Selenides and Diselenides: A Review of Their Anticancer and Chemopreventive Activity
Abstract
:1. Introduction
2. Selenides and Diselenides with Therapeutic Perspective
2.1. Naturally Occurring Selenides and Diselenides
2.2. Synthetic Diselenides
2.2.1. Antioxidant Activities of Synthetic Diselenides: GPx-Like, Metal-Binding, Chemopreventive and Free Radical Scavenging Potential
2.2.2. Prooxidant Activity and Redox Modulation Activity of Synthetic Diselenides
2.2.3. Kinase Modulation Activity of Synthetic Diselenides
2.2.4. Antiproliferative and Cytotoxic Activity of Synthetic Diselenides
2.2.5. Apoptotic Activity of Synthetic Diselenides
2.3. Synthetic Selenides
2.3.1. Redox-Modulating and Antioxidant and Chemo-Preventive Activities of Synthetic Selenides
2.3.2. Antitumoral Activity of Synthetic Selenides
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NNK | 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone |
| GSH | Reduced glutathione |
| GPx-like | Glutathione peroxidase-like |
| ROS | Reactive oxygen species |
| LDL | Low density lipoproteins |
| NF-κB | Nuclear factor-κB DNA Binding |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide |
| Trx | Thioredoxin |
| TrxR | Thioredoxin reductase |
| PDI | Disulfide isomerase |
| 2-NP | 2-Nitropropane |
| MT | Metallothionein |
| CDKs | Cyclin kinases |
| IGF-1R | Insulin-like growth factor 1 receptor |
| HDAC | Histone deacetylase |
| SAHA | Suberoylanilide hydroxamic acid |
| DPPH | 1,1-Diphenyl-2-picryl-hydrazyl |
| ABTS | 2,2-Azinobis(3-ethylbenzothiazoline- 6-sulfonic acid) |
| DMBA | 7, 12-dimethylbenz(a)anthracene |
| HPLC | High-performance liquid chromatography |
| NMR | Nuclear magnetic resonance |
| PEG | Polyethylene glycol |
| SOD2 | superoxide dismutase 2 |
| PB | peripheral blood |
| PBMC | peripheral blood mononuclear cells |
References
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. Selenoproteins. J. Biol. Chem. 2009, 284, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.H.; Hoffmann, P.R. Selenoproteins and cardiovascular stress. Throm. Haemost. 2015, 113, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, C.; Plano, D.; Sharma, A.K.; Palop, J.A. Selenium compounds, apoptosis and other types of cell death: An overview for cancer therapy. Int. J. Mol. Sci. 2012, 13, 9649–9672. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biophys. Acta 2014, 1850, 1642–1660. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, C.; Plano, D.; Font, M.; Palop, J.A. Selenium and clinical trials: New therapeutic evidence for multiple diseases. Curr. Med. Chem. 2011, 18, 4635–4650. [Google Scholar] [CrossRef] [PubMed]
- Mugesh, G.; du Mont, W.W.; Sies, H. Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 2001, 101, 2125–2179. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, B.; Koketsu, M. Recent developments in the synthesis of biologically relevant selenium-containing scaffolds. Coord. Chem. Rev. 2017, 339, 104–127. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B. Organoselenium and organotellurium compounds: Toxicology and pharmacology. Chem. Rev. 2004, 104, 6255–6285. [Google Scholar] [CrossRef] [PubMed]
- Alberto, E.E.; Nascimiento, V.; Braga, A.L. Catalytic application of selenium and tellurium compounds as glutathione peroxidase enzyme mimetics. J. Braz. Chem. Soc. 2010, 21, 2032–2041. [Google Scholar] [CrossRef]
