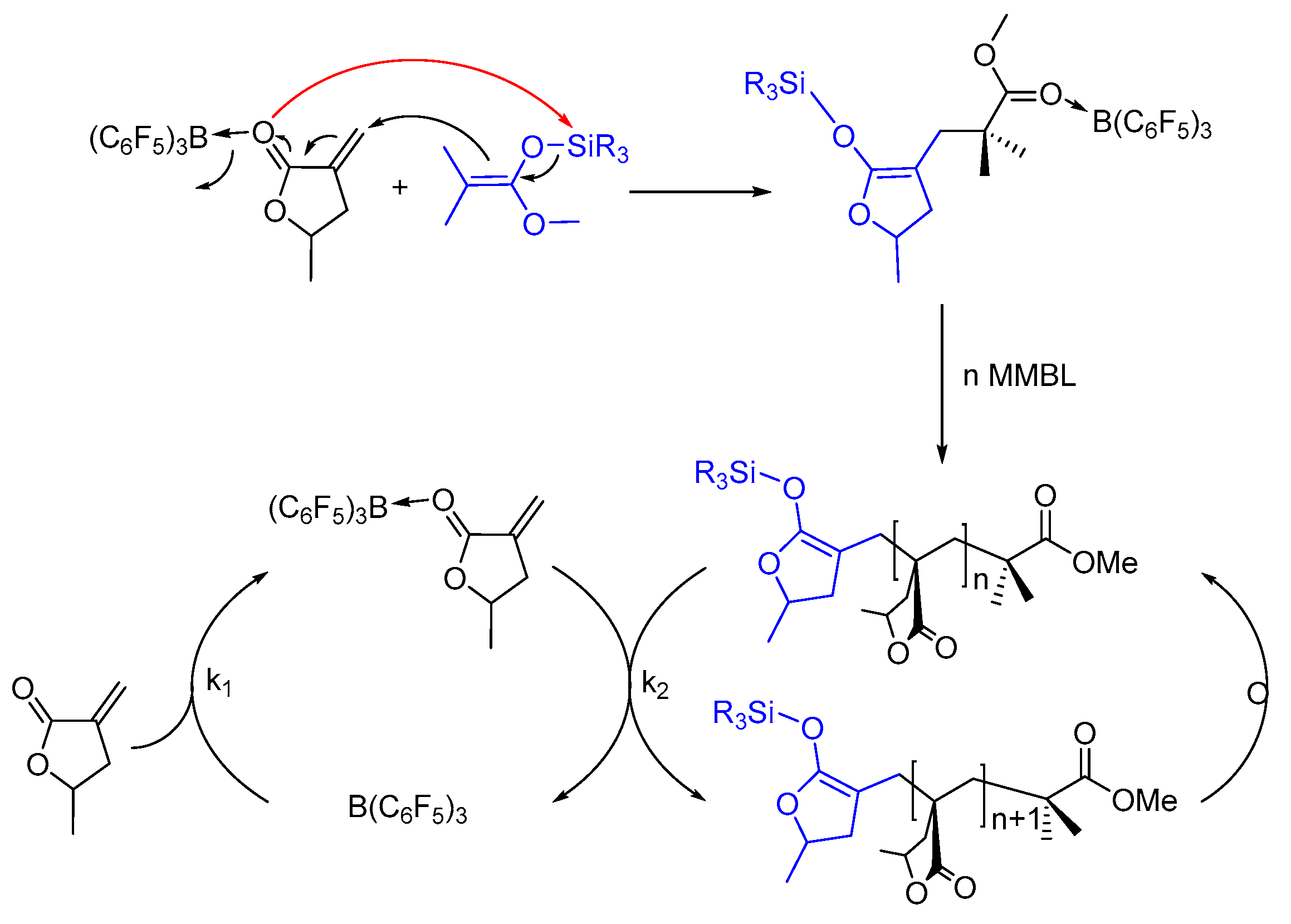
2.4. Kinetics and Mechanism of Polymerization
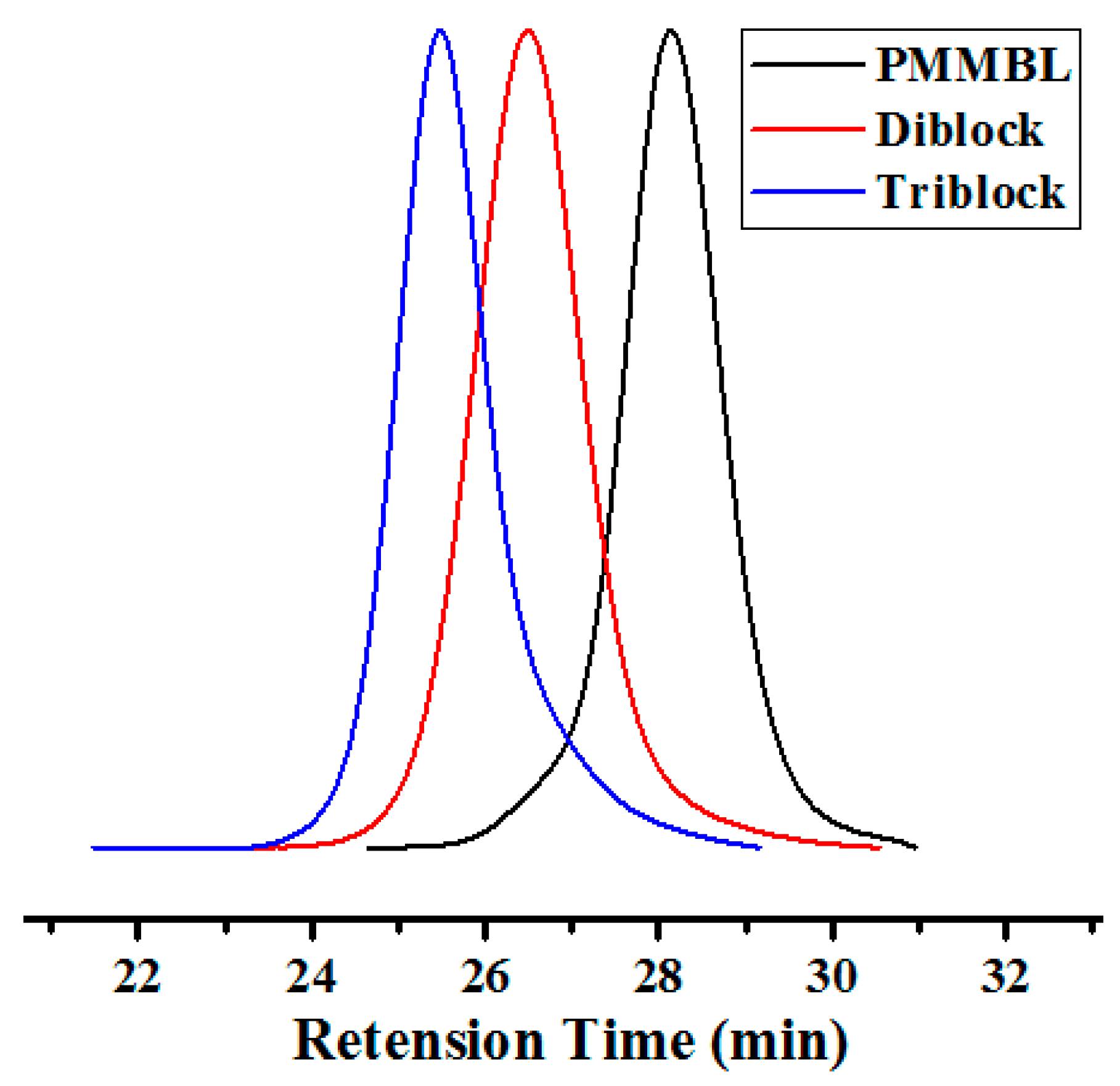
2.5. Random and Block Copolymerizations of MMBL with MBL
3. Metarials and Methods
3.1. Materials, Reagents and Methods
3.2. Synthesis of Dimethylketene Methyl Chlorodimethylsilyl Acetal Me2C=C(OMe)OSiMe2Cl (Me2ClSKA)
3.3. Synthesis of Chloro(ethoxy)dimethylsilane Me2(EtO)SiCl
3.4. Synthesis of Dimethylketene Methyl Ethoxydimethylsilyl Acetal Me2C=C-(OMe)OSiMe2(EtO) (Me2(EtO)SKA)
3.5. Isolation of Adduct B(C6F5)3·MMA
3.6. Isolation of Adduct B(C6F5)3·MMBL
3.7. NMR Reaction of Me2C=C(OMe)OSiMe3 (MeSKA) with B(C6F5)3
3.8. NMR Reaction of MeSKA with B(C6F5)3·MMA
3.9. NMR Reaction of Me2C=C(OMe)OSi(iBu)3 (iBuSKA) with B(C6F5)3
3.10. NMR Reaction of iBuSKA with B(C6F5)3·MMA
3.11. General Polymerization Procedures
3.12. Polymerization Kinetics
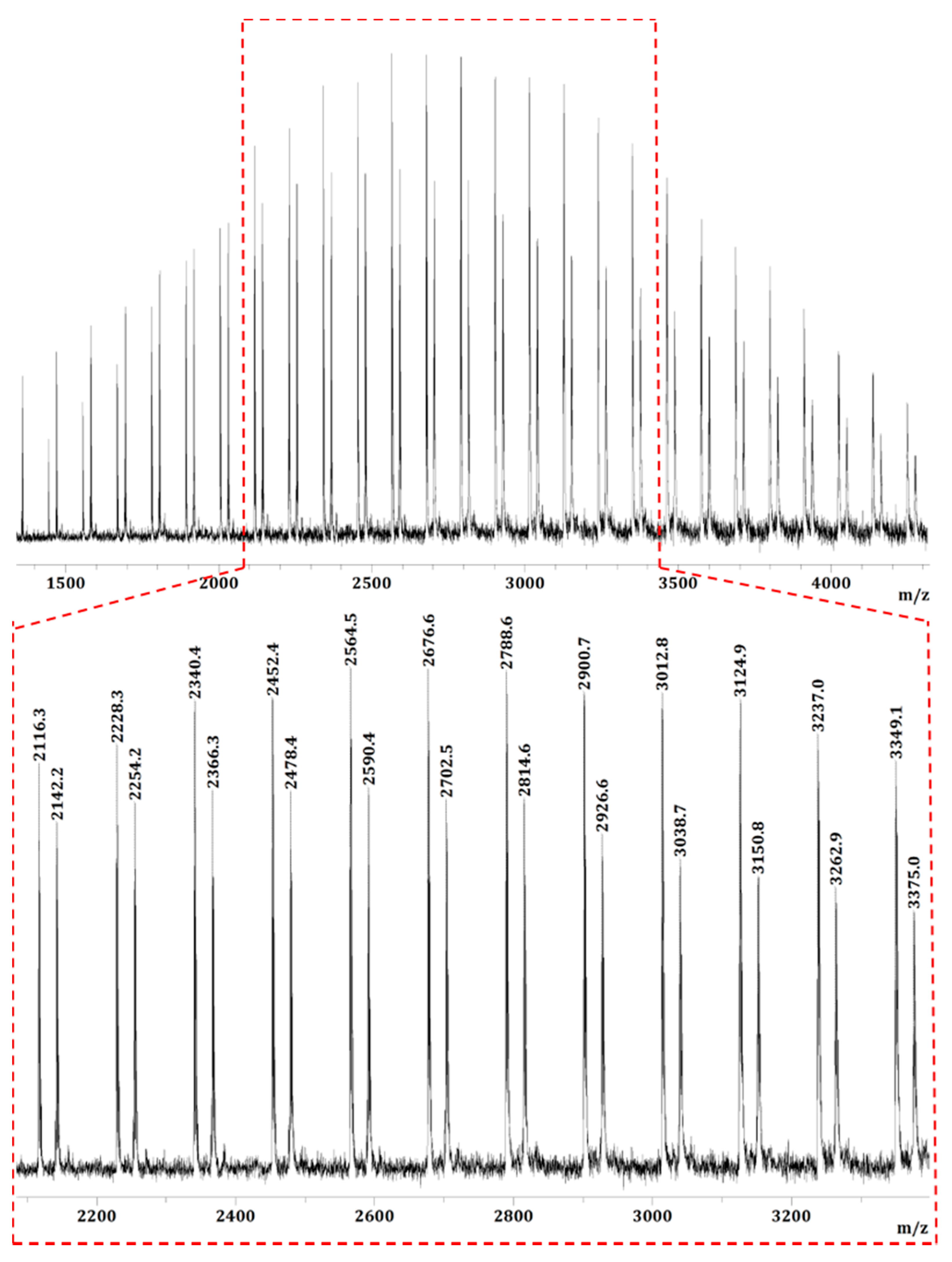
3.13. Polymer Characterizations
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xu, P.F.; Xu, X. Homoleptic Rare-Earth Aryloxide Based Lewis Pairs for Polymerization of Conjugated Polar Alkenes. ACS Catal. 2018, 8, 198–202. [Google Scholar] [CrossRef]
- Xu, P.F.; Yao, Y.M.; Xu, X. Frustrated Lewis Pair-Like Reactivity of Rare-Earth Metal Complexes: 1,4-Addition Reactions and Polymerizations of Conjugated Polar Alkenes. Chem. Eur. J. 2017, 23, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, Y.; Takasu, A.; Matsuoka, S.; Hayashi, M. N-Heterocyclic Carbene Initiated Anionic Polymerization of (E,E)-Methyl Sorbate and Subsequent Ring-Closing to Cyclic Poly(alkyl sorbate). J. Am. Chem. Soc. 2017, 139, 15005–15012. [Google Scholar] [CrossRef] [PubMed]
- Gowda, R.R.; Chen, E.Y.-X. Chemoselective Lewis Pair Polymerization of Renewable Multivinyl-Functionalized γ-Butyrolactones. Phil. Trans. R. Soc. A 2017, 375, 20170003. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, S.; Chen, Y.; Kitano, K.; Sato, S.; Satoh, T.; Kakuchi, T. B(C6F5)3-Catalyzed Group Transfer Polymerization of N,N-Disubstituted Acrylamide Using Hydrosilane: Effect of Hydrosilane and Monomer Structures, Polymerization Mechanism, and Synthesis of α-End-Functionalized Polyacrylamides. Macromolecules 2016, 49, 3049–3060. [Google Scholar] [CrossRef]
