Which Dendrimer to Attain the Desired Properties? Focus on Phosphorhydrazone Dendrimers †
Abstract
:1. Introduction
2. Phosphorhydrazone Dendrimers Compared to Other Types of Dendrimers
2.1. Positively Charged Phosphorus Dendrimers
2.1.1. Comparative Interference with Clinical Chemistry Tests
2.1.2. Comparative Efficiency as Carriers
2.1.3. Comparative Efficiency against Neurodegenerative Diseases
2.2. Negatively Charged Phosphorus Dendrimers
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers—Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. Dendritic macromolecules. Synthesis of starburst dendrimers. Macromolecules 1986, 19, 2466–2468. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Naylor, A.M.; Goddard, W.A. Starburst dendrimers—Molecular-level control of size, shape, surface-chemistry, topology, and flexibility from atoms to macroscopic matter. Angew. Chem. Int. Ed. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Tomalia, D.A. In quest of a systematic framework for unifying and defining nanoscience. J. Nanopart. Res. 2009, 11, 1251–1310. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Khanna, S.N. A Systematic Framework and Nanoperiodic Concept for Unifying Nanoscience: Hard/Soft Nanoelements, Superatoms, Meta-Atoms, New Emerging Properties, Periodic Property Patterns, and Predictive Mendeleev-like Nanoperiodic Tables. Chem. Rev. 2016, 116, 2705–2774. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A. Dendrons/dendrimers: Quantized, nano-element like building blocks for soft-soft and soft-hard nano-compound synthesis. Soft Matter 2010, 6, 456–474. [Google Scholar] [CrossRef]
- Tomalia, D.A. Dendritic effects: Dependency of dendritic nano-periodic property patterns on critical nanoscale design parameters (CNDPs). New J. Chem. 2012, 36, 264–281. [Google Scholar] [CrossRef]
- Buhleier, E.; Wehner, F.; Vögtle, F. “Cascade-” and “Nonskid-chain-like” syntheses of molecular cavity topologies. Synthesis 1978, 78, 155–158. [Google Scholar] [CrossRef]
- Worner, C.; Mulhaupt, R. Polynitrile-Functional and Polyamine-Functional Poly(Trimethylene Imine) Dendrimers. Angew. Chem. Int. Ed. 1993, 32, 1306–1308. [Google Scholar] [CrossRef]
