Peptide Nucleic Acids as a Tool for Site-Specific Gene Editing
Abstract
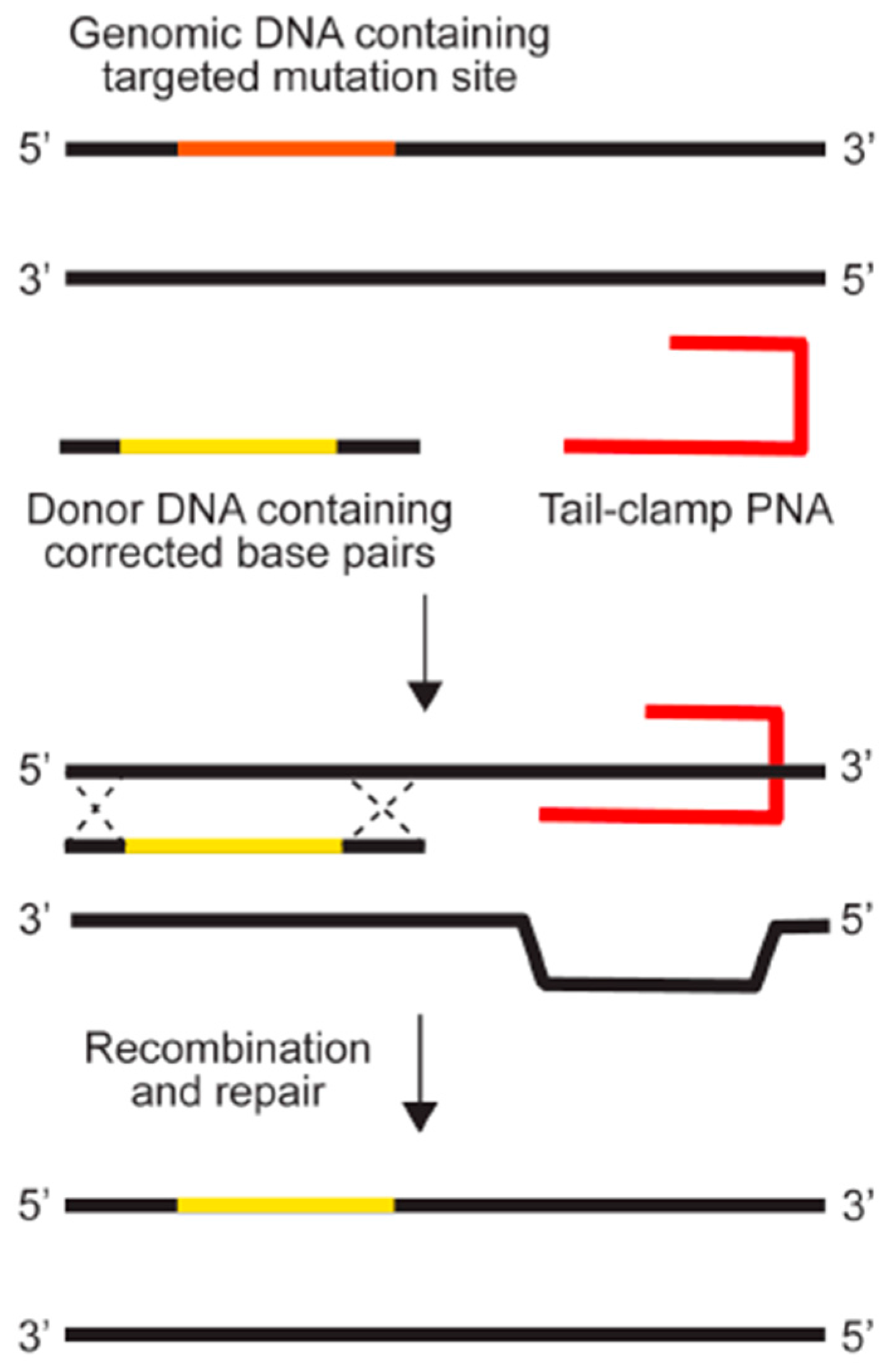
:1. Introduction
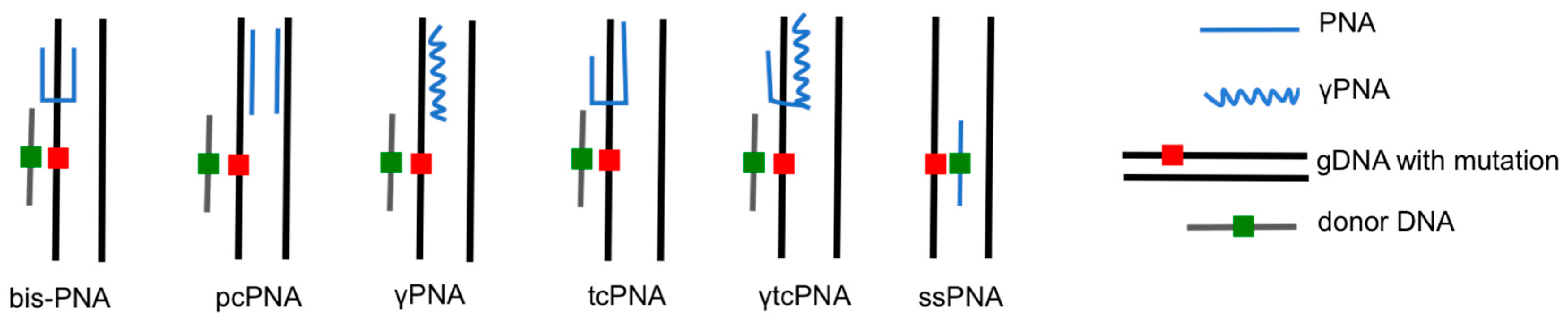
2. In Vitro Gene Editing
3. Nanoparticle-Mediated PNA Delivery
4. In Vivo Gene Editing Using PNA-Nanoparticles
5. Gene Editing without a Donor DNA
6. Off-Target Mutations and Genotoxicity
7. Mechanism of PNA Mediated Gene Editing
8. Measures to Enhance Gene Editing
9. Perspectives and Limits
10. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pauling, L.; Corey, R.B. A proposed structure for the nucleic acids. Proc. Natl. Acad. Sci. USA 1953, 39, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Felsenfeld, G.; Rich, A. Studies on the formation of two- and three-stranded polyribonucleotides. Biochim. Biophys. Acta 1957, 26, 457–468. [Google Scholar] [CrossRef]
- Nielsen, P.E.; Egholm, M.; Berg, R.H.; Buchardt, O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 1991, 254, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Egholm, M.; Buchardt, O.; Christensen, L.; Behrens, C.; Freier, S.M.; Driver, D.A.; Berg, R.H.; Kim, S.K.; Norden, B.; Nielsen, P.E. PNA hybridizes to complementary oligonucleotides obeying the Watson-Crick hydrogen-bonding rules. Nature 1993, 365, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.; Sacui, I.; Rapireddy, S.; Zanotti, K.J.; Bahal, R.; Armitage, B.A.; Ly, D.H. Synthesis and characterization of conformationally preorganized, (R)-diethylene glycol-containing gamma-peptide nucleic acids with superior hybridization properties and water solubility. J. Org. Chem. 2011, 76, 5614–5627. [Google Scholar] [CrossRef] [PubMed]
- Quijano, E.; Bahal, R.; Ricciardi, A.; Saltzman, W.M.; Glazer, P.M. Therapeutic peptide nucleic acids: Principles, limitations, and opportunities. Yale J. Biol. Med. 2017, 90, 583–598. [Google Scholar] [PubMed]
- Zarrilli, F.; Amato, F.; Morgillo, C.M.; Pinto, B.; Santarpia, G.; Borbone, N.; D’Errico, S.; Catalanotti, B.; Piccialli, G.; Castaldo, G.; et al. Peptide nucleic acids as miRNA target. Protectors for the treatment of cystic fibrosis. Molecules 2017, 22, 1144. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Seidman, M.M.; Glazer, P.M. Mutagenesis in mammalian cells induced by triple helix formation and transcription-coupled repair. Science 1996, 271, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Faruqi, A.F.; Datta, H.J.; Carroll, D.; Seidman, M.M.; Glazer, P.M. Triple-helix formation induces recombination in mammalian cells via a nucleotide excision repair-dependent pathway. Mol. Cell. Biol. 2000, 20, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Rogers, F.A.; Vasquez, K.M.; Egholm, M.; Glazer, P.M. Site-directed recombination via bifunctional PNA-DNA conjugates. Proc. Natl. Acad. Sci. USA 2002, 99, 16695–16700. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, A.S.; McNeer, N.A.; Anandalingam, K.K.; Saltzman, W.M.; Glazer, P.M. Targeted genome modification via triple helix formation. Method Mol. Biol. 2014, 1176, 89–106. [Google Scholar]
