Progress in Targeted Alpha-Particle Therapy. What We Learned about Recoils Release from In Vivo Generators
Abstract
:1. Introduction
2. Production of Alpha Emitters
3. General Radiopharmaceutical Issues
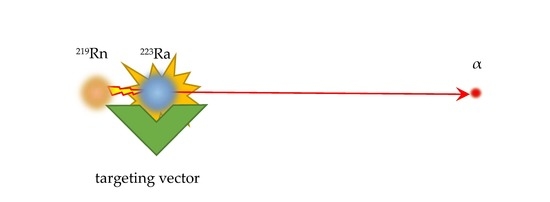
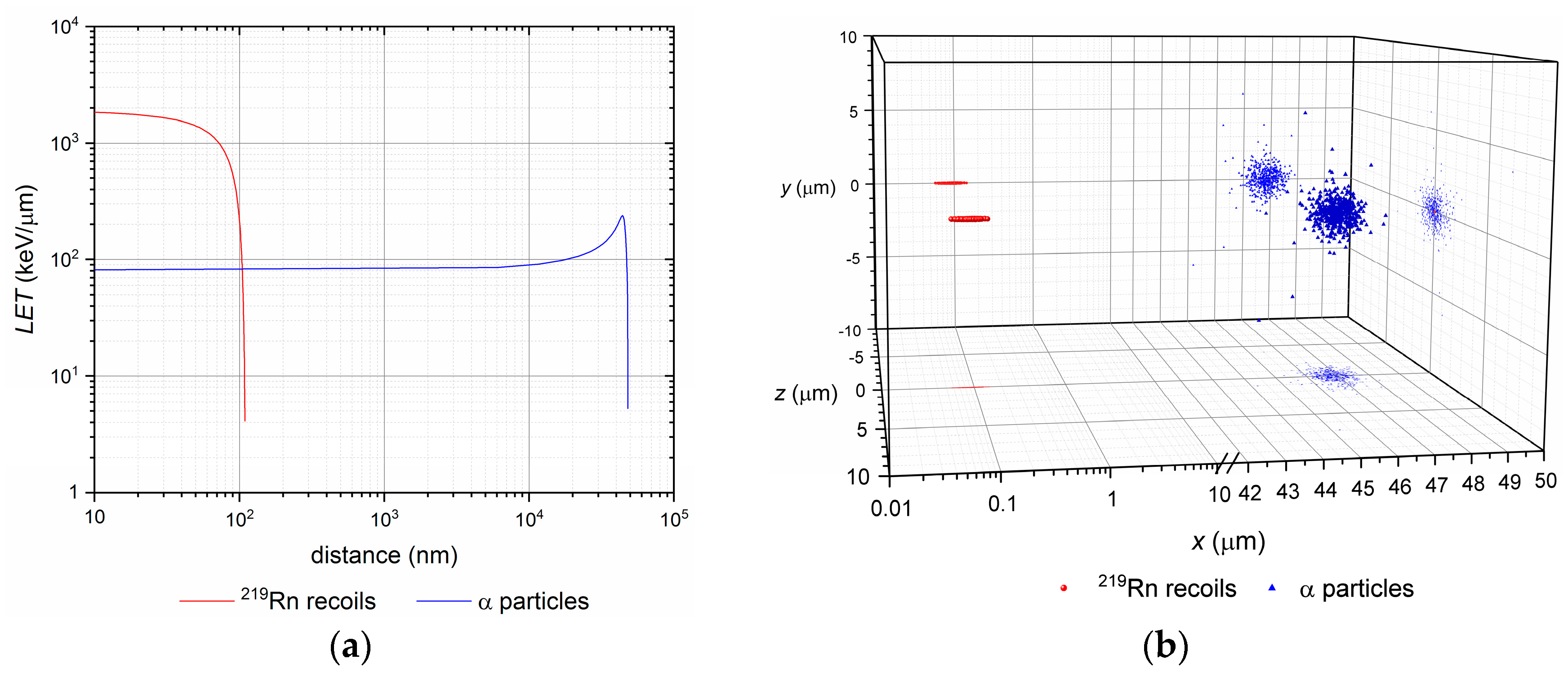
3.1. Nuclear Recoil Effect and the Release of Daughter Nuclei
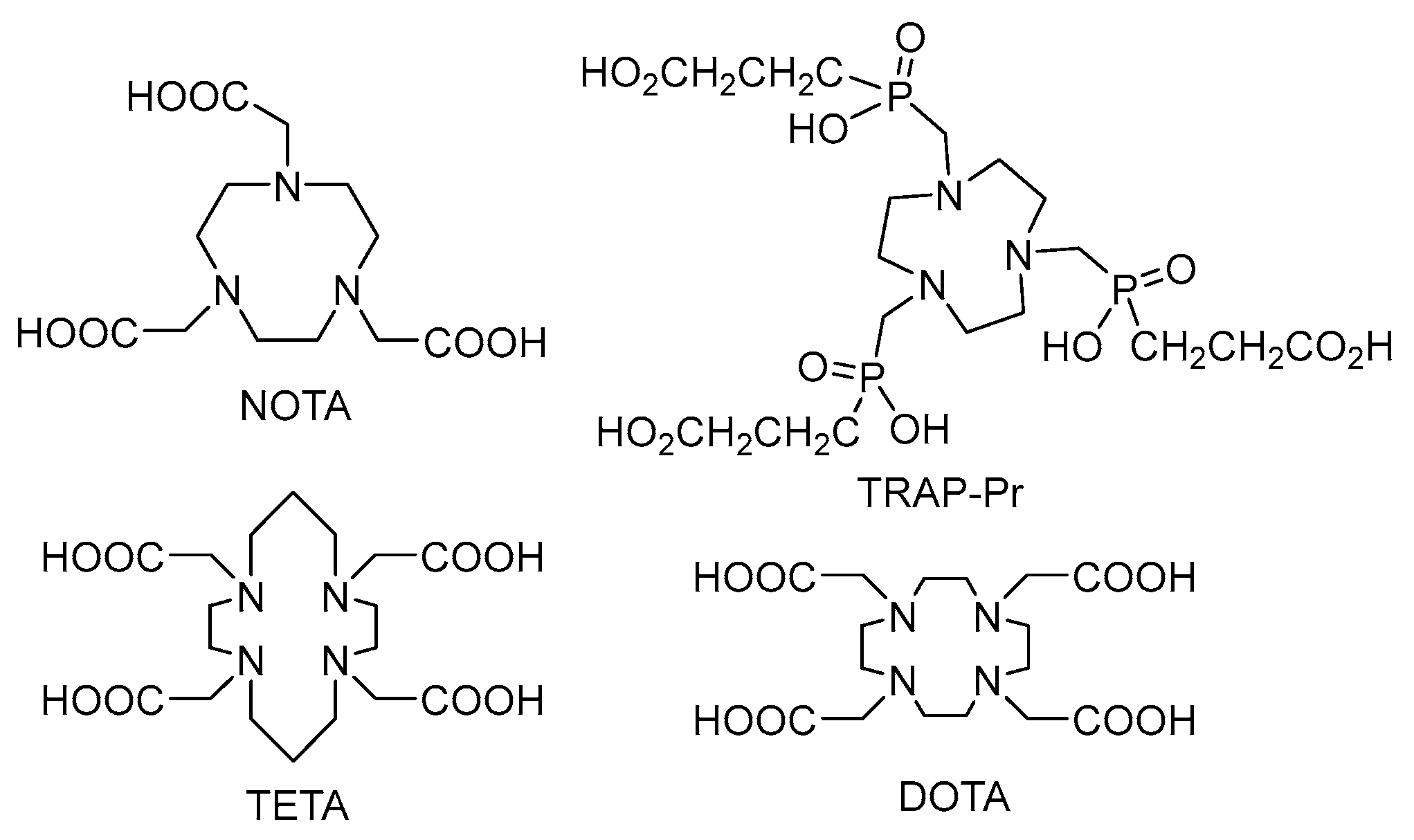
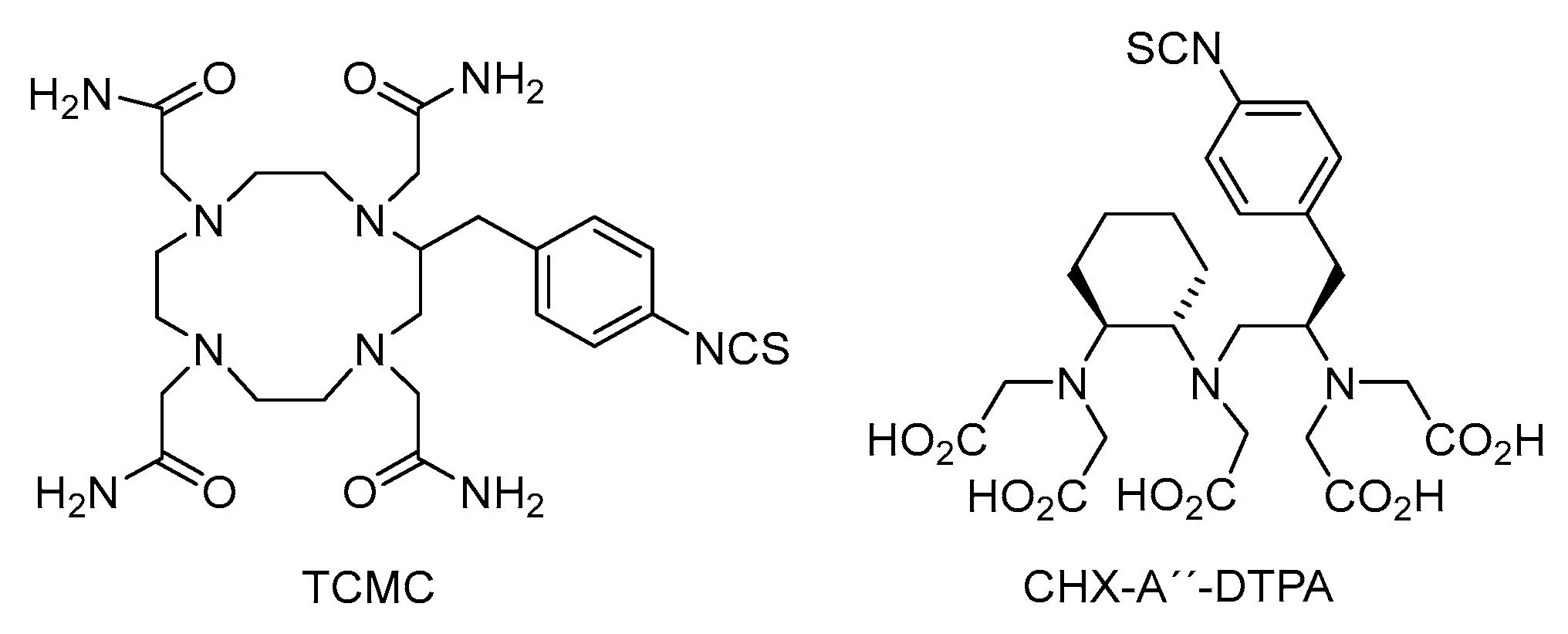
3.2. Labelling Chemistry
3.3. Targeting and Clearance
- “self-targeting” based on physiological affinity of the radioisotope to a given tissue; thus radium tends to accumulate in bones or pertechnetate, astatine or iodide in the thyroid;
