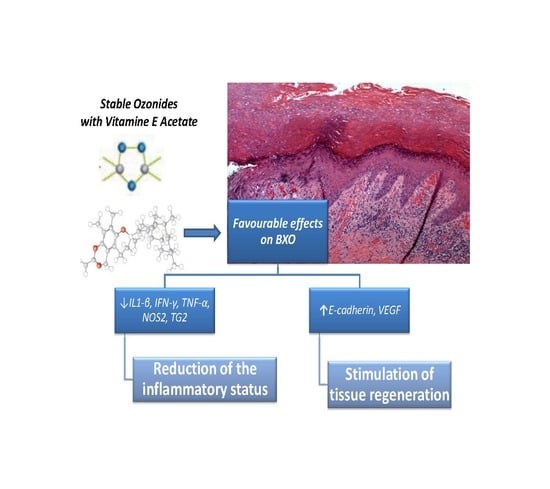
Anti-Inflammatory and Tissue Regenerative Effects of Topical Treatment with Ozonated Olive Oil/Vitamin E Acetate in Balanitis Xerotica Obliterans
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patient Recruitment
4.2. Gene Expression Analysis
4.3. EMSA (Electrophoretic Mobility Shift Assay)
4.4. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kiss, A.; Király, L.; Kutasy, B.; Merksz, M. High incidence of balanitis xerotica obliterans in boys with phimosis: Prospective 10-year study. Pediatr. Dermatol. 2005, 22, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Christman, M.S.; Chen, J.T.; Holmes, N.M. Obstructive complications of lichen sclerosus. J. Pediatr. Urol. 2009, 5, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Stuhmer, A. Balanitis xerotica obliterans (post-operationem) und ihreBeziehungenzur “Kraurosis glandis et praeputii penis”. Arch. Dermatol. Syph. 1928, 156, 613–623. [Google Scholar] [CrossRef]
- Becker, K.; Meissner, V.; Farwick, W.; Bauer, R.; Gaiser, M.R. Lichen sclerosus and atopy in boys: Coincidence or correlation? Br. J. Dermatol. 2013, 168, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Celis, S.; Reed, F.; Murphy, F.; Adams, S.; Gillick, J.; Abdelhafeez, A.H.; Lopez, P.J. Balanitis xerotica obliterans in children and adolescents: A literature review and clinical series. J. Pediatr. Urol. 2014, 10, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.X.; Sun, G.S.; Teng, J.M. Pediatric Lichen Sclerosus: A Review of the Epidemiology and Treatment Options. Pediatr. Dermatol. 2015, 32, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.M.; Lewis, F.M.; Tatnall, F.M.; Cox, N.H. British Association of Dermatologists. British Association of Dermatologists’ guidelines for the management of lichen sclerosus 2010. Br. J. Dermatol. 2010, 163, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.C.; Kirtschig, G.; Baldo, M.; Lewis, F.; Wang, S.H.; Wojnarowska, F. Systematic review and meta-analysis of randomized controlled trials on topical interventions for genital lichen sclerosus. J. Am. Acad. Dermatol. 2012, 67, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Narahari, R.; O’Riordan, A.; N’Dow, J.M.; Pickard, R. Simple Dilatation, Endoscopic Urethrotomy, and Urethroplasty for Urethral Stricture in Adults; Cochrane Database: New York, NY, USA, 2008. [Google Scholar]
- Goldstein, A.T.; Creasey, A.; Pfau, R.; Phillips, D.; Burrows, L.J. A double-blind, randomized controlled trial of clobetasol versuspimecrolimus in patients with vulvar lichen sclerosus. J. Am. Acad. Dermatol. 2011, 64, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Fortino, V.; Bocci, V. The dual action of ozone on the skin. Br. J. Dermatol. 2005, 153, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Travagli, V.; Zanardi, I.; Bocci, V. Topical applications of ozone and ozonated oils as anti-infective agents: An insight into the patent claims. Recent Pat. Anti-Infect. Drug Discov. 2009, 4, 130–142. [Google Scholar] [CrossRef]
- Lamberto, R.; Mawsouf, M.N.; Menéndez, S.; Olga, S.; León Sánchez, G.M.; Hernández, F. Ozone therapy: Clinical and basic evidence of its therapeutic potential. Arch. Med. Res. 2008, 39, 17–26. [Google Scholar]
- Travagli, V.; Zanardi, I.; Valacchi, G.; Bocci, V. Ozone and Ozonated Oils in Skin Diseases: A Review. Med. Inflamm. 2010, 6, 10–41. [Google Scholar] [CrossRef] [PubMed]