- Chin, J.Y.; Kuan, J.Y.; Lonkar, P.S.; Krause, D.S.; Seidman, M.M.; Peterson, K.R.; Nielsen, P.E.; Kole, R.; Glazer, P.M. Correction of a splice-site mutation in the beta-globin gene stimulated by triplex-forming peptide nucleic acids. Proc. Natl. Acad. Sci. USA 2008, 105, 13514–13519. [Google Scholar] [CrossRef] [PubMed]
- Peffer, N.J.; Hanvey, J.C.; Bisi, J.E.; Thomson, S.A.; Hassman, C.F.; Noble, S.A.; Babiss, L.E. Strand-invasion of duplex DNA by peptide nucleic acid oligomers. Proc. Natl. Acad. Sci. USA 1993, 90, 10648–10652. [Google Scholar] [CrossRef] [PubMed]
- Egholm, M.; Christensen, L.; Dueholm, K.L.; Buchardt, O.; Coull, J.; Nielsen, P.E. Efficient pH-independent sequence-specific DNA binding by pseudoisocytosine-containing bis-PNA. Nucleic Acid. Res. 1995, 23, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.E.; Egholm, M.; Buchardt, O. Evidence for (PNA)2/DNA triplex structure upon binding of PNA to dsDNA by strand displacement. J. Mol. Recognit. 1994, 7, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Lonkar, P.; Kim, K.; Kuan, J.Y.; Chin, J.Y.; Rogers, F.A.; Knauert, M.P.; Kole, R.; Nielsen, P.E.; Glazer, P.M. Targeted correction of a thalassemia-associated beta-globin mutation induced by pseudo-complementary peptide nucleic acids. Nucleic Acid. Res. 2009, 37, 3635–3644. [Google Scholar] [CrossRef] [PubMed]
- Schleifman, E.B.; Bindra, R.; Leif, J.; del Campo, J.; Rogers, F.A.; Uchil, P.; Kutsch, O.; Shultz, L.D.; Kumar, P.; Greiner, D.L.; et al. Targeted disruption of the CCR5 gene in human hematopoietic stem cells stimulated by peptide nucleic acids. Chem. Biol. 2011, 18, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- McNeer, N.A.; Schleifman, E.B.; Cuthbert, A.; Brehm, M.; Jackson, A.; Cheng, C.; Anandalingam, K.; Kumar, P.; Shultz, L.D.; Greiner, D.L.; et al. Systemic delivery of triplex-forming PNA and donor DNA by nanoparticles mediates site-specific genome editing of human hematopoietic cells in vivo. Gene Ther. 2013, 20, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Schleifman, E.B.; McNeer, N.A.; Jackson, A.; Yamtich, J.; Brehm, M.A.; Shultz, L.D.; Greiner, D.L.; Kumar, P.; Saltzman, W.M.; Glazer, P.M. Site-specific Genome Editing in PBMCs With PLGA Nanoparticle-delivered PNAs Confers HIV-1 Resistance in Humanized. Mice. Mol. Ther. Nucleic Acid 2013, 2, e135. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.J.; Quijano, E.; McNeer, N.A.; Caputo, C.; Bahal, R.; Anandalingam, K.; Egan, M.E.; Glazer, P.M.; Saltzman, W.M. Modified poly(lactic-co-glycolic acid) nanoparticles for enhanced cellular uptake and gene editing in the lung. Adv. Healthc. Mater. 2015, 4, 361–366. [Google Scholar] [CrossRef] [PubMed]
- McNeer, N.A.; Anandalingam, K.; Fields, R.J.; Caputo, C.; Kopic, S.; Gupta, A.; Quijano, E.; Polikoff, L.; Kong, Y.; Bahal, R.; et al. Nanoparticles that deliver triplex-forming peptide nucleic acid molecules correct F508del CFTR in airway epithelium. Nat. Commun. 2015, 6, 6952. [Google Scholar] [CrossRef] [PubMed]
- Bahal, R.; McNeer, N.A.; Quijano, E.; Liu, Y.; Sulkowski, P.; Turchick, A.; Lu, Y.; Bhunia, D.C.; Manna, A.; Greiner, D.L.; et al. In vivo correction of anaemia in beta-thalassemic mice by gammaPNA-mediated gene editing with nanoparticle delivery. Nat. Commun. 2016, 7, 13304. [Google Scholar] [CrossRef] [PubMed]
- McNeer, N.A.; Chin, J.Y.; Schleifman, E.B.; Fields, R.J.; Glazer, P.M.; Saltzman, W.M. Nanoparticles deliver triplex-forming PNAs for site-specific genomic recombination in CD34+ human hematopoietic progenitors. Mol. Ther. 2011, 19, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.R.; Keown, W.; Lowe, L.; Kirschling, D.; Kucherlapati, R. Homologous recombination involving small single-stranded oligonucleotides in human cells. New Biol. 1989, 1, 223–227. [Google Scholar] [PubMed]
- Goncz, K.K.; Kunzelmann, K.; Xu, Z.; Gruenert, D.C. Targeted replacement of normal and mutant CFTR sequences in human airway epithelial cells using DNA fragments. Hum. Mol. Genet. 1998, 7, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, T.; Nielsen, P.E. Enhanced delivery of cell-penetrating peptide-peptide nucleic acid conjugates by endosomal disruption. Nat. Protoc. 2006, 1, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Abes, S.; Williams, D.; Prevot, P.; Thierry, A.; Gait, M.J.; Lebleu, B. Endosome trapping limits the efficiency of splicing correction by PNA-oligolysine conjugates. J. Controll. Release 2006, 110, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Lohse, J.; Dahl, O.; Nielsen, P.E. Double duplex invasion by peptide nucleic acid: A general principle for sequence-specific targeting of double-stranded DNA. Proc. Natl. Acad. Sci. USA 1999, 96, 11804–11808. [Google Scholar] [CrossRef] [PubMed]