- Chen, J.W.; Chen, E.Y.-X. Elusive Silane-Alane Complex [Si-H···Al]: Isolation, Characterization, and Multifaceted Frustrated Lewis Pair Type Catalysis. Angew. Chem. Int. Ed. 2015, 54, 6842–6846. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Chen, E.X.-Y. Reactivity of Amine/E(C6F5)3 (E = B, Al) Lewis Pairs toward Linear and Cyclic Acrylic Monomers: Hydrogenation vs. Polymerization. Molecules 2015, 20, 9575–9590. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Chen, E.X.-Y. Lewis Pair Polymerization of Acrylic Monomers by N-Heterocyclic Carbenes and B(C6F5)3. Israel J. Chem. 2015, 55, 216–225. [Google Scholar] [CrossRef]
- Stephan, D.W. The broadening reach of frustrated Lewis pair chemistry. Science 2016, 354, 1248. [Google Scholar] [CrossRef] [PubMed]
- Stephan, D.W.; Erker, G. Frustrated Lewis Pair Chemistry: Development and Perspectives. Angew. Chem. Int. Ed. 2015, 54, 6400–6441. [Google Scholar] [CrossRef] [PubMed]
- Stephan, D.W. Frustrated Lewis Pairs: From Concept to Catalysis. Acc. Chem. Res. 2015, 48, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Stephan, D.W.; Erker, G. Frustrated Lewis Pairs I & II. In Topics in Current Chemistry; Springer: New York, NY, USA, 2013; Volume 332, 334. [Google Scholar]
- Chen, E.Y.-X. Polymerization by Classical and Frustrated Lewis Pairs. In Frustrated Lewis Pairs II; Erker, G., Stephan, D.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 334, pp. 239–260. [Google Scholar]
- Zhang, Y.T.; Miyake, G.M.; John, M.G.; Falivene, L.; Caporaso, L.; Cavallo, L.; Chen, E.Y.X. Lewis pair polymerization by classical and frustrated Lewis pairs: Acid, base and monomer scope and polymerization mechanism. Dalton Trans. 2012, 41, 9119–9134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Miyake, G.M.; Chen, E.Y.X. Alane-Based Classical and Frustrated Lewis Pairs in Polymer Synthesis: Rapid Polymerization of MMA and Naturally Renewable Methylene Butyrolactones into High-Molecular-Weight Polymers. Angew. Chem. Int. Ed. 2010, 49, 10158–10162. [Google Scholar] [CrossRef] [PubMed]
- He, J.H.; Zhang, Y.T.; Chen, E.Y.-X. Synthesis of Pyridineand 2-Oxazoline- Functionalized Vinyl Polymers by Alane-Based Frustrated Lewis Pairs cluster. Synlett 2014, 25, 1534–1538. [Google Scholar] [CrossRef]
