The Effects of Aronia melanocarpa ‘Viking’ Extracts in Attenuating RANKL-Induced Osteoclastic Differentiation by Inhibiting ROS Generation and c-FOS/NFATc1 Signaling
Abstract
:1. Introduction
2. Results
2.1. Quantitative HPLC Analysis of Anthocyanin Compounds from Aronia melanocarpa ‘Viking’ Extracts
2.2. Total Phenolic and Flavonoid Contents and Yields of Aronia melanocarpa ‘Viking’ Extracts
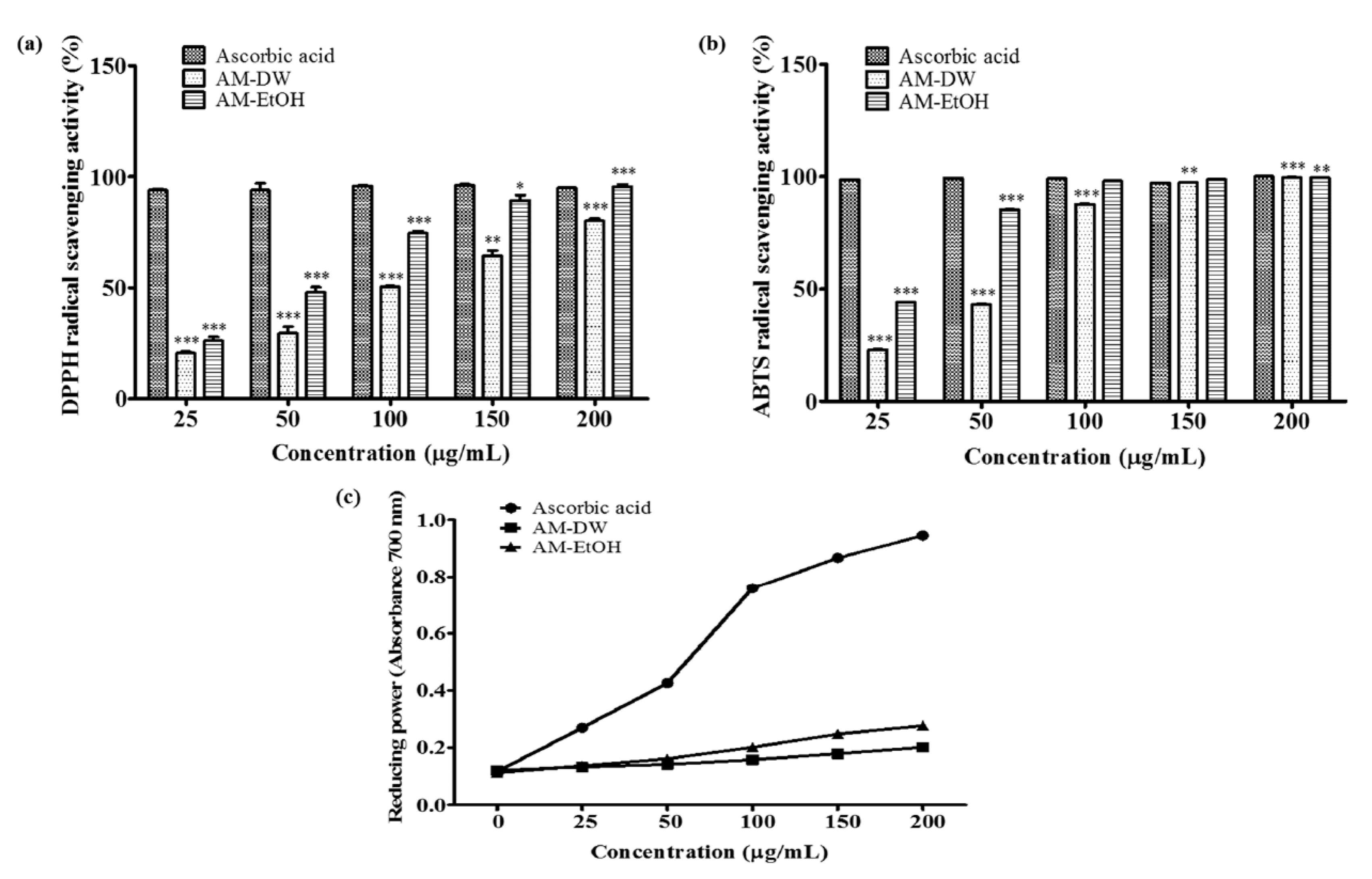
2.3. DPPH Radical Scavenging Activity of Aronia melanocarpa ‘Viking’ Extracts
2.4. ABTS Radical Scavenging Activity of Aronia melanocarpa ‘Viking’ Extracts
2.5. Determination of Reducing Power Activity of Aronia melanocarpa ‘Viking’ Extracts
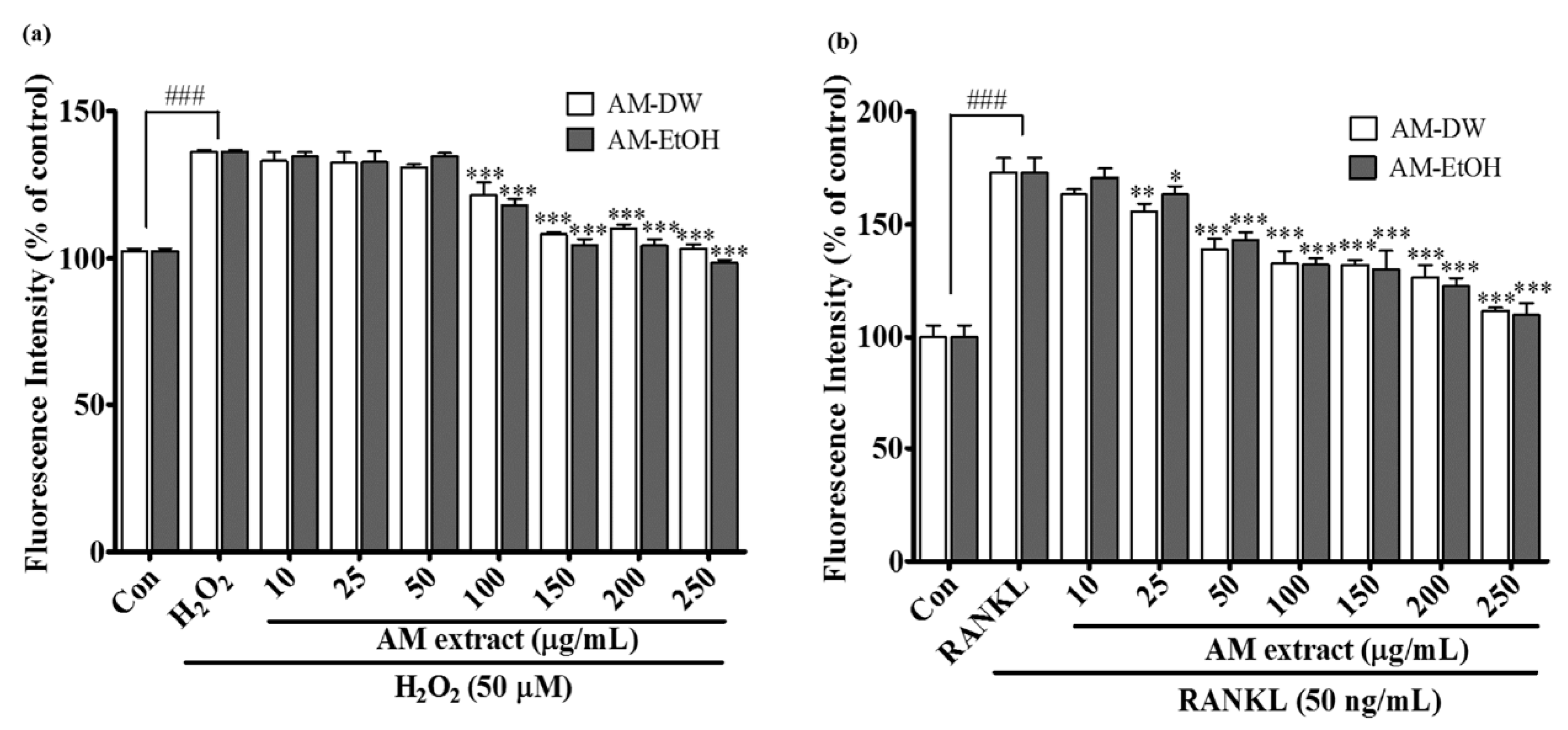
2.6. Effects of Aronia melanocarpa ‘Viking’ Extracts on Intracellular ROS Generation
2.7. Aronia melanocarpa ‘Viking’ Extracts Inhibit RANKL Induced Osteoclastic Differentiation
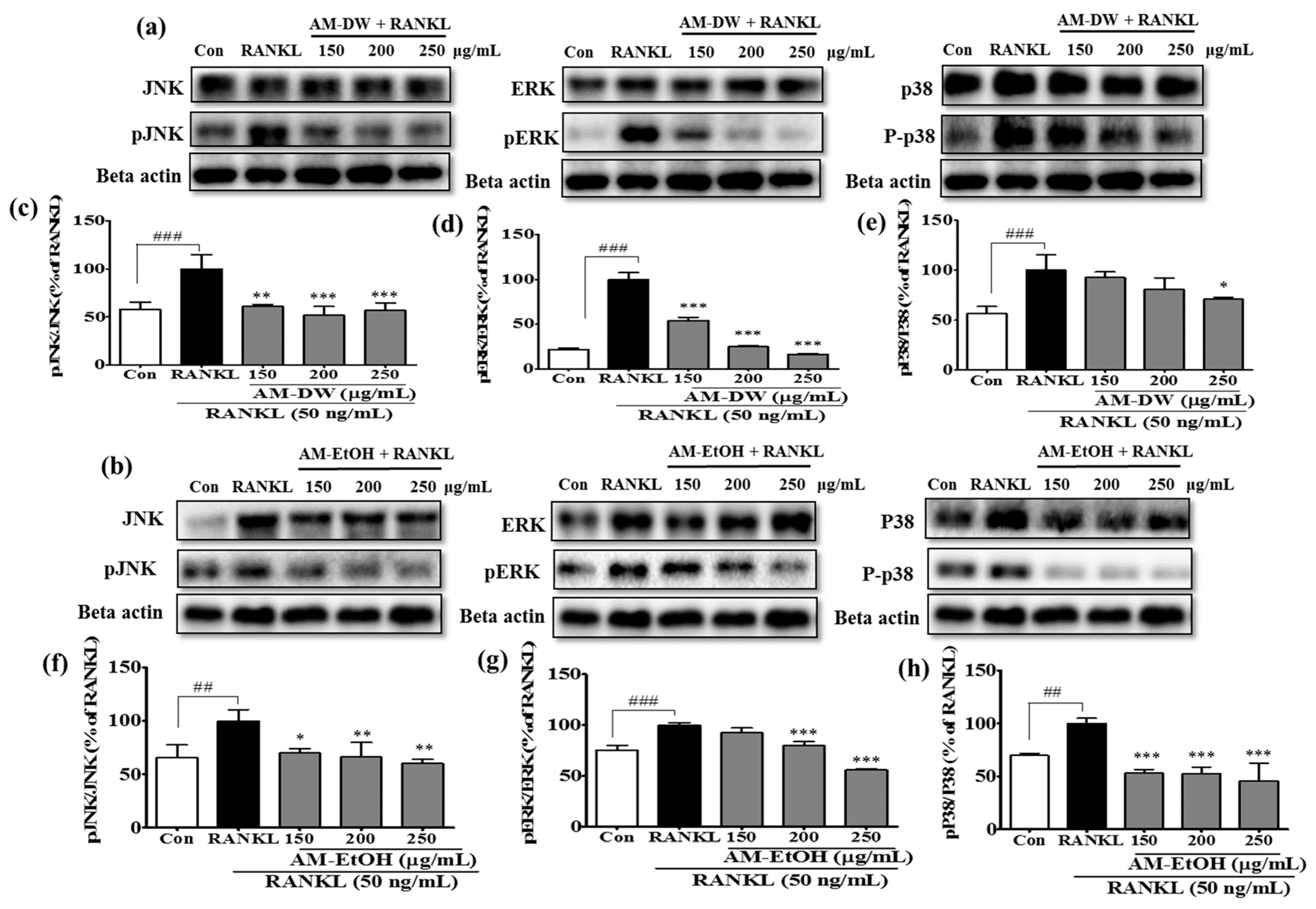
2.8. Aronia melanocarpa ‘Viking’ Extracts Inhibit MAP Kinase Activation in RANKL-Induced Osteoclasts
2.9. Aronia melanocarpa ‘Viking’ Extracts Inhibit RANKL-Induced NF-κB Activation and Its Nuclear Translocation
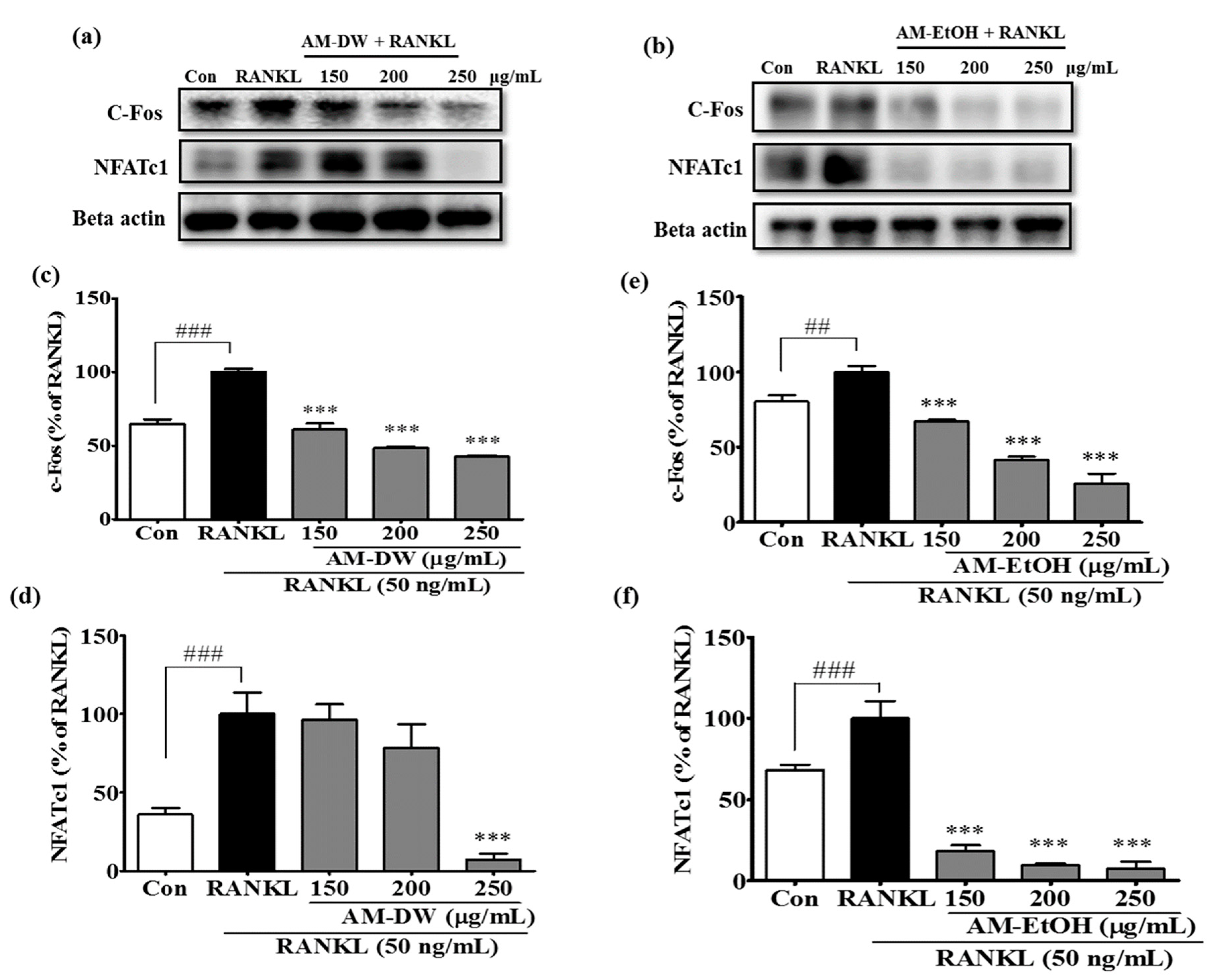
2.10. Aronia melanocarpa ‘Viking’ Extracts Suppress Transcription Factor c-Fos and NFATc1 Expression in RANKL-Induced Osteoclasts
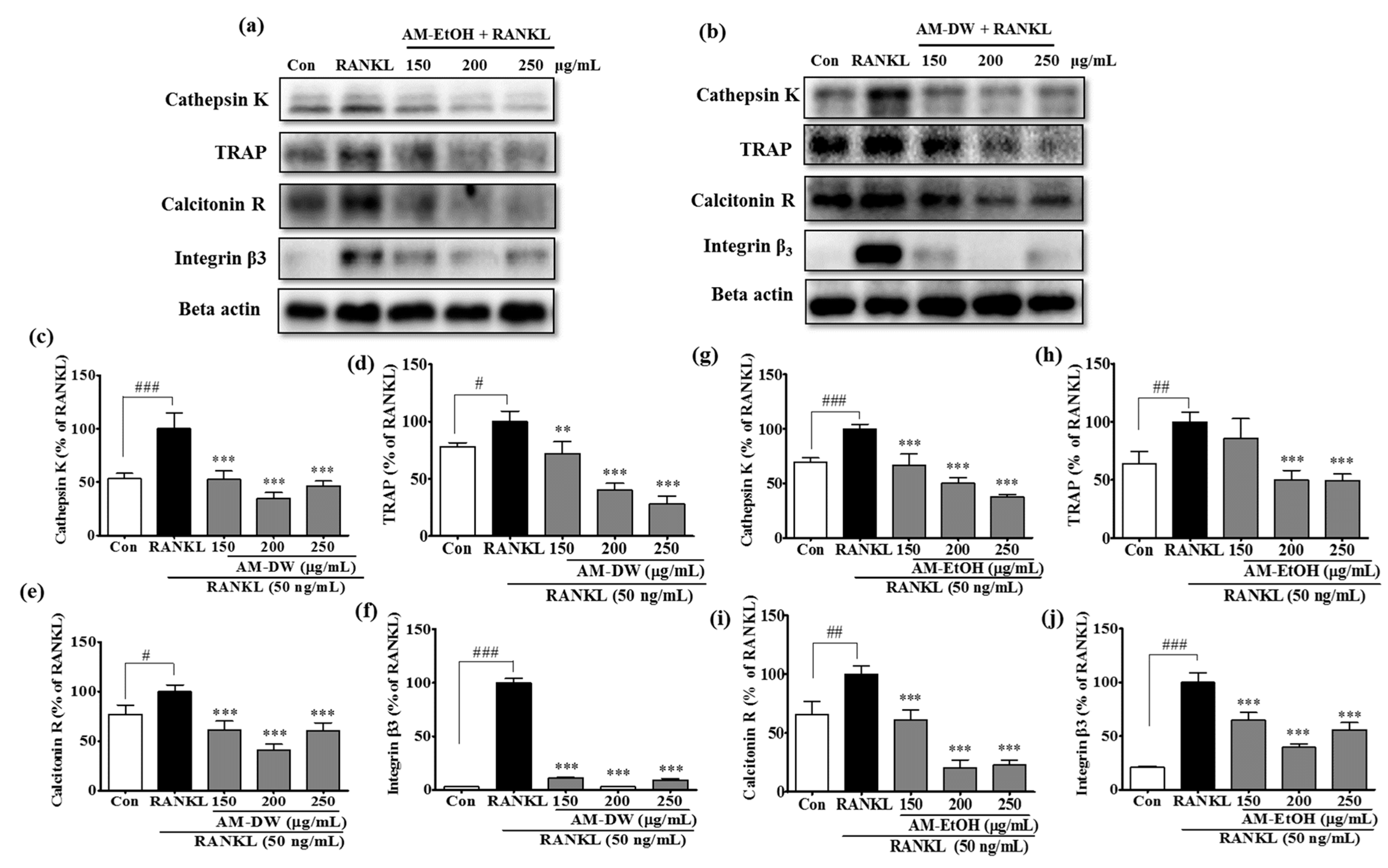
2.11. Aronia melanocarpa ‘Viking’ Extracts Reduce Osteoclast Specific Gene Expression in RANKL-Induced RAW 264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Preparation of Extracts
4.3. High-Performance Liquid Chromatography (HPLC) Analysis
4.4. Determination of Total Phenolic Content