- Hutter, G.; Nowak, D.; Mossner, M.; Ganepola, S.; Müssig, A.; Allers, K.; Schneider, T.; Hofmann, J.; Kücherer, C.; Blau, O. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 2009, 360, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Allers, K.; Hütter, G.; Hofmann, J.; Loddenkemper, C.; Rieger, K.; Thiel, E.; Schneider, T. Evidence for the cure of HIV infection by CCR5Delta32/Delta32 stem cell transplantation. Blood 2011, 117, 2791–2799. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.; Rosenberg, P.S.; Goedert, J.J.; Ashton, L.J.; Benfield, T.L.; Buchbinder, S.P.; Coutinho, R.A.; Eugen-Olsen, J.; Gallart, T.; Katzenstein, T.L.; et al. Effects of CCR5-Delta32, CCR2-64I, and SDF-1 3′A alleles on HIV-1 disease progression: An international meta-analysis of individual-patient data. Ann. Intern. Med. 2001, 135, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.Y.; Reza, F.; Glazer, P.M. Triplex-forming peptide nucleic acids induce heritable elevations in gamma-globin expression in hematopoietic progenitor cells. Mol. Ther. 2013, 21, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Musallam, K.M.; Sankaran, V.G.; Cappellini, M.D.; Duca, L.; Nathan, D.G.; Taher, A.T. Fetal hemoglobin levels and morbidity in untransfused patients with beta-thalassemia intermedia. Blood 2012, 119, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Platt, O.S.; Brambilla, D.J.; Rosse, W.F.; Milner, P.F.; Castro, O.; Steinberg, M.H.; Klug, P.P. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N. Engl. J. Med. 1994, 330, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Bahal, R.; Quijano, E.; McNeer, N.A.; Liu, Y.; Bhunia, D.C.; Lopez-Giraldez, F.; Fields, R.J.; Saltzman, W.M.; Ly, D.H.; Glazer, P.M. Single-stranded gammaPNAs for in vivo site-specific genome editing via Watson-Crick recognition. Curr. Gene Ther. 2014, 14, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Hanvey, J.C.; Peffer, N.J.; Bisi, J.E.; Thomson, S.A.; Cadilla, R.; Josey, J.A.; Ricca, D.J.; Hassman, C.F.; Bonham, M.A.; Au, K.G.; et al. Antisense and antigene properties of peptide nucleic acids. Science 1992, 258, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- Rogers, F.A.; Lin, S.S.; Hegan, D.C.; Krause, D.S.; Glazer, P.M. Targeted gene modification of hematopoietic progenitor cells in mice following systemic administration of a PNA-peptide conjugate. Mol. Ther. 2012, 20, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Koppelhus, U.; Nielsen, P.E. Cellular delivery of peptide nucleic acid (PNA). Adv. Drug Deliv. Rev. 2003, 55, 267–280. [Google Scholar] [CrossRef]