- He, J.H.; Zhang, Y.T.; Falivene, L.; Caporaso, L.; Cavallo, L.; Chen, E.Y.X. Chain Propagation and Termination Mechanisms for Polymerization of Conjugated Polar Alkenes by [Al]-Based Frustrated Lewis Pairs. Macromolecules 2014, 47, 7765–7774. [Google Scholar] [CrossRef]
- Xu, T.; Chen, E.Y.-X. Probing Site Cooperativity of Frustrated Phosphine/Borane Lewis Pairs by a Polymerization Study. J. Am. Chem. Soc. 2014, 136, 1774–1777. [Google Scholar] [CrossRef] [PubMed]
- Knaus, M.G.M.; Giuman, M.M.; Pöthig, A.; Rieger, B. End of Frustration: Catalytic Precision Polymerization with Highly Interacting Lewis Pairs. J. Am. Chem. Soc. 2016, 138, 7776–7781. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.B.; Wang, Y.B.; Ren, W.M.; Xu, T.; Wang, J.; Lu, X.B. Mechanistic Aspects of Initiation and Deactivation in N-Heterocyclic Olefin Mediated Polymerization of Acrylates with Alane as Activator. Macromolecules 2014, 47, 1966–1972. [Google Scholar] [CrossRef]
- Jia, Y.B.; Ren, W.M.; Liu, S.J.; Xu, T.; Wang, Y.B.; Lu, X.B. Controlled Divinyl Monomer Polymerization Mediated by Lewis Pairs: A Powerful Synthetic Strategy for Functional Polymers. ACS Macro Lett. 2014, 3, 896–899. [Google Scholar] [CrossRef]
- Ottou, W.N.; Conde-Mendizabal, E.; Pascual, A.; Wirotius, A.-L.; Bourichon, D.; Vignolle, J.; Robert, F.; Landais, Y.; Sotiropoulos, J.-M.; Miqueu, K.; et al. Organic Lewis Pairs Based on Phosphine and Electrophilic Silane for the Direct and Controlled Polymerization of Methyl Methacrylate: Experimental and Theoretical Investigations. Macromolecules 2017, 50, 762–774. [Google Scholar] [CrossRef]
- Webster, O.W.; Hertler, W.R.; Sogah, D.Y.; Farnham, W.B.; Rajanbabu, T.V. Group-Transfer Polymerization. 1. A New Concept for Addition Polymerization with Organo-Silicon Initiators. J. Am. Chem. Soc. 1983, 105, 5706–5708. [Google Scholar] [CrossRef]
- Webster, O.W. The Discovery and Commercialization of Group Transfer Polymerization. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 2855–2860. [Google Scholar] [CrossRef]
- Webster, O.W. Group Transfer Polymerization: A Critical Review of Its Mechanism and Comparison with Other Methods for Controlled Polymerization of Acrylic Monomers. Adv. Polym. Sci. 2003, 167, 1–34. [Google Scholar]