- “passive targeting” or “blood circulation and extravasation” is based on accumulation of nanoparticles in the areas around the tumors with leaky vasculature; commonly referred to as the enhanced permeation and retention (EPR) effect [59];
- “active induced targeting” based on specific ligand-receptor interactions between labelled small molecules, peptides, mAbs and their fragments and target cells; externally activated exposure is also possible (temperature, magnetic field or other activators) [60].
3.4. Dosimetry
3.4.1. Body Level
3.4.2. Organ and Sub-Organ Levels
3.4.3. Cellular and Subcellular Level
4. Vectors for Targeted Alpha Particle Therapy
4.1. Small Molecules
4.1.1. MABG
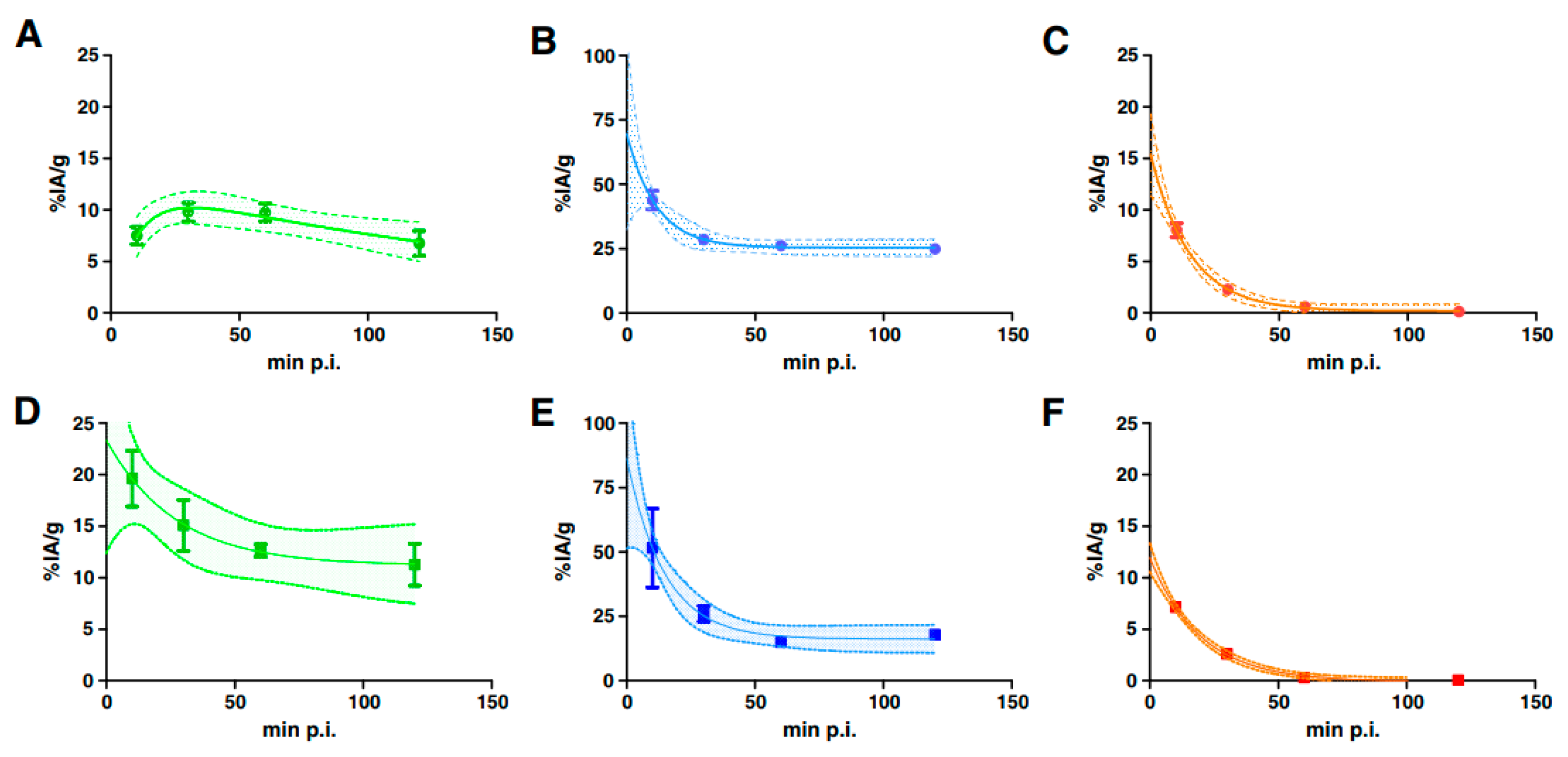
4.1.2. Prostate-Specific Membrane Antigen (PSMA)
4.1.3. Substance P