- De Brabander van den Berg, E.M.M.; Meijer, E.W. Poly(propylene imine) dendrimers—Large-scale synthesis by hetereogeneously catalyzed hydrogenations. Angew. Chem. Int. Ed. 1993, 32, 1308–1311. [Google Scholar] [CrossRef]
- Takagi, K.; Hattori, T.; Kunisada, H.; Yuki, Y. Triazine dendrimers by divergent and convergent methods. J. Polym. Sci. Part A-Polym. Chem. 2000, 38, 4385–4395. [Google Scholar] [CrossRef]
- Zhang, W.; Simanek, E.E. Dendrimers based on melamine. Divergent and orthogonal, convergent syntheses of a G3 dendrimer. Org. Lett. 2000, 2, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Ihre, H.; Hult, A.; Soderlind, E. Synthesis, characterization, and H-1 NMR self-diffusion studies of dendritic aliphatic polyesters based on 2,2-bis(hydroxymethyl)propionic acid and 1,1,1-tris(hydroxyphenyl)ethane. J. Am. Chem. Soc. 1996, 118, 6388–6395. [Google Scholar] [CrossRef]
- Ihre, H.; Hult, A.; Frechet, J.M.J.; Gitsov, I. Double-stage convergent approach for the synthesis of functionalized dendritic aliphatic polyesters based on 2,2-bis(hydroxymethyl)propionic acid. Macromolecules 1998, 31, 4061–4068. [Google Scholar] [CrossRef]
- Zhou, L.L.; Hadjichristidis, N.; Toporowski, P.M.; Roovers, J. Synthesis and properties of regular star polybutadienes with 32 arms. Rubber Chem. Technol. 1992, 65, 303–314. [Google Scholar] [CrossRef]
- Zhou, L.L.; Roovers, J. Synthesis of novel carbosilane dendritic macromolecules. Macromolecules 1993, 26, 963–968. [Google Scholar] [CrossRef]
- Richter-Egger, D.L.; Tesfai, A.; Tucker, S.A. Spectroscopic investigations of poly(propyleneimine)dendrimers using the solvatochromic probe phenol blue and comparisons to poly(Amidoamine) dendrimers. Anal. Chem. 2001, 73, 5743–5751. [Google Scholar] [CrossRef] [PubMed]
- Esumi, K.; Miyamoto, K.; Yoshimura, T. Comparison of PAMAM-Au and PPI-Au nanocomposites and their catalytic activity for reduction of 4-nitrophenol. J. Colloid Interface Sci. 2002, 254, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Donnio, B.; Barbera, J.; Gimenez, R.; Guillon, D.; Marcos, M.; Serrano, J.L. Controlled molecular conformation and morphology in poly(amidoamine) (PAMAM) and poly(propyleneimine) (DAB) dendrimers. Macromolecules 2002, 35, 370–381. [Google Scholar] [CrossRef]