4.5. Determination of Total Flavonoid Content
4.6. DPPH Radical Scavenging Activity
4.7. ABTS Radical Scavenging Activity
4.8. Determination of Reducing Power
4.9. Cell Culture
4.10. Cell Viability Analysis
4.11. Determination of Intracellular Reactive Oxygen Species (ROS)
4.12. Tartrate-Resistant Acid Phosphatase (TRAP) Activity
4.13. Tartrate-Resistant Acid Phosphatase (TRAP) Staining
4.14. Preparation of Total Cell Extracts and Immunoblot Analysis
4.15. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, Y.-B.; Zheng, C.-J.; Qin, L.-P.; Sun, L.-N.; Han, T.; Jiao, L.; Zhang, Q.-Y.; Wu, J.-Z. Antiosteoporotic activity of anthraquinones from morinda officinalis on osteoblasts and osteoclasts. Molecules 2009, 14, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Alliston, T.; Derynck, R. Medicine: Interfering with bone remodelling. Nature 2002, 416, 686–687. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G.; Wagner, E.F. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell 2002, 2, 389–406. [Google Scholar] [CrossRef]
- Park, C.K.; Lee, Y.; Chang, E.-J.; Lee, M.H.; Yoon, J.H.; Ryu, J.-H.; Kim, H.-H. Bavachalcone inhibits osteoclast differentiation through suppression of nfatc1 induction by rankl. Biochem. Pharmacol. 2008, 75, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.H.; Kim, H.-H. Signal transduction by receptor activator of nuclear factor kappa b in osteoclasts. Biochem. Biophys. Res. Commun. 2003, 305, 211–214. [Google Scholar] [CrossRef]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.-I. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Yamamoto, A.; Miyazaki, T.; Kadono, Y.; Takayanagi, H.; Miura, T.; Nishina, H.; Katada, T.; Wakabayashi, K.; Oda, H.; Nakamura, K. Possible involvement of IκB kinase 2 and MKK7 in osteoclastogenesis induced by receptor activator of nuclear factor κb ligand. J. Bone Miner. Res. 2002, 17, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Iotsova, V.; Caamaño, J.; Loy, J.; Yang, Y.; Lewin, A.; Bravo, R. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat. Med. 1997, 3, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Slimestad, R.; Torskangerpoll, K.; Nateland, H.S.; Johannessen, T.; Giske, N.H. Flavonoids from black chokeberries, aronia melanocarpa. J. Food Compos. Anal. 2005, 18, 61–68. [Google Scholar] [CrossRef]
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.M.; Sineiro, J.; Domínguez, H.; Núñez, M.a.J.; Parajó, J.C. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar]
- Brown, J.E.; Rice-Evans, C.A. Luteolin-rich artichoke extract protects low density lipoprotein from oxidation in vitro. Free Radic. Res. 1998, 29, 247–255. [Google Scholar] [PubMed]
- Kessler, M.; Ubeaud, G.; Jung, L. Anti-and pro-oxidant activity of rutin and quercetin derivatives. J. Pharm. Pharmacol. 2003, 55, 131–142. [Google Scholar] [PubMed]
- Liu, J.; Jia, L.; Kan, J.; Jin, C.-H. In vitro and in vivo antioxidant activity of ethanolic extract of white button mushroom (Agaricus bisporus). Food Chem. Toxicol. 2013, 51, 310–316. [Google Scholar] [PubMed]
- Dorman, H.J.D.; Peltoketo, A.; Hiltunen, R.; Tikkanen, M.J. Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected lamiaceae herbs. Food Chem. 2003, 83, 255–262. [Google Scholar]