- Schubert, W.; Sitz, H.D.; Bandermann, F. Group Transfer Polymerization of Methyl-Methacrylate and Methyl Acrylate in Tetrahydrofuran with Tris(piperidino)sulfonium Bifluoride as Catalyst. Makromol. Chem. 1989, 190, 2193–2201. [Google Scholar] [CrossRef]
- Schubert, W.; Bandermann, F. Group Transfer Polymerization of Methyl Acrylate in Acetonitrile. Makromol. Chem. 1989, 190, 2161–2171. [Google Scholar] [CrossRef]
- Sogah, D.Y.; Hertler, W.R.; Webster, O.W.; Cohen, G.M. Group Transfer Polymerization—Polymerization of Acrylic-Monomers. Macromolecules 1987, 20, 1473–1488. [Google Scholar] [CrossRef]
- Schubert, W.; Bandermann, F. Group Transfer Polymerization of Methyl Acrylate in Tetrahydrofuran with Tetrabutylammonium Fluoride Trihydrate and Tetrabutylammonium Cyanide as Catalysts. Makromol. Chem. 1989, 190, 2721–2726. [Google Scholar] [CrossRef]
- Schmalbrock, U.; Bandermann, F. Group-Transfer Polymerization of Methyl- Methacrylate and Butyl Acrylate in Tetrahydrofuran with Oxy Anions as Catalysts. Makromol. Chem. 1993, 194, 2543–2551. [Google Scholar] [CrossRef]
- Dicker, I.B.; Cohen, G.M.; Farnham, W.B.; Hertler, W.R.; Laganis, E.D.; Sogah, D.Y. Oxyanions Catalyze Group-Transfer Polymerization to Give Living Polymers. Macromolecules 1990, 23, 4034–4041. [Google Scholar] [CrossRef]
- Patrickios, C.S.; Hertler, W.R.; Abbott, N.L.; Hatton, T.A. Diblock, ABC Triblock, and Random Methacrylic Polyampholytes -Synthesis by Group-Transfer Polymerization and Solution Behavior. Macromolecules 1994, 27, 930–937. [Google Scholar] [CrossRef]
- Raynaud, J.; Ciolino, A.; Baceiredo, A.; Destarac, M.; Bonnette, F.; Kato, T.; Gnanou, Y.; Taton, D. Harnessing the Potential of N-Heterocyclic Carbenes for the Rejuvenation of Group-Transfer Polymerization of (Meth)acrylics. Angew. Chem. Int. Ed. 2008, 47, 5390–5393. [Google Scholar] [CrossRef] [PubMed]
- Scholten, M.D.; Hedrick, J.L.; Waymouth, R.M. Group Transfer Polymerization of Acrylates Catalyzed by N-Heterocyclic Carbenes. Macromolecules 2008, 41, 7399–7404. [Google Scholar] [CrossRef]
- Raynaud, J.; Gnanou, Y.; Taton, D. Group Transfer Polymerization of (Meth)acrylic Monomers Catalyzed by N-Heterocyclic Carbenes and Synthesis of All Acrylic Block Copolymers: Evidence for an Associative Mechanism. Macromolecules 2009, 42, 5996–6005. [Google Scholar] [CrossRef]