- Malik, N.; Wiwattanapatapee, R.; Klopsch, R.; Lorenz, K.; Frey, H.; Weener, J.W.; Meijer, E.W.; Paulus, W.; Duncan, R. Dendrimers: Relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of I-125-labelled polyamidoamine dendrimers in vivo. J. Control. Release 2000, 65, 133–148. [Google Scholar] [CrossRef]
- Janaszewska, A.; Maczynska, K.; Matuszko, G.; Appelhans, D.; Voit, B.; Klajnert, B.; Bryszewska, M. Cytotoxicity of PAMAM, PPI and maltose modified PPI dendrimers in Chinese hamster ovary (CHO) and human ovarian carcinoma (SKOV3) cells. New J. Chem. 2012, 36, 428–437. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kawamoto, S.; Saga, T.; Sato, N.; Hiraga, A.; Ishimori, T.; Akita, Y.; Mamede, M.H.; Konishi, J.; Togashi, K. Novel liver macromolecular MR contrast agent with a polypropylenimine diaminobutyl dendrimer core: Comparison to the vascular MR contrast agent with the polyamidoamine dendrimer core. Magn. Reson. Med. 2001, 46, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Mynar, J.L.; Lowery, T.J.; Wemmer, D.E.; Pines, A.; Frechet, J.M.J. Xenon biosensor amplification via dendrimer-cage supramolecular constructs. J. Am. Chem. Soc. 2006, 128, 6334–6335. [Google Scholar] [CrossRef] [PubMed]
- Boisselier, E.; Liang, L.Y.; Dalko-Csiba, M.; Ruiz, J.; Astruc, D. Interactions and Encapsulation of Vitamins C, B-3, and B-6 with Dendrimers in Water. Chem.-Eur. J. 2010, 16, 6056–6068. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.M.; Su, Y.Z.; Hu, J.J.; Zhang, J.H.; Zhang, H.F.; Cheng, Y.Y. Comparison of generation 3 polyamidoamine dendrimer and generation 4 polypropylenimine dendrimer on drug loading, complex structure, release behavior, and cytotoxicity. Int. J. Nanomed. 2011, 6, 3361–3372. [Google Scholar] [CrossRef]
- Chaudhary, S.; Gothwal, A.; Khan, I.; Srivastava, S.; Malik, R.; Gupta, U. Polypropyleneimine and polyamidoamine dendrimer mediated enhanced solubilization of bortezomib: Comparison and evaluation of mechanistic aspects by thermodynamics and molecular simulations. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 72, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Pijnenburg, N.J.M.; Lutz, M.; Siegler, M.A.; Spek, A.; van Koten, G.; Gebbink, R. The role of the dendritic support in the catalytic performance of peripheral pincer Pd-complexes. New J. Chem. 2011, 35, 2356–2365. [Google Scholar] [CrossRef]