- Brozmanová, J.; Mániková, D.; Vlčková, V.; Chovanec, M. Selenium: A double-edged sword for defense and offence in cancer. Arch. Toxicol. 2010, 84, 919–938. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Boylan, M.; Selvam, A.; Spallholz, J.E.; Björnstedt, M. Redox-active selenium compounds—From toxicity and cell death to cancer treatment. Nutrients 2016, 7, 3536. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Álvarez, E.; Plano, D.; Font, M.; Calvo, A.; Prior, C.; Jacob, C.; Palop, J.A.; Sanmartín, C. Synthesis and antiproliferative activity of novel selenoester derivatives. Eur. J. Med. Chem. 2014, 73, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, C.; Plano, D.; Domínguez, E.; Font, M.; Calvo, A.; Prior, C.; Encío, I.; Palop, J.A. Synthesis and pharmacological screening of several aroyl and heteroaroyl selenylacetic acid derivatives as cytotoxic and antiproliferative agents. Molecules 2009, 14, 3313–3338. [Google Scholar] [CrossRef] [PubMed]
- Orian, L.; Toppo, S. Organochalcogen peroxidase mimetics as potential drugs: A long story of a promise still unfulfilled. Free Radic. Biol. Med. 2014, 66, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Ronai, Z.; Tillotson, J.K.; Traganos, F.; Darzynkiewicz, Z.; Conaway, C.C.; Upadhyaya, P.; El-Bayoumy, K. Effects of organic and inorganic selenium compounds on rat mammary tumour cells. Int. J. Cancer 1995, 63, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Lipinski, B.; Błażejak, S. Application of Sodium Selenite in the Prevention and Treatment of Cancers. Cells 2017, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, B. Sodium selenite as an anticancer agent. Anticancer Agents Med. Chem. 2017, 17, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.O.; Khairuddin, P.F.; Jameson, M.B. Optimising Selenium for Modulation of Cancer Treatments. Anticancer Res. 2017, 37, 6497–6509. [Google Scholar] [PubMed]
- Jamier, V.; Ba, L.A.; Jacob, C. Selenium- and tellurium-containing multifunctional redox agents as biochemical redox modulators with selective cytotoxicity. Chemistry 2010, 16, 10920–10928. [Google Scholar] [CrossRef] [PubMed]
- Storkey, C.; Davies, M.J.; White, J.M.; Schiesser, C.H. Synthesis and antioxidant capacity of 5-selenopyranose derivatives. Chem. Commun. 2011, 47, 9693–9695. [Google Scholar] [CrossRef] [PubMed]
- Mániková, D.; Vlasáková, D.; Loduhová, J.; Letavayová, L.; Vigasová, D.; Krascsenitsová, E.; Vlcková, V.; Brozmanová, J.; Chovanec, M. Investigations on the role of base excision repair and non-homologous end-joining pathways in sodium selenite-induced toxicity and mutagenicity in Saccharomyces cerevisiae. Mutagenesis 2010, 25, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; An, B.; Lou, L.; Zhang, J.; Yan, J.; Huang, L.; Li, X.; Yin, S. Design, Synthesis, and Biological Evaluation of Novel Selenium-Containing Isocombretastatins and Phenstatins as Antitumor Agents. J. Med. Chem. 2017, 60, 7300–7314. [Google Scholar] [CrossRef] [PubMed]
- Domracheva, I.; Kanepe-Lapsa, I.; Jackevica, L.; Vasiljeva, J.; Arsenyan, P. Selenopheno quinolinones and coumarins promote cancer cell apoptosis by ROS depletion and caspase-7 activation. factor 2. Life Sci. 2017, 186, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Álvarez, E.; Gajdács, M.; Spengler, G.; Palop, J.A.; Marć, M.A.; Kieć-Kononowicz, K.; Amaral, L.; Molnár, J.; Jacob, C.; Handzlik, J.; et al. Identification of selenocompounds with promising properties to reverse cancer multidrug resistance. Bioorg. Med. Chem. Lett. 2016, 26, 2821–2824. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guo, M.; Liu, Y.; Mu, W.; Deng, G.; Li, C.; Qiu, C. Selenium induces an anti-tumor effect via inhibiting intratumoral angiogenesis in a mouse model of transplanted canine mammary tumor cells. Biol. Trace Elem. Res. 2016, 171, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Spengler, G.; Sanmartín, C.; Marć, M.A.; Handzlik, J.; Domínguez-Álvarez, E. Selenoesters and selenoanhydrides as novel multidrug resistance reversing agents: A confirmation study in a colon cancer MDR cell line. Bioorg. Med. Chem. Lett. 2017, 27, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Sakalli Çetin, E.; Nazıroğlu, M.; Çiğ, B.; Övey, İ.S.; Aslan Koşar, P. Selenium potentiates the anticancer effect of cisplatin against oxidative stress and calcium ion signaling-induced intracellular toxicity in MCF-7 breast cancer cells: Involvement of the TRPV1 channel. J. Recept. Signal. Transduct. Res. 2017, 37, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Emmert, S.W.; El-Bayoumy, K.; Das, A.; Sun, Y.W.; Amin, S.; Desai, D.; Aliaga, C.; Richie, J.P., Jr. Induction of lung glutathione and glutamylcysteine ligase by 1,4-phenylenebis(methylene)selenocyanate and its glutathione conjugate: Role of nuclear factor-erythroid 2-related factor 2. Free Radic. Biol. Med. 2012, 52, 2064–2071. [Google Scholar] [CrossRef] [PubMed]
- Alcolea, V.; Plano, D.; Karelia, D.N.; Palop, J.A.; Amin, S.; Sanmartín, C.; Sharma, A.K. Novel seleno- and thio-urea derivatives with potent in vitro activities against several cancer cell lines. Eur. J. Med. Chem. 2016, 113, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Argelich, N.; Encío, I.; Plano, D.; Fernandes, A.P.; Palop, J.; Sanmartín, C.; Sanmartín, C. Novel Methylselenoesters as Antiproliferative Agents. Molecules 2017, 22, 1288. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Feng, Q.; Kumagai, T.; Torikai, K.; Ohigashi, H.; Osawa, T.; Noguchi, N.; Niki, E.; Uchida, K. Ebselen, a glutathione peroxidase mimetic seleno-organic compound, as a multifunctional antioxidant. Implication for inflammation-associated carcinogenesis. J. Biol. Chem. 2002, 277, 2687–2694. [Google Scholar] [CrossRef] [PubMed]
- Estevam, E.C.; Griffin, S.; Nasim, M.J.; Denezhkin, P.; Schneider, R.; Lilischkis, R.; Dominguez-Alvarez, E.; Witek, K.; Latacz, G.; Keck, C.; et al. Natural selenium particles from Staphylococcus carnosus: Hazards or particles with particular promise? J. Hazard. Mater. 2017, 324, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, C.; Font, M.; Palop, J.A. Molecular symmetry: A structural property frequently present in new cytotoxic and proapoptotic drugs. Mini Rev. Med. Chem. 2006, 6, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, C.; Plano, D.; Palop, J.A. Selenium compounds and apoptotic modulation: A new perspective in cancer therapy. Mini Rev. Med. Chem. 2008, 8, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xie, Y.; El-Sayed, W.M.; Szakacs, J.G.; Franklin, M.R.; Roberts, J.C. Chemopreventive activity of selenocysteine prodrugs against tobacco-derived nitrosamine (NNK) induced lung tumors in the A/J mouse. J. Biochem. Mol. Toxicol. 2005, 19, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.F.; Wong, Y.S. Selenocystine induces S-phase arrest and apoptosis in human breast adenocarcinoma MCF-7 cells by modulating ERK and Akt phosphorylation. J. Agric. Food Chem. 2008, 56, 10574–10581. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-S.; Yun, B.Y.; Kim, I.Y. Induction of the mitochondrial permeability transition by selenium compounds mediated by oxidation of the protein thiol groups and generation of the superoxide. Biochem. Pharmacol. 2003, 66, 2301–2311. [Google Scholar] [CrossRef] [PubMed]