- Koppelhus, U.; Shiraishi, T.; Zachar, V.; Pankratova, S.; Nielsen, P.E. Improved cellular activity of antisense peptide nucleic acids by conjugation to a cationic peptide-lipid (CatLip) domain. Bioconjug. Chem. 2008, 19, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Fang, H.; Song, Y.; Bielska, A.A.; Wang, Z.; Taylor, J.S. Phospholipid conjugate for intracellular delivery of peptide nucleic acids. Bioconjug. Chem. 2009, 20, 1729–1736. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, G.D.; Arzumanov, A.; Abes, R.; Yin, H.; Wood, M.J.; Lebleu, B.; Gait, M.J. Improved cell-penetrating peptide-PNA conjugates for splicing redirection in HeLa cells and exon skipping in mdx mouse muscle. Nucleic Acid Res. 2008, 36, 6418–6428. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.J.; Bahal, R.; Babar, I.A.; Pincus, Z.; Barrera, F.; Liu, C.; Svoronos, A.; Braddock, D.T.; Glazer, P.M.; Engelman, D.M.; et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature 2015, 518, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Papapetrou, E.P.; Zoumbos, N.C.; Athanassiadou, A. Genetic modification of hematopoietic stem cells with nonviral systems: Past progress and future prospects. Gene Ther. 2005, 12 (Suppl. 1), S118–S130. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Temsamani, J.; Tang, J.Y. Pharmacokinetics, biodistribution, and stability of oligodeoxynucleotide phosphorothioates in mice. Proc. Natl. Acad. Sci. USA 1991, 88, 7595–7599. [Google Scholar] [CrossRef] [PubMed]
- Dragulescu-Andrasi, A.; Rapireddy, S.; Frezza, B.M.; Gayathri, C.; Gil, R.R.; Ly, D.H. A simple gamma-backbone modification preorganizes peptide nucleic acid into a helical structure. J. Am. Chem. Soc. 2006, 128, 10258–10267. [Google Scholar] [CrossRef] [PubMed]
- Bahal, R.; Sahu, B.; Rapireddy, S.; Lee, C.M.; Ly, D.H. Sequence-unrestricted, Watson-Crick recognition of double helical B-DNA by (R)-miniPEG-gammaPNAs. ChemBioChem 2012, 13, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Kamat, C.D.; Shmueli, R.B.; Connis, N.; Rudin, C.M.; Green, J.J.; Hann, C.L. Poly(beta-amino ester) nanoparticle delivery of TP53 has activity against small cell lung cancer in vitro and in vivo. Mol. Cancer Ther. 2013, 12, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Green, J.J.; Langer, R.; Anderson, D.G. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc. Chem. Res. 2008, 41, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.J.; Cheng, C.J.; Quijano, E.; Weller, C.; Kristofik, N.; Duong, N.; Hoimes, C.; Egan, M.E.; Saltzman, W.M. Surface modified poly(beta amino ester)-containing nanoparticles for plasmid DNA delivery. J. Contro. Release 2012, 164, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Trabulo, S.; Cardoso, A.L.; Mano, M.; de Lima, M.C.P. Cell-Penetrating Peptides-Mechanisms of Cellular Uptake and Generation of Delivery Systems. Pharmaceuticals 2010, 3, 961–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, M.C.; Vidal, P.; Chaloin, L.; Heitz, F.; Divita, G. A new peptide vector for efficient delivery of oligonucleotides into mammalian cells. Nucleic Acid Res. 1997, 25, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.G.; Olsen, J.C.; Sarkadi, B.; Moore, K.L.; Swanstrom, R.; Boucher, R.C. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat. Genet. 1992, 2, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Kayali, R.; Bury, F.; Ballard, M.; Bertoni, C. Site-directed gene repair of the dystrophin gene mediated by PNA-ssODNs. Hum. Mol. Genet. 2010, 19, 3266–3281. [Google Scholar] [CrossRef] [PubMed]
- Nik-Ahd, F.; Bertoni, C. Ex vivo gene editing of the dystrophin gene in muscle stem cells mediated by peptide nucleic acid single stranded oligodeoxynucleotides induces stable expression of dystrophin in a mouse model for Duchenne muscular dystrophy. Stem Cells 2014, 32, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.E.; Egholm, M.; Berg, R.H.; Buchardt, O. Sequence specific inhibition of DNA restriction enzyme cleavage by PNA. Nucleic Acid Res. 1993, 21, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Knauert, M.P.; Lloyd, J.A.; Rogers, F.A.; Datta, H.J.; Bennett, M.L.; Weeks, D.L.; Glazer, P.M. Distance and affinity dependence of triplex-induced recombination. Biochemistry 2005, 44, 3856–3864. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.L.; Yang, L.X. Gamma-H2AX—A novel biomarker for DNA double-strand breaks. In Vivo 2008, 22, 305–309. [Google Scholar] [PubMed]
- Petit, C.; Sancar, A. Nucleotide excision repair: From E. coli to man. Biochimie 1999, 81, 15–25. [Google Scholar] [CrossRef]
- Batty, P.D.; Wood, R.D. Damage recognition in nucleotide excision repair of DNA. Gene 2000, 241, 193–204. [Google Scholar] [CrossRef]

| Drug Delivery System | Reagent | Study Design (Model, Target Gene) | Efficiency (Assay) | Year, Ref. |
|---|---|---|---|---|
| N/A | bis-PNA and donor DNA | human cell free extract (pSupFG1/G144C) | 0.08% (β-galactosidase assay) | 2002, [10] |
| electroporation or nucleofection | bis-PNA and donor DNA with chloroquine | cell culture (Chinese hamster ovary cells containing a human β-globin splice-site mutation, CHO-GFP/IVS2–1G→A and human CD34+ progenitor cells) | 0.4%—CHO cells (FACS) | 2008, [12] |
| electroporation | pcPNA and donor DNA with SAHA | cell culture (Chinese hamster ovary cells containing a human β-globin splice-site mutation, CHO-GFP/IVS2–1G→A) | 0.78% (FACS) | 2009, [16] |
| PLGA NPs | bis-PNA and donor DNA | cell culture (human CD34+ cells, β-globin) | 0.91% (limiting dilution, allele specific PCR) | 2011, [23] |