- Enciso, A.E.; Neun, B.; Rodriguez, J.; Ranjan, A.P.; Dobrovolskaia, M.A.; Simanek, E.E. Nanoparticle Effects on Human Platelets in Vitro: A Comparison between PAMAM and Triazine Dendrimers. Molecules 2016, 21, 428. [Google Scholar] [CrossRef] [PubMed]
- Feliu, N.; Walter, M.V.; Montanez, M.I.; Kunzmann, A.; Hult, A.; Nystrom, A.; Malkoch, M.; Fadeel, B. Stability and biocompatibility of a library of polyester dendrimers in comparison to polyamidoamine dendrimers. Biomaterials 2012, 33, 1970–1981. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Turrin, C.O.; Majoral, J.P. Biological properties of phosphorus dendrimers. New J. Chem. 2010, 34, 1512–1524. [Google Scholar] [CrossRef]
- Caminade, A.M. Phosphorus dendrimers for nanomedicine. Chem. Commun. 2017, 53, 9830–9838. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Hameau, A.; Majoral, J.P. Multicharged and/or Water-Soluble Fluorescent Dendrimers: Properties and Uses. Chem.-Eur. J. 2009, 15, 9270–9285. [Google Scholar] [CrossRef] [PubMed]
- Loup, C.; Zanta, M.A.; Caminade, A.M.; Majoral, J.P.; Meunier, B. Preparation of water-soluble cationic phosphorus-containing dendrimers as DNA transfecting agents. Chem.-Eur. J. 1999, 5, 3644–3650. [Google Scholar] [CrossRef]
- Solassol, J.; Crozet, C.; Perrier, V.; Leclaire, J.; Beranger, F.; Caminade, A.M.; Meunier, B.; Dormont, D.; Majoral, J.P.; Lehmann, S. Cationic phosphorus-containing dendrimers reduce prion replication both in cell culture and in mice infected with scrapie. J. Gen. Virol. 2004, 85, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Shcharbin, D.; Shcharbina, N.; Milowska, K.; de la Mata, F.J.; Munoz-Fernandez, M.A.; Mignani, S.; Gomez-Ramirez, R.; Majoral, J.P.; Bryszewska, M. Interference of cationic polymeric nanoparticles with clinical chemistry tests-Clinical relevance. Int. J. Pharm. 2014, 473, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Padie, C.; Maszewska, M.; Majchrzak, K.; Nawrot, B.; Caminade, A.M.; Majoral, J.P. Polycationic phosphorus dendrimers: Synthesis, characterization, study of cytotoxicity, complexation of DNA, and transfection experiments. New J. Chem. 2009, 33, 318–326. [Google Scholar] [CrossRef]