- Cronheim, G. Organic ozonides as chemotherapeutic agents. II. Antiseptic properties. J. Am. Pharm. Assoc. 1947, 36, 278–281. [Google Scholar] [CrossRef]
- Tara, F.; Zand-Kargar, Z.; Rajabi, O.; Berenji, F.; Akhlaghi, F.; Shakeri, M.T.; Azizi, H. The Effects of Ozonated Olive Oil and Clotrimazole Cream for Treatment of Vulvovaginal Candidiasis. Altern. Ther. Health Med. 2016, 22, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.V.; Kumar, V.; Kumar, S.; Patel, G.D. Therapeutic Effect of Topical Ozonated Oil on Epithelial Healing of Palatal Wound Site: A Planimetrical and Cytological Study. J. Investig. Clin. Dent. 2011, 2, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.V.; Patel, A.; Kumar, S.; Holmes, J.C. Effect of subgingival application of topical ozonated olive oil in the treatment of chronic periodontitis: A randomized, controlled, double blind, clinical and microbiological study. Minerva Stomatol. 2012, 61, 381–398. [Google Scholar] [PubMed]
- Berger, M.B.; Damico, N.J.; Menees, S.B.; Fenner, D.E.; Haefner, H.K. Rates of self-reported urinary, gastrointestinal, and pain comorbidities in women with vulvar lichen sclerosus. J. Low. Genit. Tract Dis. 2012, 16, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Karadag, A.S.; Kavala, M.; Ozlu, E.; Zindanc, İ.; Ozkanl, S.; Turkoglu, Z.; Zemheri, E. The co-occurrence of lichen sclerosus et atrophicus and celiac disease. Indian Dermatol. Online J. 2014, 5, S106–S108. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, L.; Gilliam, A.; Khavari, N.; Bass, D. Association between lichen sclerosus and celiac disease: A report of three pediatric cases. Pediatr. Dermatol. 2014, 31, e128–e131. [Google Scholar] [CrossRef] [PubMed]
- Oyama, N.; Chan, I.; Neill, S.; Hamada, T. Autoantibodies to extracellular matrix protein 1 in lichen sclerosus. Lancet 2003, 362, 118–123. [Google Scholar] [CrossRef]
- Edmonds, E.; Barton, G.; Buisson, S.; Francis, N.; Gotch, F.; Game, L.; Haddad, M.; Dinneen, M.; Bunker, C. Gene expression profiling in male genital lichen sclerosus. Int. J. Exp. Pathol. 2011, 92, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Kantere, D.; Löwhagen, G.B.; Alvengren, G.; Månesköld, A.; Gillstedt, M.; Tunbäck, P. The clinical spectrum of lichen sclerosus in male patients—A retrospective study. Acta Derm. Venereol. 2014, 94, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Pilatz, A.; Altinkilic, B.; Schormann, E.; Maegel, L.; Izykowski, N.; Becker, J.; Weidner, W.; Kreipe, H.; Jonigk, D. Congenital phimosis in patients with and without lichen sclerosus: Distinct expression patterns of tissue remodeling associated genes. J. Urol. 2013, 189, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.; Currò, M.; Barbera, A.; Caccamo, D.; Antonuccio, P.; Arena, S.; Montalto, A.S.; Parisi, S.; Marseglia, L.; Gitto, E.; et al. Expression of transglutaminase in foreskin of children with Balanitis Xerotica Obliterans. Int. J. Mol. Sci. 2016, 17, 1551. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.; Csontai, A.; Pirot, L.; Nyrady, P.; Merksz, M.; Kiraly, L. The response of balanitis xerotica obliterans to local steroid application compared with placebo in children. J. Urol. 2001, 165, 219–220. [Google Scholar] [CrossRef] [PubMed]
- Dahlman-Ghozlan, K.; Hedblad, M.A.; Von Krogh, G. Penile lichen sclerosus et atrophicus treated with Clobetasol dipropionate 0.05% cream: A retrospective clinical and histopathological study. J. Am. Acad. Dermatol. 1999, 40, 451–457. [Google Scholar] [CrossRef]