- Li, G.X.; Hu, H.; Jiang, C.; Schuster, T.; Lü, J. Differential involvement of reactive oxygen species in apoptosis induced by two classes of selenium compounds in human prostate cancer cells. Int. J. Cancer 2007, 120, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Umemoto, D.; Matsunaga, A.; Sato, T.; Chikuma, M. Antioxidant activities of synthesized selenocompounds without selenol groups. Biomed. Res. Trace Elem. 2006, 17, 423–426. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, H.B. Thiol peroxidase-like activity of some intramolecularly coordinated diorganyl diselenides. J. Chem. Sci. 2005, 117, 621–628. [Google Scholar] [CrossRef]
- Meotti, F.C.; Stangherlin, E.C.; Zeni, G.; Nogueira, C.W.; Rocha, J.B. Protective role of aryl and alkyl diselenides on lipid peroxidation. Environ. Res. 2004, 94, 276–282. [Google Scholar] [CrossRef]
- Rosa, R.M.; Moura, D.J.; Romano e Silva, A.C.; Saffi, J.; Pegas-Henriques, J.A. Antioxidant activity of diphenyl diselenide prevents the genotoxicity of several mutagens in Chinese hamster V79 cells. Mutat. Res. 2007, 631, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Brandão, R.; Acker, C.I.; Leite, M.R.; Barbosa, N.B.; Nogueira, C.W. Diphenyl diselenide protects against glycerol-induced renal damage in rats. J. Appl. Toxicol. 2009, 29, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Straliotto, M.R.; Hort, M.A.; Fiuza, B.; Rocha, J.B.; Farina, M.; Chiabrando, G.; de Bem, A.F. Diphenyl diselenide modulates oxLDL-induced cytotoxicity in macrophage by improving the redox signaling. Biochimie 2013, 95, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.T.; de Oliveira, I.M.; Grivicich, I.; Guecheva, T.N.; Saffi, J.; Henriques, J.A.; Rosa, R.M. Diphenyl diselenide protects cultured MCF-7 cells against tamoxifen-induced oxidative DNA damage. Biomed. Pharmacother. 2013, 67, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Machado, M.; Villela, I.V.; Moura, D.J.; Rosa, R.M.; Salvador, M.; Lopes, N.P.; Braga, A.L.; Roesler, R.; Saffi, J.; Henriques, J.A. 3,3-Ditrifluoromethyldiphenyl diselenide: A new organoselenium compound with interesting antigenotoxic and antimutagenic activities. Mutat. Res. 2009, 673, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Parkin, K.L. Induction of phase II enzyme activity by various selenium compounds. Nutr. Cancer 2006, 55, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Reich, H.J.; Hondal, R.J. Why Nature Chose Selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Reddy, B.S.; el-Bayoumy, K. Inhibition by dietary organoselenium, p-methoxybenzene-selenol, of hepatocarcinogenesis induced by azoxymethane in rats. Jpn. J. Cancer Res. 1985, 76, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.S.; Tanaka, T.; el-Bayoumy, K. Inhibitory effect of dietary p-methoxybenzeneselenol on azoxymethane-induced colon and kidney carcinogenesis in female F344 rats. J. Natl. Cancer Inst. 1985, 74, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, A.; Mishra, B.; Barik, A.; Kumbhare, L.B.; Pandey, R.; Jain, V.K.; Priyadarsini, K.I. 3,3’-Diselenodipropionic acid, an efficient peroxyl radical scavenger and GPx mimic, protects erythrocytes (RBCs) from AAPH-induced hemolysis. Chem. Res. Toxicol. 2007, 20, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, A.; Bansal, P.; Kumar, S.J.; Bag, P.P.; Paul, P.; Reddy, N.D.; Kumbhare, L.B.; Jain, V.K.; Chaubey, R.C.; Unnikrishnan, M.K.; et al. In vivo radioprotection studies of 3,3′-diselenodipropionic acid, a selenocystine derivative. Free Radic. Biol. Med. 2010, 48, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Prigol, M.; Hassan, W.; Nogueira, C.W.; Rocha, J.B. Protective effect of binaphthyl diselenide, a synthetic organoselenium compound, on 2-nitropropane-induced hepatotoxicity in rats. Cell Biochem. Funct. 