- Shakhbazau, A.; Mohanty, C.; Shcharbin, D.; Bryszewska, M.; Caminade, A.M.; Majoral, J.P.; Alant, J.; Midha, R. Doxycycline-regulated GDNF expression promotes axonal regeneration and functional recovery in transected peripheral nerve. J. Control. Release 2013, 172, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Ionov, M.; Lazniewska, J.; Dzmitruk, V.; Halets, I.; Loznikova, S.; Novopashina, D.; Apartsin, E.; Krasheninina, O.; Venyaminova, A.; Milowska, K.; et al. Anticancer siRNA cocktails as a novel tool to treat cancer cells. Part (A). Mechanisms of interaction. Int. J. Pharm. 2015, 485, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Dzmitruk, V.; Szulc, A.; Shcharbin, D.; Janaszewska, A.; Shcharbina, N.; Lazniewska, J.; Novopashina, D.; Buyanova, M.; Ionov, M.; Klajnert-Maculewicz, B.; et al. Anticancer siRNA cocktails as a novel tool to treat cancer cells. Part (B). Efficiency of pharmacological action. Int. J. Pharm. 2015, 485, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Klajnert, B.; Cangiotti, M.; Calici, S.; Majoral, J.P.; Caminade, A.M.; Cladera, J.; Bryszewska, M.; Ottaviani, M.F. EPR study of the interactions between dendrimers and peptides involved in Alzheimer’s and prion diseases. Macromol. Biosci. 2007, 7, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Klajnert, B.; Cangiotti, M.; Calici, S.; Ionov, M.; Majoral, J.P.; Caminade, A.M.; Cladera, J.; Bryszewska, M.; Ottaviani, M.F. Interactions between dendrimers and heparin and their implications for the anti-prion activity of dendrimers. New J. Chem. 2009, 33, 1087–1093. [Google Scholar] [CrossRef]
- Katir, N.; Majoral, J.P.; El Kadib, A.; Caminade, A.M.; Bousmina, M. Molecular and Macromolecular Engineering with Viologens as Building Blocks. Rational Design of Phosphorus-Viologen Dendritic Structures. Eur. J. Org. Chem. 2012, 2012, 269–273. [Google Scholar] [CrossRef]
- Ciepluch, K.; Katir, N.; El Kadib, A.; Felczak, A.; Zawadzka, K.; Weber, M.; Klajnert, B.; Lisowska, K.; Caminade, A.M.; Bousmina, M.; et al. Biological Properties of New Viologen-Phosphorus Dendrimers. Mol. Pharm. 2012, 9, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Milowska, K.; Szwed, A.; Zablocka, M.; Caminade, A.M.; Majoral, J.P.; Mignani, S.; Gabryelak, T.; Bryszewska, M. In vitro PAMAM, phosphorus and viologen-phosphorus dendrimers prevent rotenone-induced cell damage. Int. J. Pharm. 2014, 474, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Boggiano, M.K.; Soler-Illia, G.J.A.A.; Rozes, L.; Sanchez, C.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P. New mesostructured hybrid materials made from assemblies of dendrimers and titanium (IV) oxo-organo clusters. Angew. Chem. Int. Ed. 2000, 39, 4249–4254. [Google Scholar] [CrossRef]