- Zhao, H.; Dong, J.; Lu, J.; Chen, J.; Li, Y.; Shan, L.; Lin, Y.; Fan, W.; Gu, G. Effects of extraction solvent mixtures on antioxidant activity evaluation and their extraction capacity and selectivity for free phenolic compounds in barley(Hordeum vulgare L.). J. Agric. Food Chem. 2006, 54, 7277–7286. [Google Scholar] [PubMed]
- Lee, N.K.; Choi, Y.G.; Baik, J.Y.; Han, S.Y.; Jeong, D.-W.; Bae, Y.S.; Kim, N.; Lee, S.Y. A crucial role for reactive oxygen species in rankl-induced osteoclast differentiation. Blood 2005, 106, 852–859. [Google Scholar] [PubMed]
- Lander, H.M. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997, 11, 118–124. [Google Scholar] [PubMed]
- Chang, E.-J.; Kim, H.J.; Ha, J.; Kim, H.J.; Ryu, J.; Park, K.-H.; Kim, U.-H.; Lee, Z.H.; Kim, H.-M.; Fisher, D.E.; et al. Hyaluronan inhibits osteoclast differentiation via toll-like receptor 4. J. Cell Sci. 2007, 120, 166–176. [Google Scholar] [PubMed]
- Takatsuna, H.; Asagiri, M.; Kubota, T.; Oka, K.; Osada, T.; Sugiyama, C.; Saito, H.; Aoki, K.; Ohya, K.; Takayanagi, H. Inhibition of rankl-induced osteoclastogenesis by (−)-dhmeq, a novel NF-κB inhibitor, through downregulation of NFATc1. J. Bone Miner. Res. 2005, 20, 653–662. [Google Scholar] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 1990, 186, 1–85. [Google Scholar] [PubMed]
- Moskovitz, J.; Yim, M.B.; Chock, P.B. Free radicals and disease. Arch. Biochem. Biophys. 2002, 397, 354–359. [Google Scholar] [PubMed]
- Lai, L.-S.; Chou, S.-T.; Chao, W.-W. Studies on the antioxidative activities of hsian-tsao (Mesona procumbens hemsl) leaf gum. J. Agric. Food Chem. 2001, 49, 963–968. [Google Scholar] [PubMed]