2010, 28, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.A.; Fry, F.H.; Holme, A.L.; Yiakouvaki, A.; Al-Qenaei, A.; Pourzand, C.; Jacob, C. Towards multifunctional antioxidants: Synthesis, electrochemistry, in vitro and cell culture evaluation of compounds with ligand/catalytic properties. Org. Biomol. Chem. 2005, 3, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Battin, E.E.; Perron, N.E.; Brumaghim, J.L. The central role of metal coordination in selenium antioxidant activity. Inorg. Chem. 2006, 45, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Mugesh, G.; Panda, A.; Singh, H.B.; Punekar, N.S.; Butcher, R.J. Glutathione peroxidase-like antioxidant activity of diaryl diselenides: A Mechanistic Study. J. Am. Chem. Soc. 2001, 123, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Mugesh, G.; Panda, A.; Singh, H.B.; Punekar, N.S.; Butcher, R.J. Diferrocenyl diselenides: Excellent thiol peroxidase-like antioxidants. Chem. Commun. 1998, 2227–2228. [Google Scholar] [CrossRef]
- Bhabak, K.P.; Mugesh, G. A simple and efficient strategy to enhance the antioxidant activities of amino-substituted glutathione peroxidase mimics. Chem. Eur. J. 2008, 14, 8640–8651. [Google Scholar] [CrossRef] [PubMed]
- Bhabak, K.P.; Mugesh, G. Synthesis and structure–activity correlation studies of secondary-and tertiary-amine-based glutathione peroxidase mimics. Chem. Eur. J. 2009, 15, 9846–9854. [Google Scholar] [CrossRef] [PubMed]
- Bailly, F.; Azaroual, N.; Bernier, J.L. Design, synthesis and glutathione peroxidase-like properties of ovothiol-derived diselenides. Bioorg. Med. Chem. 2003, 11, 4623–4630. [Google Scholar] [CrossRef]
- Romano, B.; Plano, D.; Encio, I.; Palop, J.A.; Sanmartín, C. In vitro radical scavenging and cytotoxic activities of novel hybrid selenocarbamates. Bioorg. Med. Chem. 2015, 23, 1716–1727. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Aguilar, A.; Romero-Hernández, L.L.; Arenas-González, A.; Merino-Montiel, P.; Montiel-Smith, S.; Meza-Reyes, S.; Vega-Báez, J.L.; Plata, G.B.; Padrón, J.M.; López, Ó.; et al. New selenosteroids as antiproliferative agents. Org. Biomol. Chem. 2017, 15, 5041–5054. [Google Scholar] [CrossRef] [PubMed]
- Press, D.J.; Back, T.G. Enhanced glutathione peroxidase activity of conformationally restricted naphthalene peri-dichalcogenides. Org. Lett. 2011, 13, 4104–4107. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.M.; Picada, J.D.; Saffi, J.; Henriques, J.A.P. Cytotoxic, genotoxic and mutagenic effects of diphenyl diselenide in Chinese hamster lung fibroblasts. Mutat. Res. Genet. Toxicol. Environ. Mutagen 2007, 628, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Meotti, F.C.; Curte, E.; Pilissão, C.; Zeni, G.; Rocha, J.B. Investigations into the potential neurotoxicity induced by diselenides in mice and rats. Toxicology 2003, 183, 29–37. [Google Scholar] [CrossRef]
- Olm, E.; Fernandes, A.P.; Hebert, C.; Rundlöf, A.K.; Larsen, E.H.; Danielsson, O.; Björnstedt, M. Extracellular thiol-assisted selenium uptake dependent on the x(c)- cystine transporter explains the cancer-specific cytotoxicity of selenite. Proc. Natl. Acad. Sci. USA 2009, 106, 11400–11405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Nitteranon, V.; Guo, S.; Qiu, P.; Wu, X.; Li, F.; Xiao, H.; Hu, Q.; Parkin, K.L. Organoselenium compounds modulate extracellular redox by induction of extracellular cysteine and cell surface thioredoxin reductase. Chem. Res. Toxicol. 2013, 26, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, C.; Plano, D.; Font, M.; Palop, J.A. Kinase regulation by sulfur and selenium containing compounds. Curr. Cancer Drug Targets 2011, 11, 496–523. [Google Scholar] [CrossRef] [PubMed]