- Kim, D.H.; Karan, P.; Göring, P.; Leclaire, J.; Caminade, A.M.; Majoral, J.P.; Gösele, U.; Steinhart, M.; Knoll, W. Formation of dendrimer nanotubes by layer-by-layer deposition. Small 2005, 1, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Poupot, M.; Griffe, L.; Marchand, P.; Maraval, A.; Rolland, O.; Martinet, L.; L’Faqihi-Olive, F.E.; Turrin, C.O.; Caminade, A.M.; Fournie, J.J.; et al. Design of phosphorylated dendritic architectures to promote human monocyte activation. FASEB J. 2006, 20, 2339–2351. [Google Scholar] [CrossRef] [PubMed]
- Fruchon, S.; Poupot, M.; Martinet, L.; Turrin, C.O.; Majoral, J.P.; Fournie, J.J.; Caminade, A.M.; Poupot, R. Anti-inflammatory and immunosuppressive activation of human monocytes by a bioactive dendrimer. J. Leukoc. Biol. 2009, 85, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Griffe, L.; Poupot, M.; Marchand, P.; Maraval, A.; Turrin, C.O.; Rolland, O.; Metivier, P.; Bacquet, G.; Fournie, J.J.; Caminade, A.M.; et al. Multiplication of human natural killer cells by nanosized phosphonate-capped dendrimers. Angew. Chem. Int. Ed. 2007, 46, 2523–2526. [Google Scholar] [CrossRef] [PubMed]
- Rolland, O.; Griffe, L.; Poupot, M.; Maraval, A.; Ouali, A.; Coppel, Y.; Fournie, J.J.; Bacquet, G.; Turrin, C.O.; Caminade, A.M.; et al. Tailored control and optimisation of the number of phosphonic acid termini on phosphorus-containing dendrimers for the ex-vivo activation of human monocytes. Chem.-Eur. J. 2008, 14, 4836–4850. [Google Scholar] [CrossRef] [PubMed]
- Hayder, M.; Varilh, M.; Turrin, C.O.; Saoudi, A.; Caminade, A.M.; Poupot, R.; Liblau, R.S. Phosphorus-Based Dendrimer ABP Treats Neuroinflammation by Promoting IL-10-Producing CD4(+) T Cells. Biomacromolecules 2015, 16, 3425–3433. [Google Scholar] [CrossRef] [PubMed]
- Hayder, M.; Poupot, M.; Baron, M.; Nigon, D.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P.; Eisenberg, R.A.; Fournie, J.J.; Cantagrel, A.; et al. A Phosphorus-Based Dendrimer Targets Inflammation and Osteoclastogenesis in Experimental Arthritis. Sci. Transl. Med. 2011, 3, 81. [Google Scholar] [CrossRef] [PubMed]