- Lee, J.-H.; Jin, H.; Shim, H.-E.; Kim, H.-N.; Ha, H.; Lee, Z.H. Epigallocatechin-3-gallate inhibits osteoclastogenesis by down-regulating c-fos expression and suppressing the nuclear factor-κb signal. Mol. Pharmacol. 2010, 77, 17–25. [Google Scholar] [PubMed]
- Lee, J.-W.; Ahn, J.-Y.; Hasegawa, S.-I.; Cha, B.-Y.; Yonezawa, T.; Nagai, K.; Seo, H.-J.; Jeon, W.-B.; Woo, J.-T. Inhibitory effect of luteolin on osteoclast differentiation and function. Cytotechnology 2009, 61, 125–134. [Google Scholar] [PubMed]
- Kim, M.H.; Ryu, S.Y.; Bae, M.A.; Choi, J.-S.; Min, Y.K.; Kim, S.H. Baicalein inhibits osteoclast differentiation and induces mature osteoclast apoptosis. Food Chem. Toxicol. 2008, 46, 3375–3382. [Google Scholar] [PubMed]
- Sakai, E.; Shimada-Sugawara, M.; Yamaguchi, Y.; Sakamoto, H.; Fumimoto, R.; Fukuma, Y.; Nishishita, K.; Okamoto, K.; Tsukuba, T. Fisetin inhibits osteoclastogenesis through prevention of rankl-induced ros production by NRF2-mediated up-regulation of phase ii antioxidant enzymes. J. Pharmacol. Sci. 2013, 121, 288–298. [Google Scholar] [PubMed]
- Lee, S.-H.; Jang, H.-D. Scoparone attenuates rankl-induced osteoclastic differentiation through controlling reactive oxygen species production and scavenging. Exp. Cell Res. 2015, 331, 267–277. [Google Scholar] [PubMed]
- Gironés-Vilaplana, A.; Valentão, P.; Andrade, P.B.; Ferreres, F.; Moreno, D.A.; García-Viguera, C. Phytochemical profile of a blend of black chokeberry and lemon juice with cholinesterase inhibitory effect and antioxidant potential. Food Chem. 2012, 134, 2090–2096. [Google Scholar] [PubMed]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [PubMed]