- Raynaud, J.; Liu, N.; Gnanou, Y.; Taton, D. Expanding the Scope of Group Transfer Polymerization Using N-Heterocyclic Carbenes as Catalysts: Application to Miscellaneous (Meth)acrylic Monomers and Kinetic Investigations. Macromolecules 2010, 43, 8853–8861. [Google Scholar] [CrossRef]
- Raynaud, J.; Liu, N.; Fevre, M.; Gnanou, Y.; Taton, D. No Matter the Order of Monomer Addition for the Synthesis of Well-Defined Block Copolymers by Sequential Group Transfer Polymerization using N-Heterocyclic Carbenes as Catalysts. Polym. Chem. 2011, 2, 1706–1712. [Google Scholar] [CrossRef]
- Fevre, M.; Pinaud, J.; Gnanou, Y.; Vignolle, J.; Taton, D. N-Heterocyclic Carbenes (NHCs) as Organocatalysts and Structural Components in Metal-Free Polymer Synthesis. Chem. Soc. Rev. 2013, 42, 2142–2172. [Google Scholar] [CrossRef] [PubMed]
- Kakuchi, T.; Chen, Y.G.; Kitakado, J.; Mori, K.; Fuchise, K.; Satoh, T. Organic Superbase as an Efficient Catalyst for Group Transfer Polymerization of Methyl Methacrylate. Macromolecules 2011, 44, 4641–4647. [Google Scholar] [CrossRef]
- Fevre, M.; Vignolle, J.; Heroguez, V.; Taton, D. Tris(2,4,6-trimethoxyphenyl) phosphine (TTMPP) as Potent Organocatalyst for Group Transfer Polymerization of Alkyl (Meth)acrylates. Macromolecules 2012, 45, 7711–7718. [Google Scholar] [CrossRef]
- Chen, Y.G.; Fuchise, K.; Narumi, A.; Kawaguchi, S.; Satoh, T.; Kakuchi, T. Core-First Synthesis of Three-, Four-, and Six-Armed Star-Shaped Poly(methyl methacrylate)s by Group Transfer Polymerization Using Phosphazene Base. Macromolecules 2011, 44, 9091–9098. [Google Scholar] [CrossRef]
- Hsu, J.C.; Chen, Y.G.; Kakuchi, T.; Chen, W.C. Synthesis of Linear and Star-Shaped Poly[4-(diphenylamino)benzyl methacrylate]s by Group Transfer Polymerization and Their Electrical Memory Device Applications. Macromolecules 2011, 44, 5168–5177. [Google Scholar] [CrossRef]
- Kikuchi, S.; Chen, Y.; Fuchise, K.; Takada, K.; Kitakado, J.; Sato, S.; Satoh, T.; Kakuchi, T. Thermoresponsive Properties of 3-, 4-, 6-, and 12-Armed Star-Shaped Poly[2-(dimethylamino)ethyl methacrylate]s Prepared by Core-First Group Transfer Polymerization. Polym. Chem. 2014, 5, 4701–4709. [Google Scholar] [CrossRef]