- Poupot, M.; Turrin, C.O.; Caminade, A.M.; Fournie, J.J.; Attal, M.; Poupot, R.; Fruchon, S. Poly(phosphorhydrazone) dendrimers: Yin and yang of monocyte activation for human NK cell amplification applied to immunotherapy against multiple myeloma. Nanomed.-Nanotechnol. Biol. Med. 2016, 12, 2321–2330. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Fruchon, S.; Turrin, C.O.; Poupot, M.; Ouali, A.; Maraval, A.; Garzoni, M.; Maly, M.; Furer, V.; Kovalenko, V.; et al. The key role of the scaffold on the efficiency of dendrimer nanodrugs. Nat. Commun. 2015, 6, 7722. [Google Scholar] [CrossRef] [PubMed]
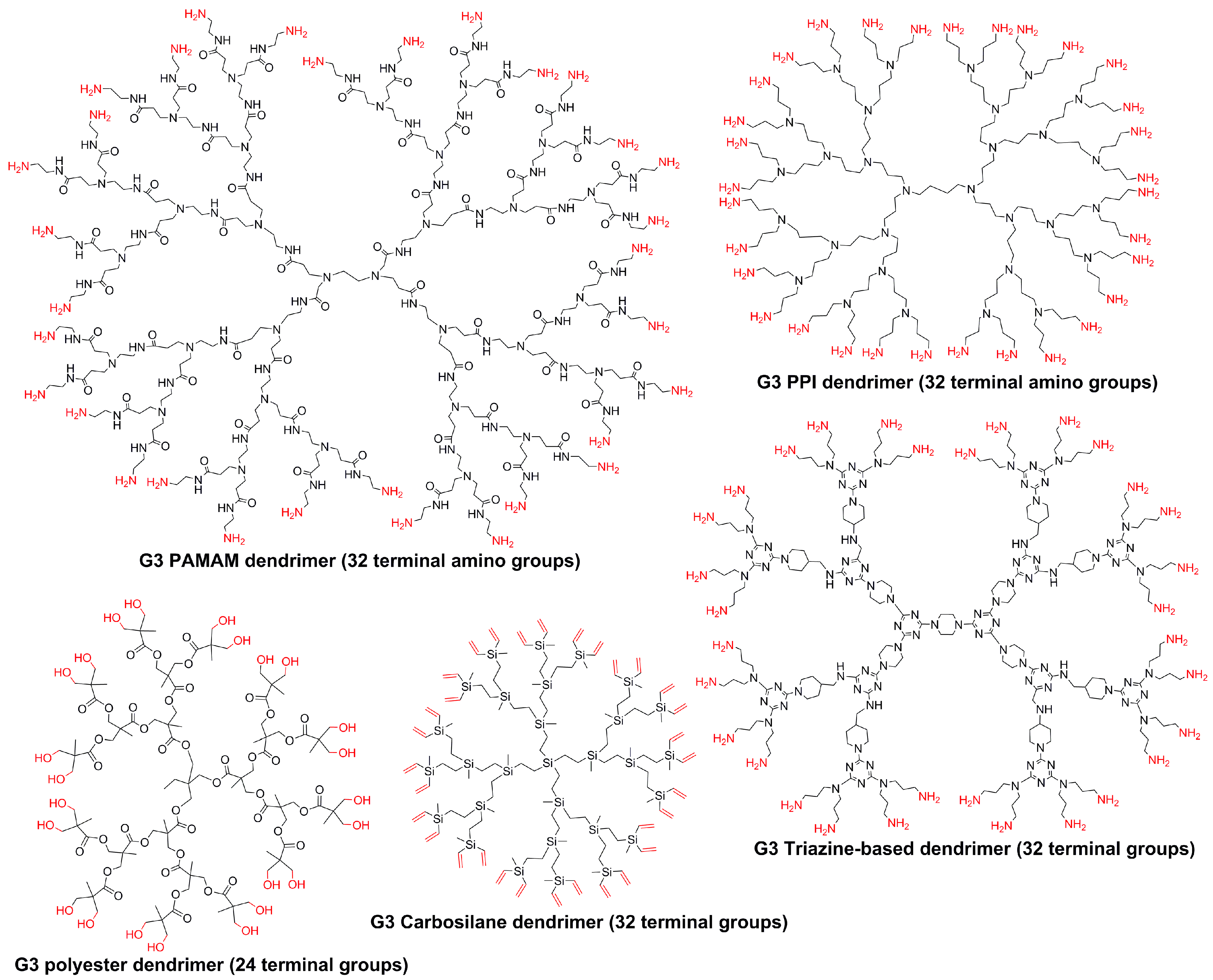

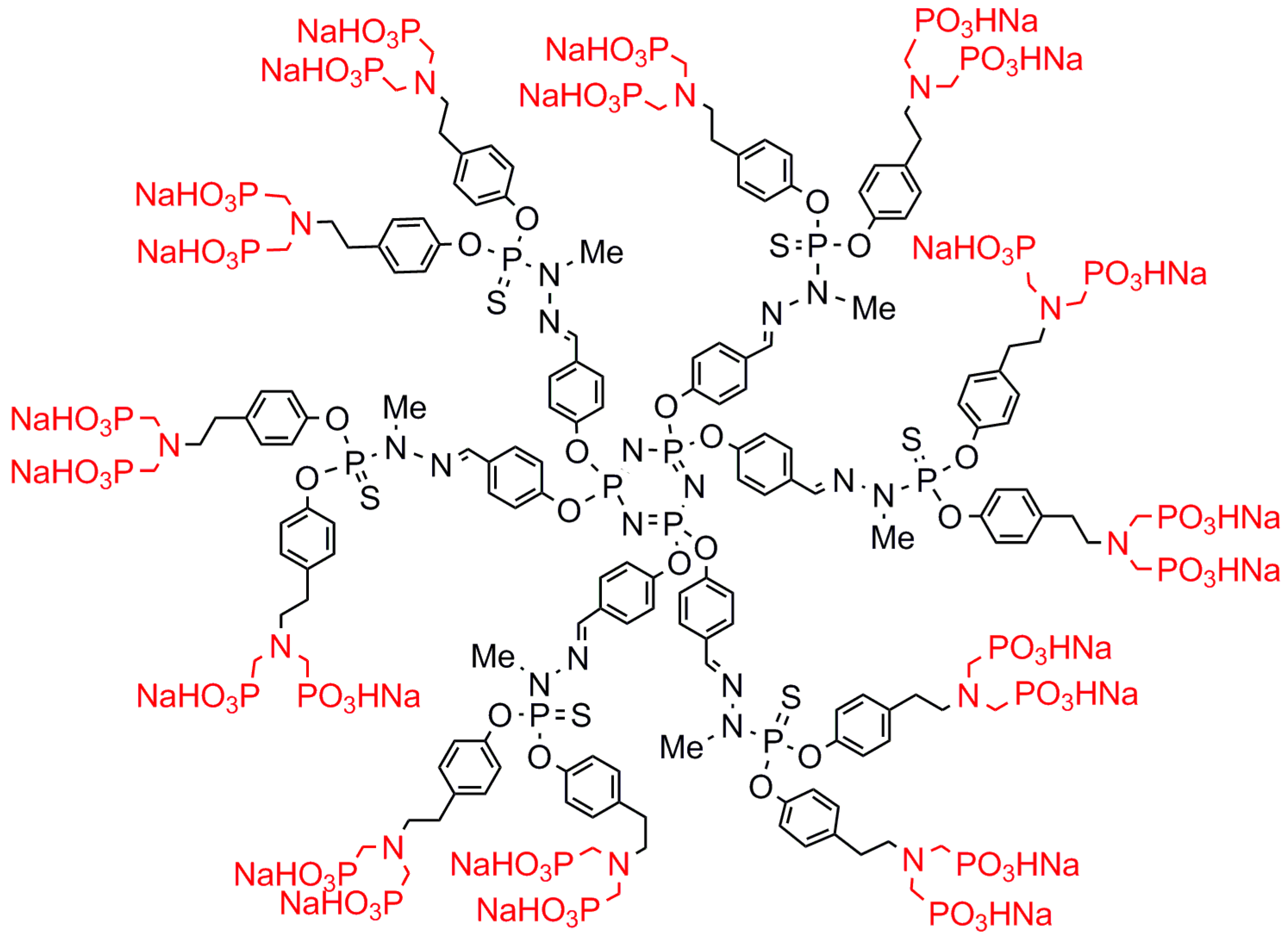

| PPH | PAMAM | PPI | PCSi | P-Lys | Experiment | Ref. |
|---|---|---|---|---|---|---|
| -NEt2H)96 | -NH3)64 | -NMe3)24 | Clinical tests | [35] | ||
| -NEt2H)96 | -NH3)64 | Transfection | [37] | |||
| -NEt2H)48/ -NEt2H)96 | -NH3)32/ -NH3)64 | -NMe3)8 | Protection SiRNA 1 | [38] | ||
| -NEt2H)48/ -NEt2H)96 | -NH3)32/ -NH3)64 | -NMe3)8 | Carrier of Si RNA | [39] | ||
| -NEt2H)96 | -NH3)64/ -NH3)128 | -NH3)16 | Peptide aggregation scavenger | [40] | ||
| -NEt2H)96 | -NH3)64/ -NH3)128 | -NH3)16 | Interaction with heparin | [41] | ||
| -NEt2H)48/ -NEt2H)96 | -NH3)32/ -NH3)64 | Decrease ROS 2 levels | [44] | |||
| (PO3HNa)2]12 | (PO3HNa)2]8 | Against RA 3 | [53] | |||
| (PO3HNa)2]12 4 | (PO3HNa)2]8 | (PO3HNa)2]8 | (PO3HNa)2]8 | (PO3HNa)2]8 | Activation of monocytes | [54] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caminade, A.-M.; Majoral, J.-P. Which Dendrimer to Attain the Desired Properties? Focus on Phosphorhydrazone Dendrimers. Molecules 2018, 23, 622. https://doi.org/10.3390/molecules23030622
Caminade A-M, Majoral J-P. Which Dendrimer to Attain the Desired Properties? Focus on Phosphorhydrazone Dendrimers. Molecules. 2018; 23(3):622. https://doi.org/10.3390/molecules23030622
Chicago/Turabian StyleCaminade, Anne-Marie, and Jean-Pierre Majoral. 2018. "Which Dendrimer to Attain the Desired Properties? Focus on Phosphorhydrazone Dendrimers" Molecules 23, no. 3: 622. https://doi.org/10.3390/molecules23030622