- Bonizzi, G.; Piette, J.; Schoonbroodt, S.; Greimers, R.; Havard, L.; Merville, M.-P.; Bours, V. Reactive oxygen intermediate-dependent NF-κB activation by interleukin-1β requires 5-lipoxygenase or nadph oxidase activity. Mol. Cell. Biol. 1999, 19, 1950–1960. [Google Scholar] [PubMed]
- Hall, T.; Schaeublin, M.; Jeker, H.; Fuller, K.; Chambers, T. The role of reactive oxygen intermediates in osteoclastic bone resorption. Biochem. Biophys. Res. Commun. 1995, 207, 280–287. [Google Scholar] [PubMed]
- Takayanagi, H. The role of nfat in osteoclast formation. Ann. N. Y. Acad. Sci. 2007, 1116, 227–237. [Google Scholar] [PubMed]
- Kwak, H.B.; Lee, B.K.; Oh, J.; Yeon, J.-T.; Choi, S.-W.; Cho, H.J.; Lee, M.S.; Kim, J.-J.; Bae, J.-M.; Kim, S.H. Inhibition of osteoclast differentiation and bone resorption by rotenone, through down-regulation of rankl-induced c-fos and nfatc1 expression. Bone 2010, 46, 724–731. [Google Scholar] [PubMed]
- Doyle, S.L.; Jefferies, C.A.; O’Neill, L.A. Bruton’s tyrosine kinase is involved in p65-mediated transactivation and phosphorylation of p65 on serine 536 during nfκb activation by lipopolysaccharide. J. Biol. Chem. 2005, 280, 23496–23501. [Google Scholar] [PubMed]
- Asagiri, M.; Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone 2007, 40, 251–264. [Google Scholar] [PubMed]
- Takayanagi, H. Mechanistic insight into osteoclast differentiation in osteoimmunology. J. Mol. Med. 2005, 83, 170–179. [Google Scholar] [PubMed]
- Wang, Z.-Q.; Ovitt, C.; Grigoriadis, A.E.; Möhle-Steinlein, U.; Rüther, U.; Wagner, E.F. Bone and haematopoietic defects in mice lacking c-fos. Nature 1992, 360, 741–745. [Google Scholar] [PubMed]