- Plano, D.; Ibáñez, E.; Calvo, A.; Palop, J.A.; Sanmartín, C. Novel library of selenocompounds as kinase modulators. Molecules 2011, 16, 6349–6364. [Google Scholar] [CrossRef] [PubMed]
- Yanochko, G.M.; Eckhart, W. Type I insulin-like growth factor receptor over-expression induces proliferation and anti-apoptotic signaling in a three-dimensional culture model of breast epithelial cells. Breast Cancer Res. 2006, 8, R18. [Google Scholar] [CrossRef] [PubMed]
- Gowda, R.; Madhunapantula, S.V.; Desai, D.; Amin, S.; Robertson, G.F. Selenium-containing histone deacetylase inhibitors for melanoma management. Cancer Biol. Ther. 2012, 13, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Font, M.; Plano, D.; Sanmartín, C.; Palop, J.A. Topological and quantum molecular descriptors as effective tools for analyzing cytotoxic activity achieved by a series of (diselanediyldibenzene-4,1 diylnide)biscarbamate derivatives. J. Mol. Graph. Model. 2017, 73, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, J.; Park, M.S. Synthesis of new diorganodiselenides from organic halides: Their antiproliferative effects against human breast cancer MCF-7 cells. Arch. Pharm. Res. 2015, 38, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Weekley, C.M.; Harris, H.H. Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem. Soc. Rev. 2013, 42, 8870–8894. [Google Scholar] [CrossRef] [PubMed]
- Nedel, F.; Campos, V.F.; Alves, D.; McBride, A.J.; Dellagostin, O.A.; Collares, T.; Savegnago, L.; Seixas, F.K. Substituted diaryl diselenides: Cytotoxic and apoptotic effect in human colon adenocarcinoma cells. Life Sci. 2012, 91, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, M.A.; Guru, S.; Naqvi, T.; Kumar, M.; Kumbhar, N.; Akhoon, S.; Banday, S.; Singh, S.K.; Bhushan, S.; Mustafa Peerzada, G.; et al. An investigation of in vitro cytotoxicity and apoptotic potential of aromatic diselenides. Bioorg. Med. Chem. Lett. 2014, 24, 3440–3446. [Google Scholar] [CrossRef] [PubMed]
- Plano, D.; Baquedano, Y.; Ibáñez, E.; Jiménez, I.; Palop, J.A.; Spallholz, J.E.; Sanmartín, C. Antioxidant-prooxidant properties of a new organoselenium compound. Molecules 2010, 15, 7292–7312. [Google Scholar] [CrossRef] [PubMed]
- El-Bayoumy, K.; Chae, Y.H.; Upadhyaya, P.; Ip, C. Chemoprevention of mammary cancer by diallyl selenide, a novel organoselenium compound. Anticancer Res. 1996, 16, 2911–2915. [Google Scholar] [PubMed]
- Mecklenburg, S.; Shaaban, S.; Ba, L.A.; Burkholz, T.; Schneider, T.; Diesel, B.; Kiemer, A.K.; Röseler, A.; Becker, K.; Reichrath, J.; et al. Exploring synthetic avenues for the effective synthesis of selenium-and tellurium-containing multifunctional redox agents. Org. Biomol. Chem. 2009, 7, 4753–4762. [Google Scholar] [CrossRef] [PubMed]
- Kumakura, F.; Mishra, B.; Priyadarsini, K.I.; Iwaoka, M. A water-soluble cyclic selenide with enhanced glutathione peroxidase-like catalytic activities. Eur. J. Org. Chem. 2010, 440–445. [Google Scholar] [CrossRef]
- Nascimento, V.; Alberto, E.E.; Tondo, D.W.; Dambrowski, D.; Detty, M.R.; Nome, F.; Braga, A.L. GPx-Like activity of selenides and selenoxides: Experimental evidence for the involvement of hydroxy perhydroxy selenane as the active species. J. Am. Chem. Soc. 2012, 134, 138–141. [Google Scholar] [CrossRef] [PubMed]
- McNeil, N.M.; Press, D.J.; Mayder, D.M.; Garnica, P.; Doyle, L.M.; Back, T.G. Enhanced Glutathione Peroxidase Activity of Water-Soluble and Polyethylene Glycol-Supported Selenides, Related Spirodioxyselenuranes and Pincer Selenuranes. J. Org. Chem. 2016, 81, 7884–7897. [Google Scholar] [CrossRef] [PubMed]