- Chen, Y.; Takada, K.; Kubota, N.; Eric, O.T.; Ito, T.; Isono, T.; Satoh, T.; Kakuchi, T. Synthesis of End-Functionalized Poly-(methyl methacrylate) by Organocatalyzed Group Transfer Polymerization Using Functional Silyl Ketene Acetals and Alpha-Phenylacrylates. Polym. Chem. 2015, 6, 1830–1837. [Google Scholar] [CrossRef]
- Fuchise, K.; Chen, Y.G.; Satoh, T.; Kakuchi, T. Recent Progressin Organocatalytic Group Transfer Polymerization. Polym. Chem. 2013, 4, 4278–4291. [Google Scholar] [CrossRef]
- Hertler, W.R.; Sogah, D.Y.; Webster, O.W.; Trost, B.M. Group Transfer Polymerization. 3. Lewis Acid Catalysis. Macromolecules 1984, 17, 1415–1419. [Google Scholar] [CrossRef]
- Zhuang, R.; Müller, A.H.E. Group Transfer Polymerization of n-Butyl Acrylate with Lewis Acid Catalysts. 1. Kinetic Investigation Using HgI2 as a Catalyst in Toluene. Macromolecules 1995, 28, 8035–8042. [Google Scholar] [CrossRef]
- Zhuang, R.; Müller, A.H.E. Group Transfer Polymerization of n-Butyl Acrylate with Lewis Acid Catalysts. 2. Kinetic Investigation Using the HgI2/Me3SiI Catalyst System in Toluene and Methylene Chloride. Macromolecules 1995, 28, 8043–8050. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, E.Y.-X. Controlled Polymerization of Methacrylates to High Molecular Weight Polymer Using Oxidatively Activated Group Transfer Polymerization Initiators. Macromolecules 2008, 41, 36–42. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, E.Y.-X. Structure-Reactivity Relationships in Bimolecular-Ativated Monomer Polymerization of (Meth)acrylates Using Oxidatively Activated Group 14 Ketene Acetals. Macromolecules 2008, 41, 6353–6360. [Google Scholar] [CrossRef]
- Miyake, G.M.; Zhang, Y.T.; Chen, E.Y.-X. Living Polymerization of Naturally Renewable Butyrolactone-Based Vinylidene Monomers by Ambiphilic Silicon Propagators. Macromolecules 2010, 43, 4902–4908. [Google Scholar] [CrossRef]
- Zhang, Y.; Gustafson, L.O.; Chen, E.Y.-X. Dinuclear Silylium-Enolate Bifunctional Active Species: Remarkable Activity and Stereoselectivity toward Polymerization of Methacrylate and Renewable Methylene Butyrolactone Monomers. J. Am. Chem. Soc. 2011, 133, 13674–13684. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Chen, E.Y.-X. Silylium Dual Catalysis in Living Polymerization of Methacrylates via In Situ Hydrosilylation of Monomer. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1895–1903. [Google Scholar] [CrossRef]