- Shinohara, M.; Koga, T.; Okamoto, K.; Sakaguchi, S.; Arai, K.; Yasuda, H.; Takai, T.; Kodama, T.; Morio, T.; Geha, R.S.; et al. Tyrosine kinases btk and tec regulate osteoclast differentiation by linking rank and itam signals. Cell 2008, 132, 794–806. [Google Scholar] [PubMed]
- Takayanagi, H.; Kim, S.; Taniguchi, T. Signaling crosstalk between rankl and interferons in osteoclast differentiation. Arthritis Res. Ther. 2002, 4, S227–S232. [Google Scholar]
- Teitelbaum, S.L.; Ross, F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003, 4, 638–649. [Google Scholar] [PubMed]
- Elzaawely, A.A.; Xuan, T.D.; Tawata, S. Antioxidant and antibacterial activities of rumex japonicus houtt. Aerial parts. Biol. Pharm. Bull. 2005, 28, 2225–2230. [Google Scholar] [PubMed]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 89, 569–575. [Google Scholar]
- Lee, J.H.; Whang, J.B.; Youn, N.R.; Lee, S.Y.; Lee, H.J.; Kim, Y.J.; Koh, K.H. Antioxidant and oxygen radical scavenging capacities of the extracts of pear cactus, mulberry and korean black raspberry fruits. Prev. Nutr. Food Sci. 2009, 14, 188–194. [Google Scholar]
- Jeong, C.-H.; Choi, G.-N.; Kim, J.-H.; Kwak, J.-H.; Heo, H.-J.; Shim, K.-H.; Cho, B.-R.; Bae, Y.-I.; Choi, J.-S. In vitro antioxidative activities and phenolic composition of hot water extract from different parts of cudrania tricuspidata. Prev. Nutr. Food Sci. 2009, 14, 283–289. [Google Scholar]
- Atmani, D.; Chaher, N.; Berboucha, M.; Ayouni, K.; Lounis, H.; Boudaoud, H.; Debbache, N.; Atmani, D. Antioxidant capacity and phenol content of selected algerian medicinal plants. Food Chem. 2009, 112, 303–309. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