| electroporation | tcPNA and donor DNA | cell culture (THP-1 and human CD34+ cells, CCR5) | 2.8% (limiting dilution, allele specific PCR) | 2011, [17] |
| electroporation | bis-PNA and donor DNA | cell culture (human CD34+ cells, γ-globin) | 1.63% (allele specific qPCR) | 2013, [32] |
| PLGA NPs | tcPNA and donor DNA or bis-PNA and donor DNA | in vivo (humanized NOD-scid IL2rγnull mice, CCR5 and β-globin) | 0.43% (deep sequencing) | 2013, [18] |
| PLGA NPs | tcPNA and donor DNA | ex vivo (human PBMCs engrafted into NOD-scid IL2rγnull mice, CCR5) | 0.97% (deep sequencing) | 2013, [19] |
| PLGA NPs and PLGA/PBAE NPs, IV delivery | γPNA and donor DNA | in vivo (β-globin/eGFP transgenic mouse) | 0.1% (deep sequencing) | 2014, [35] |
| PLGA/PBAE/MPG NPs, intranasal delivery | tcPNA and donor DNA | in vivo (β-globin/eGFP transgenic mouse, intranasal delivery) | 0.4% (FACS) | 2015, [20] |
| PLGA/PBAE/MPG NPs, intranasal delivery | tcPNA and donor DNA | in vivo (F508del mice, CFTR, intranasal delivery) | 5.7% (deep sequencing) | 2015, [21] |
| PLGA NPs, IV delivery | γtcPNA and donor DNA with SCF | in vivo (IVS2-654 thalassemic mice, β-globin) | 3.4% (deep sequencing) | 2016, [22] |
| Gene | Reagents | Source of gDNA | On-Target Modification Frequency | Off-Target Modification Frequency | Reference |
|---|---|---|---|---|---|
| CCR5 | tcPNA/DNA | THP-1 cells | 2.8% | <0.057% | [17] |
| CCR5 | tcPNA/DNA PLGA NPs | human PBMCs | 0.97% | 0.004% | [19] |
| CCR5 | tcPNA/DNA PLGA NPs | humanized NOD-scid IL2rγnull mouse bone marrow | 0.43% | 0.004% | [18] |
| CFTR | tcPNA/DNA PBAE/PLGA/MPG NPs | CFBE cells | 9.2% | <0.00001% | [21] |
| CFTR | tcPNA/DNA PBAE/PLGA/MPG NPs | mouse nasal epithelium | 5.7% | <0.0001% | [21] |
| β-globin | γtcPNA/DNA PLGA NPs with SCF | total mouse bone marrow cells | 3.9% | 0.0032% | [22] |
| β-globin | γtcPNA/DNA PLGA NPs with SCF | human CD34+ cells | 5.02% | 0.000012% | [22] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricciardi, A.S.; Quijano, E.; Putman, R.; Saltzman, W.M.; Glazer, P.M. Peptide Nucleic Acids as a Tool for Site-Specific Gene Editing. Molecules 2018, 23, 632. https://doi.org/10.3390/molecules23030632
Ricciardi AS, Quijano E, Putman R, Saltzman WM, Glazer PM. Peptide Nucleic Acids as a Tool for Site-Specific Gene Editing. Molecules. 2018; 23(3):632. https://doi.org/10.3390/molecules23030632
Chicago/Turabian StyleRicciardi, Adele S., Elias Quijano, Rachael Putman, W. Mark Saltzman, and Peter M. Glazer. 2018. "Peptide Nucleic Acids as a Tool for Site-Specific Gene Editing" Molecules 23, no. 3: 632. https://doi.org/10.3390/molecules23030632