- Chen, Y.G.; Kitano, K.; Tsuchida, S.; Kikuchi, S.; Takada, K.; Satoh, T.; Kakuchi, T. B(C6F5)3-Catalyzed Group Transfer Polymerization of Alkyl Methacrylates with Dimethylphenylsilane through In Situ Formation of Silyl Ketene Acetal by B(C6F5)3-Catalyzed 1,4-Hydrosilylation of Methacrylate Monomer. Polym. Chem. 2015, 6, 3502–3511. [Google Scholar] [CrossRef]
- Fuchise, K.; Tsuchida, S.; Takada, K.; Chen, Y.G.; Satoh, T.; Kakuchi, T. B(C6F5)3-Catalyzed Group Transfer Polymerization of n-Butyl Acrylate with Hydrosilane through In Situ Formation of Initiator by 1,4-Hydrosilylation of n-Butyl Acrylate. ACS Macro Lett. 2014, 3, 1015–1019. [Google Scholar] [CrossRef]
- Allen, R.D.; Long, T.E.; McGrath, J.E. Preparation of high purity, anionic polymerization grade alkyl methacrylate monomers. Polym. Bull. 1986, 15, 127–134. [Google Scholar] [CrossRef]
- Lehmann, M.; Schulz, A.; Villinger, A. Bissilylated Halonium Ions: [Me3Si−X−SiMe3][B(C6F5)4] (X = F, Cl, Br, I). Angew. Chem. Int. Ed. 2009, 48, 7444–7447. [Google Scholar] [CrossRef] [PubMed]
- Karsch, M.; Lund, H.; Schulz, A.; Villinger, A.; Voss, K. Molecular Networks Based on CN Coordination Bonds. Eur. J. Inorg. Chem. 2012, 2012, 5542–5553. [Google Scholar] [CrossRef]
- Chen, J.; Gowda, R.R.; He, J.; Zhang, Y.; Chen, E.Y.-X. Controlled or High-Speed Group Transfer Polymerization by Silyl Ketene Acetals without Catalyst. Macromolecules 2016, 49, 8075–8087. [Google Scholar] [CrossRef]
- Oisaki, K.; Suto, Y.; Kanai, M.; Shibasaki, M. A New Method for the Catalytic Aldol Reaction to Ketones. J. Am. Chem. Soc. 2003, 125, 5644–5645. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, C.; Chen, F.; Kuo, Y.-N. Ketene alkyltrialkylsilyl acetals: Synthesis, pyrolysis and NMR studies. J. Organomet. Chem. 1972, 46, 59–71. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

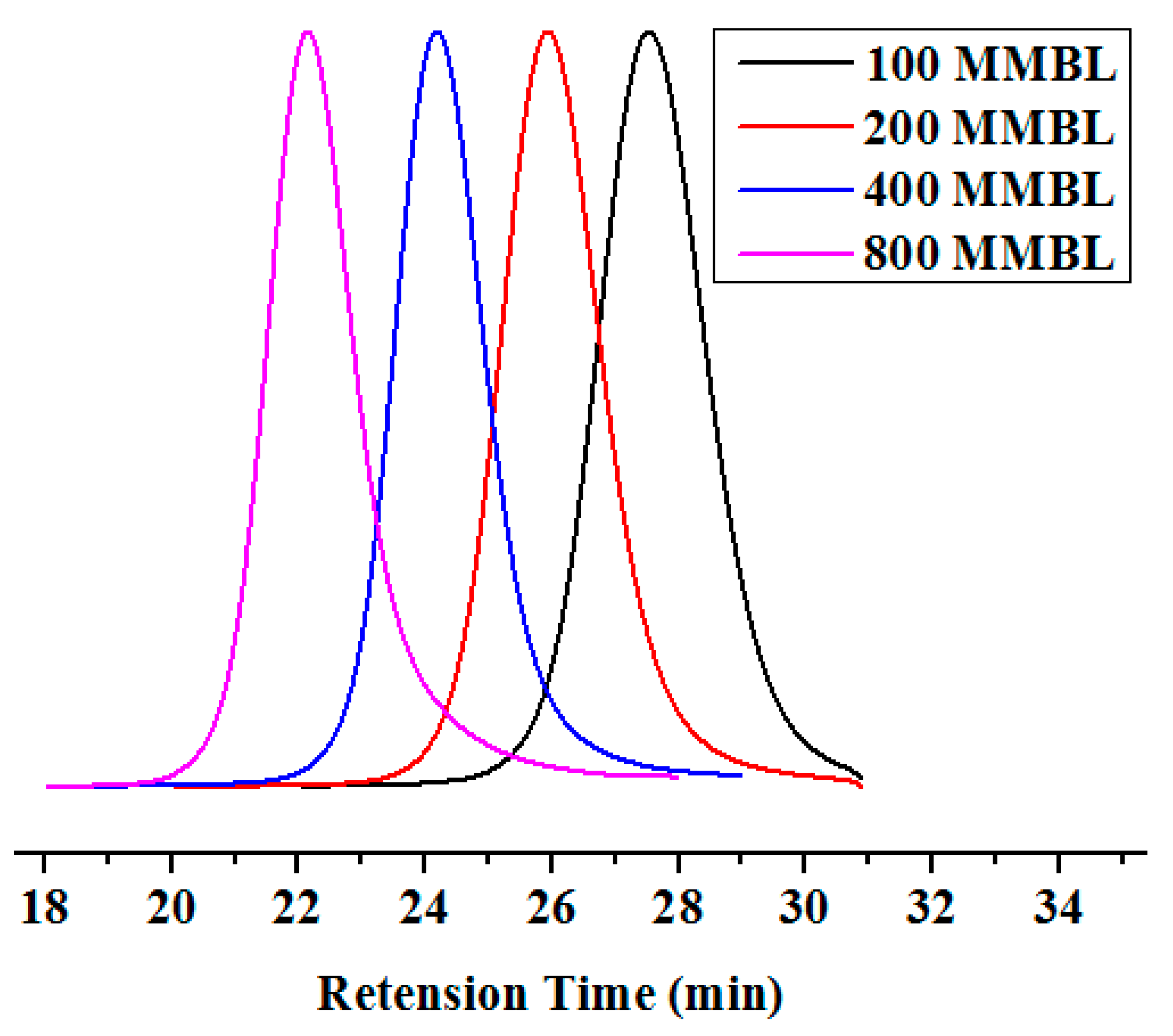

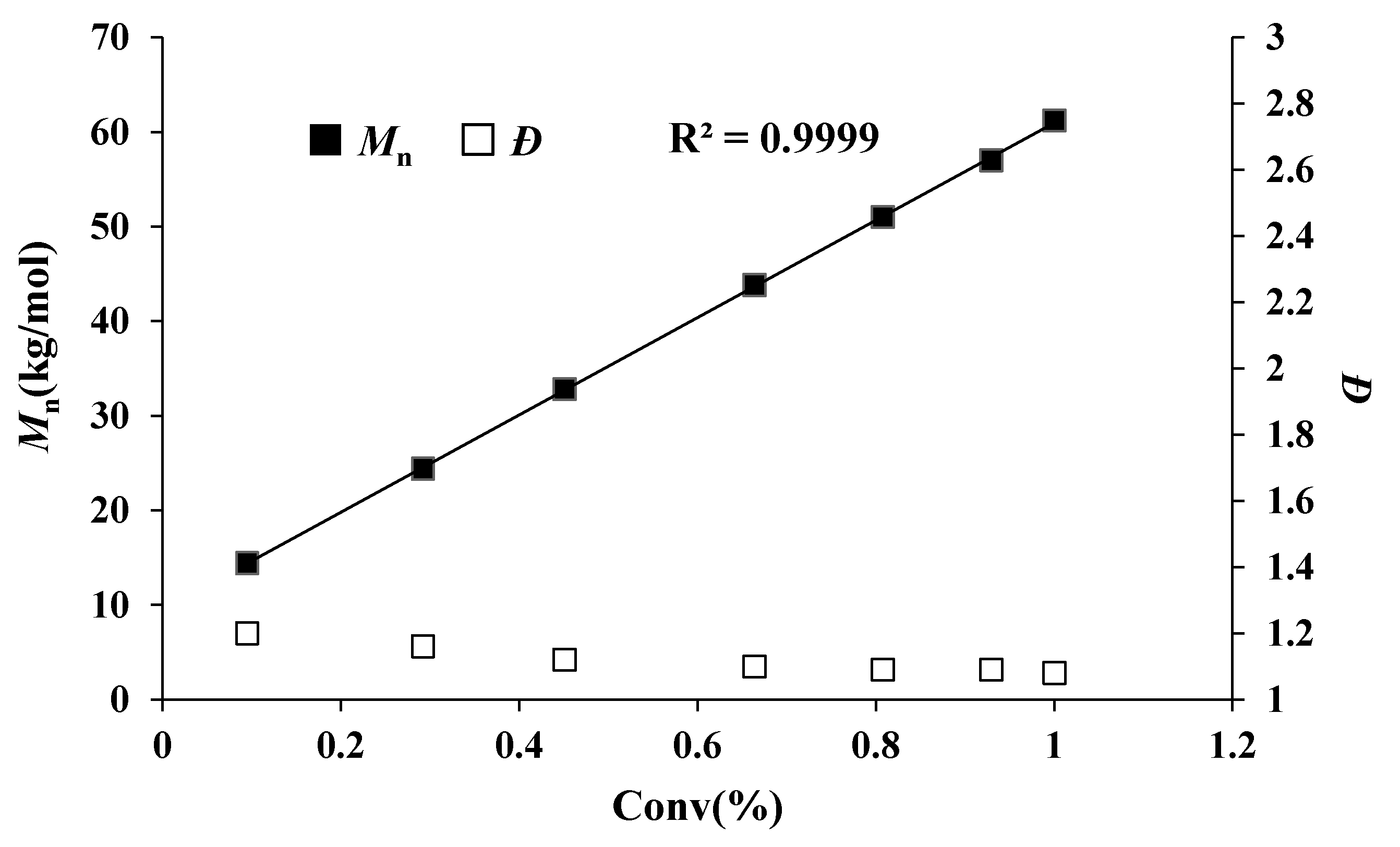

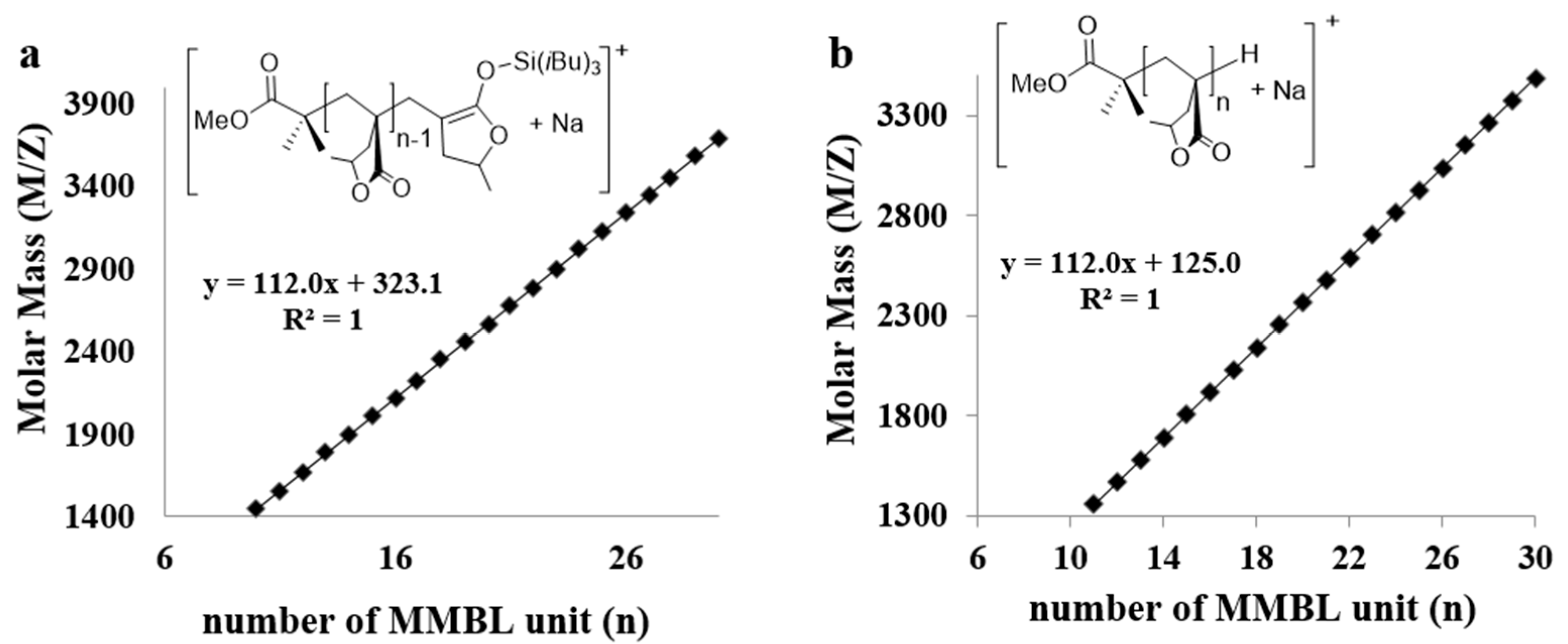