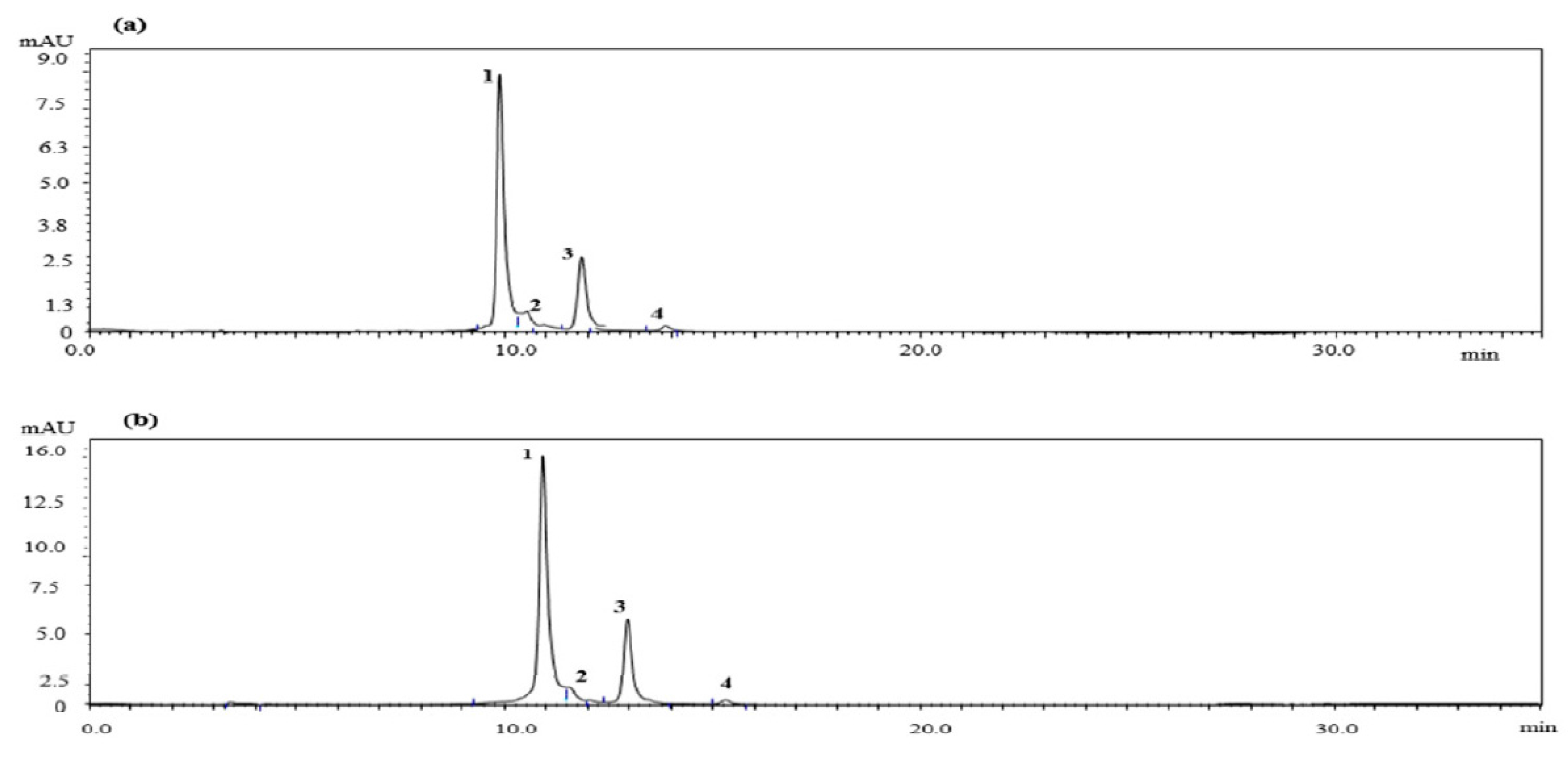


| Peak | Compound | Content (mg/g) | |
|---|---|---|---|
| AM-DW | AM-EtOH | ||
| 1 | Cyanidin-3-galactoside | 4.7131 | 9.9636 |
| 2 | Cyanidin-3-glucoside | 0.4560 | 0.9497 |
| 3 | Cyanidin-3-arabinoside | 1.2700 | 3.5179 |
| 4 | Cyanidin-3-xyloside | 0.1860 | 1.1423 |
| Extract | Total Phenolic (mg GAE †/100 g of Dry Mass) | Total Flavonoid (mg CE ‡/100 g of Dry Mass) | Yield (%) |
|---|---|---|---|
| AM-DW | 2617.25 ± 14.96 | 2004.99 ± 26.87 | 62.85 |
| AM-EtOH | 4004.46 ± 49.21 | 3896.11 ± 13.74 | 64.30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, M.; Kim, I.S.; Lee, Y.M.; Hong, S.M.; Lee, T.H.; Lim, J.H.; Debnath, T.; Lim, B.O. The Effects of Aronia melanocarpa ‘Viking’ Extracts in Attenuating RANKL-Induced Osteoclastic Differentiation by Inhibiting ROS Generation and c-FOS/NFATc1 Signaling. Molecules 2018, 23, 615. https://doi.org/10.3390/molecules23030615
Ghosh M, Kim IS, Lee YM, Hong SM, Lee TH, Lim JH, Debnath T, Lim BO. The Effects of Aronia melanocarpa ‘Viking’ Extracts in Attenuating RANKL-Induced Osteoclastic Differentiation by Inhibiting ROS Generation and c-FOS/NFATc1 Signaling. Molecules. 2018; 23(3):615. https://doi.org/10.3390/molecules23030615
Chicago/Turabian StyleGhosh, Mithun, In Sook Kim, Young Min Lee, Seong Min Hong, Taek Hwan Lee, Ji Hong Lim, Trishna Debnath, and Beong Ou Lim. 2018. "The Effects of Aronia melanocarpa ‘Viking’ Extracts in Attenuating RANKL-Induced Osteoclastic Differentiation by Inhibiting ROS Generation and c-FOS/NFATc1 Signaling" Molecules 23, no. 3: 615. https://doi.org/10.3390/molecules23030615