- Bracco, G.L.; Carli, P.; Sonni, L.; Maestrini, G.; De Marco, A.; Taddei, G.L. Clinical and histologic effects of topical treatments of vulval lichen sclerosus. A critical evaluation. J. Reprod. Med. 1993, 38, 37–40. [Google Scholar] [PubMed]
- Poindexter, G.; Morrell, D.S. Anogenital pruritus: Lichen sclerosus in children. Pediatr. Ann. 2007, 36, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.V.; Kumar, S.; Vidya, G.D.; Patel, A.; Holmes, J.C.; Kumar, V. Cytological Assessment of Healing Palatal Donor Site wound and Grafted Gingival Wound after Application of Ozonated Oil: An Eighteen Months Randomized Controlled Clinical Trial. Acta Cytol. 2012, 56, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Sechi, L.A.; Lezcano, I.; Nunez, N.; Espim, M.; Duprè, I.; Pinna, A.; Molicotti, P.; Fadda, G.; Zanetti, S. Antibacterial activity of ozonized sunflower oil. J. Appl. Microbiol. 2001, 90, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Quan, G.; Choi, J.Y.; Lee, D.S.; Lee, S.C. TGF-β1 upregulates transglutaminase 2 and fibronectin in dermal fibroblasts: A possible mechanism for the stabilization of tissue inflammation. Arch. Dermatol. Res. 2005, 297, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Hashimoto, S.; Lotz, M.; Pritzker, K.; Terkeltaub, R. Interleukin-1 induces pro-mineralizing activity of cartilage tissue transglutaminase and factor XIIIa. Am. J. Pathol. 2001, 159, 149–163. [Google Scholar] [CrossRef]
- Chen, D.; Bassi, J.K.; Walther, T.; Thomas, W.G.; Allen, A.M. Expression of angiotensin type 1A receptors in C1 neurons restores the sympathoexcitation to angiotensin in the rostral ventrolateral medulla of angiotensin type 1A knockout mice. Hypertension 2010, 56, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.D.; Vasievich, M.P.; Ruiz, P.; Gould, S.K.; Parsons, K.K.; Pazmino, A.K.; Facemire, C.; Chen, B.J.; Kim, H.S.; Tran, T.T.; et al. Glomerular type 1 angiotensin receptors augment kidney injury and inflammation in murine autoimmune nephritis. J. Clin. Investig. 2009, 119, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Green, S.J.; Nancy, C.A.; Meltzer, M.S. Cytokine-induced synthesis of nitrogen oxides in macrophages: A protective host response to Leishmania and other intracellular pathogens. J. Leukoc. Biol. 1991, 50, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y. Molecular design and biological activities of NF-κB inhibitors. Front. Biosci. 2006, 11, 3026–3035. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, M.; Brennan, F.M.; Maini, R.N. Human Cytokines: Their Role in Disease and Therapy, Role of Cytokines in Autoimmune Diseases; Blackwell Science: Boston, MA, USA, 1995; pp. 185–194. [Google Scholar]
- Kim, H.S.; Noh, S.U.; Han, Y.W. Therapeutic effects of topical application of ozone on acute cutaneous wound healing. J. Korean Med. Sci. 2009, 24, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Papanas, N.; Maltezos, E. Growth factors in the treatment of diabetic foot ulcers: New technologies, any promises? Int. J. Low. Extrem. Wounds 2007, 6, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Lim, Y.; Zanardi, I.; Bocci, V.; Travagli, V. Evaluation of ozonated sesame oil effect in wound healing using the SKH1 mice as a model. In Proceedings of the 7th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology, Valletta, Malta, 8–11 March 2010. [Google Scholar]