| Run No. | Initiator (I) | Monomer (M) | [M]:[I]:[B] b | Time (h) | Conv. c (%) | Mn. d (kg/mol) | Ð | I* e (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Me2EtSiH | MMBL | 200:1:1 | 0.5 | 100 | 48.1 | 1.26 | 47 |
| 2 | Me2PhSiH | MMBL | 200:1:1 | 0.5 | 100 | 57.4 | 1.39 | 39 |
| 3 | Me2ClSiH | MMBL | 200:1:1 | 2 | 100 | 78.6 | 1.44 | 29 |
| 4 | Et3SiH | MMBL | 200:1:1 | 0.5 | 100 | 33 | 1.27 | 68 |
| 5 | Ph3SiH | MMBL | 200:1:1 | 6 | 100 | 105 | 1.61 | 21 |
| 6 | iBu3SiH | MMBL | 200:1:1 | 24 | 100 | 345 | 1.51 | 7 |
| 7 | Me2PhSKA | MMBL | 200:1:1 | 0.5 | 100 | 45.5 | 1.33 | 50 |
| 8 | Me2(EtO)SKA | MMBL | 200:1:1 | 0.5 | 100 | 41.5 | 1.37 | 54 |
| 9 | Me2ClSKA | MMBL | 200:1:1 | 6 | 100 | 85.1 | 1.82 | 27 |
| 10 | PhSKA | MMBL | 200:1:1 | 2 | 100 | 68.8 | 1.37 | 33 |
| 11 | MeSKA | MMBL | 200:1:1 | 1 | 100 | 31.9 | 1.08 | 71 |
| 12 | EtSKA | MMBL | 200:1:1 | 0.5 | 100 | 37 | 1.21 | 61 |
| 13 | iBuSKA | MMBL | 200:1:1 | 1 | 100 | 32.2 | 1.09 | 70 |
| 14 | iBuSKA | MMBL | 100:1:1 | 0.17 | 100 | 13.2 | 1.08 | 86 |
| 15 | MeSKA | MBL | 200:1:1 | 24 | 100 | 14.6 (58%) 98.0 (42%) | 1.20, 1.80 | - |
| 16 | MeSKA | MBL | 100:1:1 | 0.5 | 100 | 12.7 | 1.12 | 78 |
| 17 | iBuSKA | MBL | 200:1:1 | 24 | 100 | 23.4 | 1.95 | 84 |
| 18 | iBuSKA | MBL | 100:1:1 | 0.5 | 100 | 13.4 | 1.05 | 74 |
| Run No. | M1/M2 | Conv. b(%) | Mw c (kg/mol) | Đ |
|---|---|---|---|---|
| 1 d | 100 MMBL + 100 MBL | MBL:100 MMBL:100 | 28.3 | 1.03 |
| 2 | 100 MMBL/100 MBL | MBL:100 MMBL:100 | 25.7 | 1.07 |
| 3 | 100 MMBL/100 MBL/100 MMBL | MBL:100 MMBL:100 | 37.1 | 1.06 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Zhao, W.; He, J.; Zhang, Y. Silyl Ketene Acetals/B(C6F5)3 Lewis Pair-Catalyzed Living Group Transfer Polymerization of Renewable Cyclic Acrylic Monomers. Molecules 2018, 23, 665. https://doi.org/10.3390/molecules23030665
Hu L, Zhao W, He J, Zhang Y. Silyl Ketene Acetals/B(C6F5)3 Lewis Pair-Catalyzed Living Group Transfer Polymerization of Renewable Cyclic Acrylic Monomers. Molecules. 2018; 23(3):665. https://doi.org/10.3390/molecules23030665
Chicago/Turabian StyleHu, Lu, Wuchao Zhao, Jianghua He, and Yuetao Zhang. 2018. "Silyl Ketene Acetals/B(C6F5)3 Lewis Pair-Catalyzed Living Group Transfer Polymerization of Renewable Cyclic Acrylic Monomers" Molecules 23, no. 3: 665. https://doi.org/10.3390/molecules23030665