- Casaril, A.M.; Domingues, M.; Fronza, M.; Vieira, B.; Begnini, K.; Lenardão, E.J.; Seixas, F.K.; Collares, T.; Nogueira, C.W.; Savegnago, L. Antidepressant-like effect of a new selenium-containing compound is accompanied by a reduction of neuroinflammation and oxidative stress in lipopolysaccharide-challenged mice. J. Psychopharmacol. 2017, 31, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.F.B.; Oliveira, R.L.; Rodrigues, K.C.; Voss, G.T.; Godoi, B.; Schumacher, R.F.; Perin, G.; Wilhelm, E.A.; Luchese, C.; Alves, D. Organoselenium compounds from purines: Synthesis of 6-arylselanylpurines with antioxidant and anticholinesterase activities and memory improvement effect. Bioorg. Med. Chem. 2017, 25, 6718–6723. [Google Scholar] [CrossRef] [PubMed]
- Mániková, D.; Letavayová, L.M.; Vlasáková, D.; Košík, P.; Estevam, E.C.; Nasim, M.J.; Gruhlke, M.; Slusarenko, A.; Burkholz, T.; Jacob, C.; et al. Intracellular diagnostics: Hunting for the mode of action of redox-modulating selenium compounds in selected model systems. Molecules 2014, 19, 12258–12279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doering, M.; Ba, L.A.; Lilienthal, N.; Nicco, C.; Scherer, C.; Abbas, M.; Zada, A.A.; Coriat, R.; Burkholz, T.; Wessjohann, L.; et al. Synthesis and selective anticancer activity of organochalcogen based redox catalysts. J. Med. Chem. 2010, 53, 6954–6963. [Google Scholar] [CrossRef] [PubMed]
- De Souza, D.; Mariano, D.O.; Nedel, F.; Schultze, E.; Campos, V.F.; Seixas, F.; da Silva, R.S.; Munchen, T.S.; Ilha, V.; Dornelles, L.; et al. New organochalcogen multitarget drug: Synthesis and antioxidant and antitumoral activities of chalcogenozidovudine derivatives. J. Med. Chem. 2015, 58, 3329–3339. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.J.B.; Valença, W.O.; Lima, D.J.B.; Cavalcanti, B.C.; Pessoa, C.; Rafique, J.; Braga, A.L.; Jacob, C.; da Silva Júnior, E.N.; et al. Synthesis of selenium-quinone hybrid compounds with potential antitumor activity via Rh-catalyzed C-H bond activation and click reactions. Molecules. 2018, 23, 83. [Google Scholar] [CrossRef]
- Plano, D.; Karelia, D.N.; Pandey, M.K.; Spallholz, J.E.; Amin, S.; Sharma, A.K. Design, synthesis and biological evaluation of novel selenium (Se-NSAID) molecules as anticancer agents. J. Med. Chem. 2016, 59, 1946–1959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; An, B.; Li, J.; Hu, J.; Huang, L.; Li, X.; Chan, A.S.C. Synthesis and evaluation of selenium-containing indole chalcone and diarylketone derivatives as tubulin polymerization inhibition agents. Org. Biomol. Chem. 2017, 15, 7404–7410. [Google Scholar] [CrossRef] [PubMed]

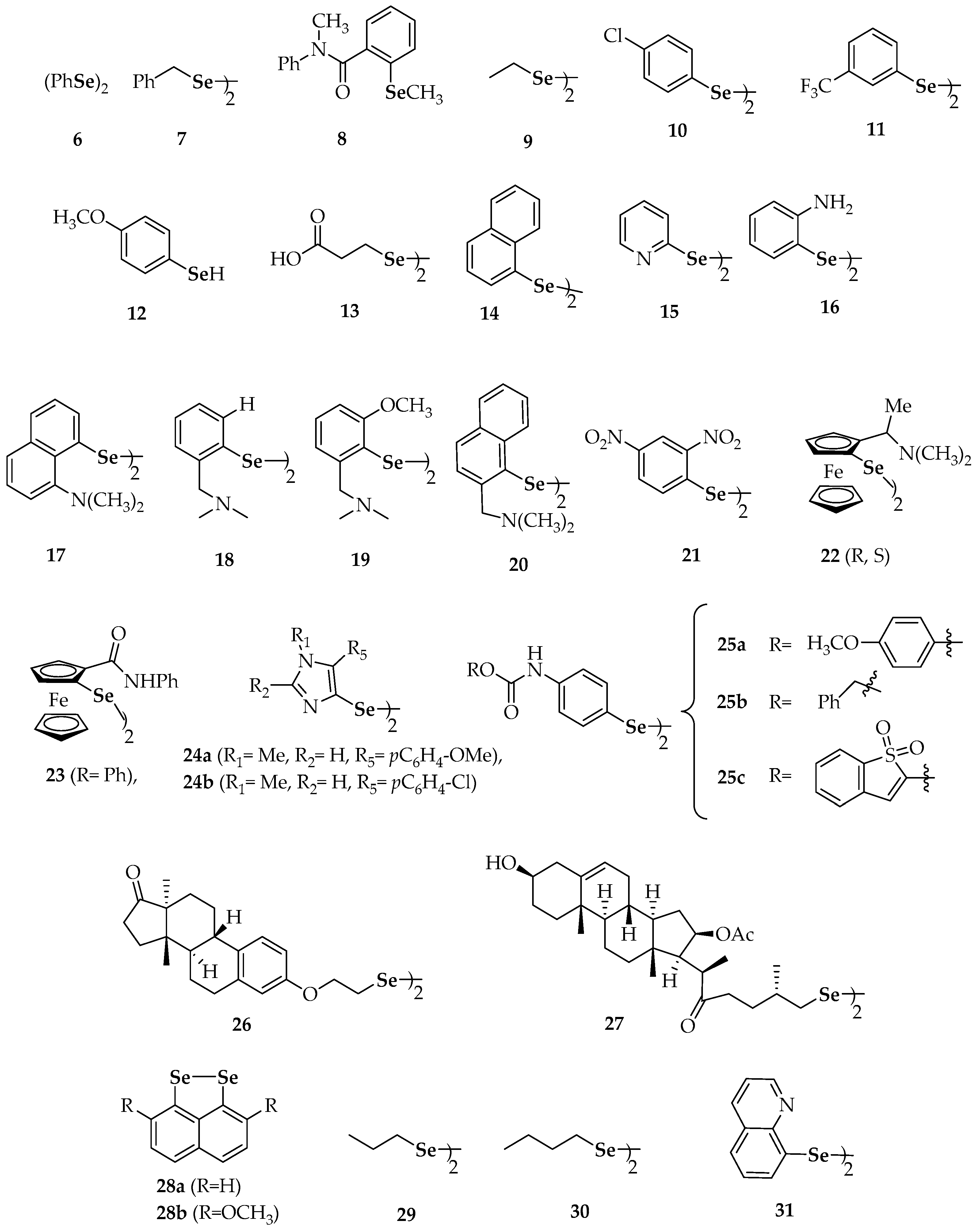
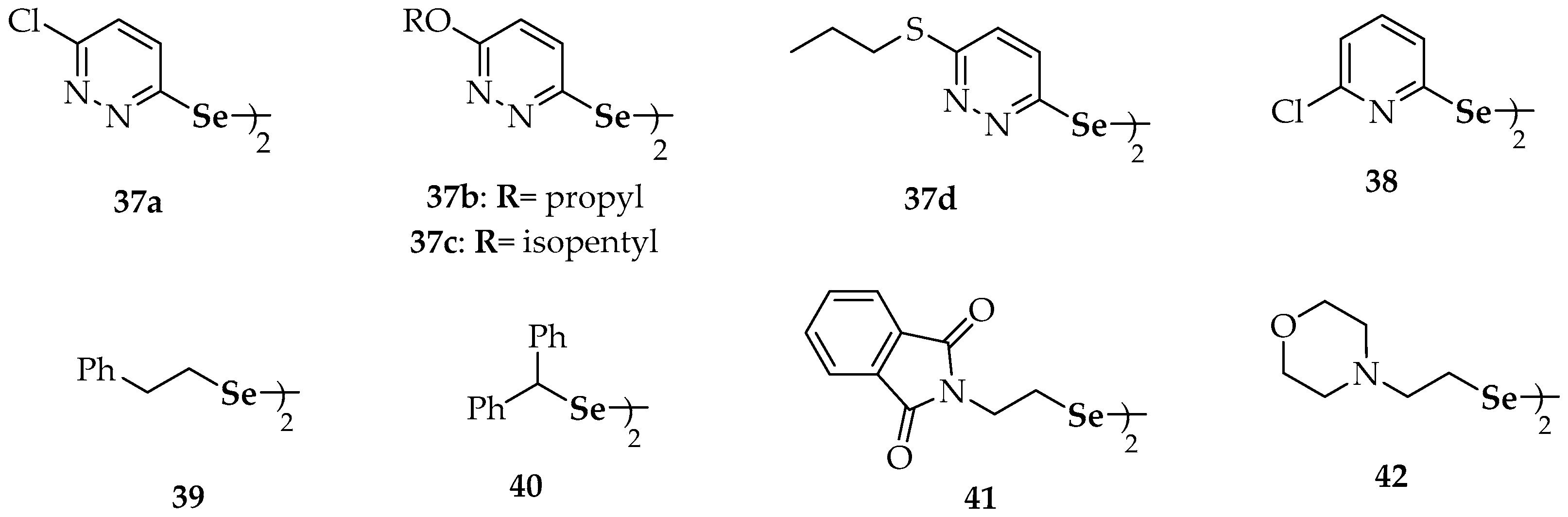
| Compound | Compound | ||
|---|---|---|---|
| 6 | diphenyl diselenide | 26 | bis[2-(estra-1,3,5(10)-trien-1 7-one-3-yl)oxyethyl]diselenide |
| 7 | dibenzyl diselenide | 27 | bis[(25R)-16β-acetoxy-3β-hydroxy-22-oxocholest-5-en-26-yl]diselenide |
| 9 | diethyl diselenide, | 28a | naphtho[1,8-cd][1,2]diselenole |
| 10 | bis(4-clorophenyl) diselenide | 28b | 3,8-dimethoxynaphtho[1,8-cd][1,2]diselenole |
| 11 | 3′3-ditrifluoromethyldiphenyl diselenide | 29 | dipropyl diselenide |
| 12 | p-methoxybenzeneselenol | 30 | dibutyl diselenide |
| 13 | 3,3′-diselenodipropionic acid | 31 | diquinolyl-8-yl diselenide |
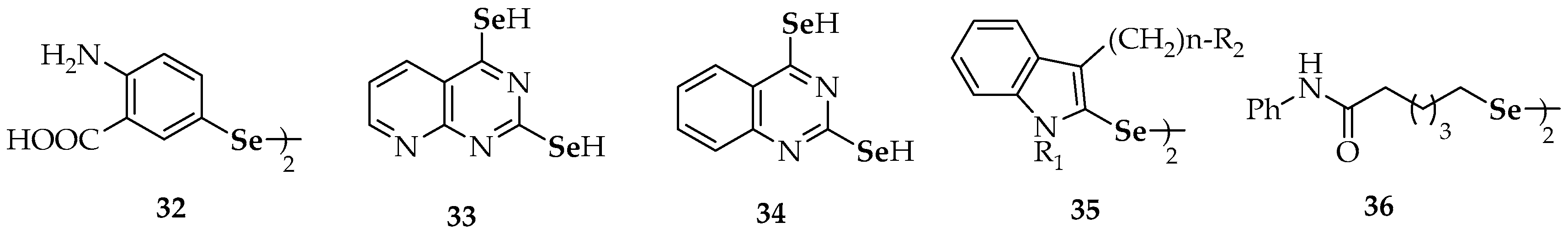
| 14 | binaphthyl diselenide | 32 | 4,4′-diamino-3′,3-dicarboxydiphenyl diselenide |
| 15 | dipyrid-2-yl diselenide | 36 | 6,6′-diselanediylbis(N-phenylhexanamide) |