- Sagai, M.; Bocci, V. Mechanisms of Action Involved in Ozone Therapy: Is healing induced via a mild oxidative stress? Med. Gas Res. 2011, 1, 29. [Google Scholar] [CrossRef] [PubMed]
- Amina, A.; Aleksander, Q.; Popel, S. Reactive Oxygen Species Regulate Hypoxia-Inducible Factor 1α Differentially in Cancer and Ischemia. Mol. Cell. Biol. 2008, 28, 5106–5119. [Google Scholar]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, C.; Muthana, M.; Lewis, C.E. Hypoxia regulates macrophage functions in inflammation. J. Immunol. 2005, 175, 6257–6263. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Bamji, S.X.; Rico, B.; Kimes, N.; Reichardt, L.F. BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin-β-catenin interactions. J. Cell Biol. 2006, 174, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; van de Wetering, M.; Clevers, H. The intestinal stem cell. Genes Dev. 2008, 22, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, U.; Funke, R.; Ebnet, K.; Vorschmitt, H.; Koch, S.; Vestweber, D. Association of Csk to VE-cadherin and inhibition of cell proliferation. EMBO J. 2005, 24, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, W.; Hinterdorfer, P.; Ness, W.; Raab, A.; Vestweber, D.; Schindler, H.; Drenckhahn, D. Cadherin interaction probed by atomic force microscopy. Proc. Natl. Acad. Sci. USA 2000, 97, 4005–4010. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, D.; Campisi, A.; Currò, M.; Aguennouz, M.; Li Volti, G.; Avola, R.; Ientile, R. Nuclear factor κb activation is associated with glutamate evoked tissue transglutaminase up-regulation in primary astrocyte cultures. J. Neurosci. Res. 2005, 82, 858–865. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound (OZOILE®) are available from the authors. |





| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| ACT-β | TGGTTACAGGAAGTCCCTTGCC | ATGCTATCACCTCCCCTGTGTG |
| IL1-β | GCTTATTACAGTGGCAATGA | TAGTGGTGGTCGGAGATT |
| TNF-α | GTGAGGAGGACGAACATC | GAGCCAGAAGAGGTTGAG |
| IFN-γ | GCAGCCAACCTAAGCAAGAT | TCACCTGACACATTCAAGTTCTG |
| TG2 | CCTTACGGAGTCCAACCTCA | CCGTCTTCTGCTCCTCAGTC |
| NOS2 | TGACCTCCTAACAAGTAGCA | CAGCAGCAAGTTCCATCT |
| HIF-1α | CGTTCCTTCGATCAGTTGTC | TCAGTGGTGGCAGTGGTAGT |
| VEGF | AGGAGGAGGGCAGAATCATCA | CTCGATTGGATGGCAGTAGCT |
| E-cadherin | TGAGTGTCCCCCGGTATCTTC | CAGTATCAGCCGCTTTCAGATTTT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Currò, M.; Russo, T.; Ferlazzo, N.; Caccamo, D.; Antonuccio, P.; Arena, S.; Parisi, S.; Perrone, P.; Ientile, R.; Romeo, C.; et al. Anti-Inflammatory and Tissue Regenerative Effects of Topical Treatment with Ozonated Olive Oil/Vitamin E Acetate in Balanitis Xerotica Obliterans. Molecules 2018, 23, 645. https://doi.org/10.3390/molecules23030645
Currò M, Russo T, Ferlazzo N, Caccamo D, Antonuccio P, Arena S, Parisi S, Perrone P, Ientile R, Romeo C, et al. Anti-Inflammatory and Tissue Regenerative Effects of Topical Treatment with Ozonated Olive Oil/Vitamin E Acetate in Balanitis Xerotica Obliterans. Molecules. 2018; 23(3):645. https://doi.org/10.3390/molecules23030645
Chicago/Turabian StyleCurrò, Monica, Tiziana Russo, Nadia Ferlazzo, Daniela Caccamo, Pietro Antonuccio, Salvatore Arena, Saveria Parisi, Patrizia Perrone, Riccardo Ientile, Carmelo Romeo, and et al. 2018. "Anti-Inflammatory and Tissue Regenerative Effects of Topical Treatment with Ozonated Olive Oil/Vitamin E Acetate in Balanitis Xerotica Obliterans" Molecules 23, no. 3: 645. https://doi.org/10.3390/molecules23030645