| 16 | 2′2-diaminodiphenyl diselenide | 37a | 6,6′-dichlorodipyridazyn-2-yl diselenide |
| 17 | 8-(2-(8-(dimethylamino)naphthalen-1-yl)diselanyl)-N,N-dimethylnaphthalen-1-amine | 37b | 6,6′-di(propoxy)dipyridazyn-2-yl diselenide |
| 18 | (2-(2-(2-((dimethylamino)methyl)phenyl)diselanyl) phenyl)-N,N-dimethylmethanamine | 37c | 6,6′-di(isopentyloxy)dipyridazyn-2-yl diselenide |
| 19 | 2-(2-(2-((dimethylamino)methyl)-6-methoxyphenyl) diselanyl)-3-methoxyphenyl)-N,N-dimethylmethanamine | 37d | 6,6′-di(propylthio)dipyridazyn-2-yl diselenide |
| 20 | (1-(2-(2-((dimethylamino)methyl)naphthalen-1-yl)diselanyl)naphthalen-2-yl)-N,N-dimethylmethanamine | 38 | 2-chloro-6-(2-(6-chloropyridin-2-yl)diselanyl)pyridine |
| 21 | 1,2-bis(2,4-dinitrophenyl)diselane | 39 | 2,2′- di(phenylethyl) diselenide |
| 22 | bis(2-(1(R,S)-(N,N-dimethylamino)ethyl)ferrocen-1-yl) diselenide | 40 | dibenzhydryl diselenide |
| 23 | bis(2-(N-phenylcarbamoyl)ferrocen-1-yl) diselenide | 41 | 1,2-bis(isoindoline-1,3-dione-2 ethyl)diselane |
| 24a | 5-(4-methoxyphenyl)-4-(2-(5-(4-methoxyphenyl)-1-methyl-1H-imidazol-4-yl)diselanyl)-1-methyl-1H-imidazole | 42 | 1,2-bis(2-morpholinoethyl)diselane |
| 24b | 5-(4-chlorophenyl)-4-(2-(5-(4-chlorophenyl)-1-methyl-1H-imidazol-4-yl)diselanyl)-1-methyl-1H-imidazole | 43 | 4′,4-dimethoxydiphenyl diselenide |
| 25a | bis-4-[1-[(4′-methoxy)phenyl]-4-seleno-imidazole] | 44 | 3′,5′,3,5-tetratrifluoromethyl-diphenyl diselenide |
| 25b | 4,4′-diselanediylbis(N-benzylbenzamide) | 45 | 4′,4-diaminodiphenyl diselenide |
| 25c | N-(1,1-dioxidobenzo[b]thiophen-2-yl)-4-((4-((1,1-dioxidobenzo[b]thiophen-2-yl)carbamoyl)phenyl) diselaneyl)benzamide | ||
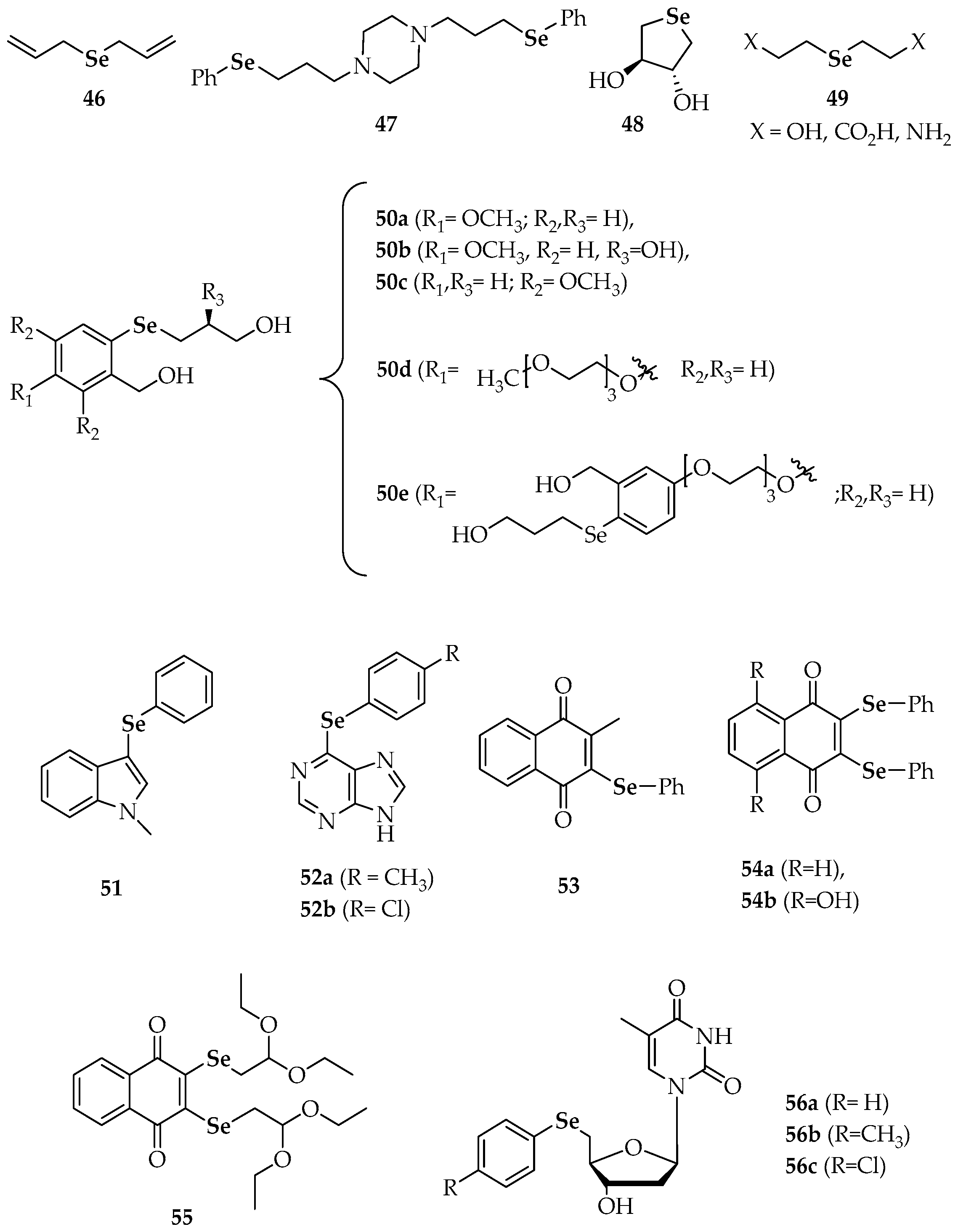
| Compound | |
|---|---|
| 46 | diallyl selenide |
| 47 | 1,4-bis(3-(phenylselanyl)propyl)piperazine |
| 48 | trans-3,4-dihydroxyselenolane |
| 50 a–e | different substituted phenyl 3-hydroxypropyl selenides |
| 51 | 3-(phenylselanyl)-1-methyl-1H-indole |
| 52a, 52b | 6-(4-methylphenyl)selanylpurine 6-(4-chlorophenyl)selanylpurine |
| 53 | 2-methyl-3-(phenylselanyl) naphthalene-1,4-dione |
| 54a | 2,3-bis(phenylselanyl)naphthalene-1,4-dione |
| 54b | 5,8-dihydroxy-2,3-bis(phenyl-selanyl)naphthalene-1,4-dione |
| 55 | 2,3-bis((2,2-diethoxyethyl)selanyl)naphthalene-1,4-dione |
| 56a | 5′-(phenylseleno)zidovudine |
| 56b | 5′-(4-methylphenylseleno)zidovudine |
| 56c | 5′-(4-chlorophenylseleno)zidovudine |
| 57 | 3-bromo-4-(4-(((4-fluorophenyl)selanyl)methyl)-1H-1,2,3-triazol-1-yl)-2,2-dimethyl-3,4-dihydro-2H-benzo[h]chromene-5,6-dione |
| 58 | 2-(2-acetoxybenzamido)-3-(methylselanyl)propanoic acid |
| 59 | selenide-containing indole chalcone derivatives |
| 60 | (3,4-dimethoxy-5-(methylselanyl)phenyl)(1H-indol-5-yl)methanone |
| 61 | (3,4-dimethoxy-5-(methylselanyl)phenyl)(1H-indol-4-yl)methanone |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Pérez, M.; Ali, W.; Marć, M.A.; Handzlik, J.; Domínguez-Álvarez, E. Selenides and Diselenides: A Review of Their Anticancer and Chemopreventive Activity. Molecules 2018, 23, 628. https://doi.org/10.3390/molecules23030628
Álvarez-Pérez M, Ali W, Marć MA, Handzlik J, Domínguez-Álvarez E. Selenides and Diselenides: A Review of Their Anticancer and Chemopreventive Activity. Molecules. 2018; 23(3):628. https://doi.org/10.3390/molecules23030628
Chicago/Turabian StyleÁlvarez-Pérez, Mónica, Wesam Ali, Małgorzata Anna Marć, Jadwiga Handzlik, and Enrique Domínguez-Álvarez. 2018. "Selenides and Diselenides: A Review of Their Anticancer and Chemopreventive Activity" Molecules 23, no. 3: 628. https://doi.org/10.3390/molecules23030628