4.2. Biomolecules—Antibodies
4.3. Macromolecules and Nanoconstructs
5. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Song, H.; Senthamizhchelvan, S.; Hobbs, R.F.; Sgouros, G. Alpha Particle Emitter Radiolabeled Antibody for Metastatic Cancer: What Can We Learn from Heavy Ion Beam Radiobiology? Antibodies 2012, 1, 124–148. [Google Scholar] [CrossRef]
- Borchardt, P.E.; Yuan, R.R.; Miederer, M.; McDevitt, M.R.; Scheinberg, D.A. Targeted Actinium-225 In Vivo Generators for Therapy of Ovarian Cancer. Cancer Res. 2003, 63, 5084–5090. [Google Scholar] [PubMed]
- Jaggi, J.S.; Kappel, B.J.; McDevitt, M.R.; Sgouros, G.; Flombaum, C.D.; Cabassa, C.; Scheinberg, D.A. Efforts to control the errant products of a targeted in vivo generator. Cancer Res. 2005, 65, 4888–4895. [Google Scholar] [CrossRef] [PubMed]
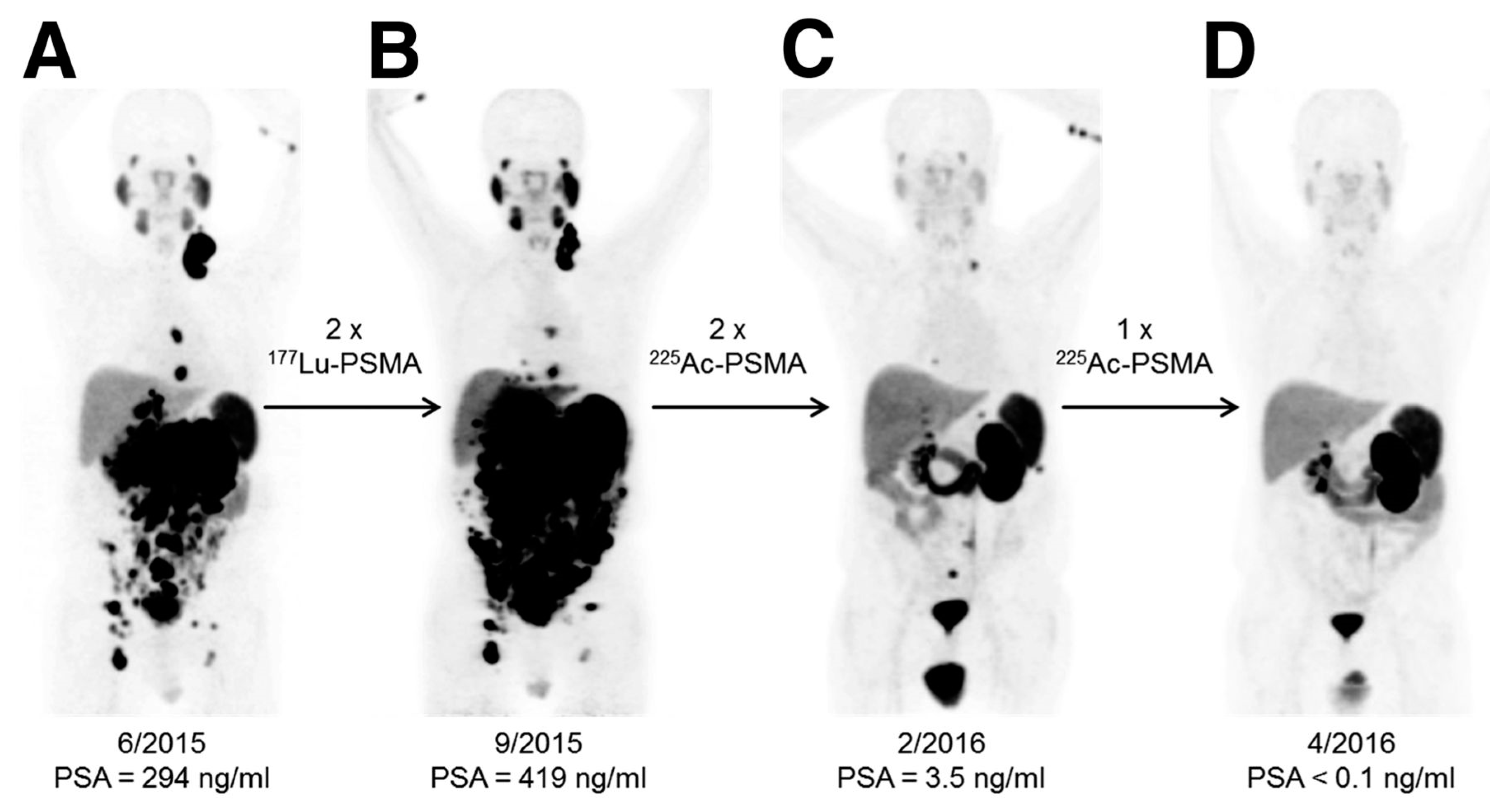
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Habekorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Woodward, J.; Kennel, S.J.; Stuckey, A.; Osborne, D.; Wall, J.; Rondinone, A.J.; Standaert, R.F.; Mirzadeh, S. LaPO4 nanoparticles doped with actinium-225 that partially sequester daughter radionuclides. Bioconj. Chem. 2011, 22, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Kozempel, J.; Vlk, M. Nanoconstructs in Targeted Alpha-Therapy. Rec. Pat. Nanomed. 2014, 4, 71–76. [Google Scholar] [CrossRef]
- Mokhodoeva, O.; Vlk, M.; Málková, E.; Kukleva, E.; Mičolová, P.; Štamberg, K.; Šlouf, M.; Dzhenloda, R.; Kozempel, J. Study of Ra-223 uptake mechanism by Fe3O4 nanoparticles: Towards new prospective theranostic SPIONs. J. Nanopart. Res. 2016, 18, 301. [Google Scholar] [CrossRef]
- Piotrowska, A.; Leszczuk, E.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Functionalized NaA nanozeolites labeled with Ra-224,Ra-225 for targeted alpha therapy. J. Nanopart. Res. 2013, 15, 2082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Máthé, D.; Szigeti, K.; Hegedűs, N.; Horváth, I.; Veres, D.S.; Kovács, B.; Szűcs, Z. Production and in vivo imaging of 203Pb as a surrogate isotope for in vivo 212Pb internal absorbed dose studies. Appl. Radiat. Isot. 2016, 114, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R. History and current uses of 224Ra in ankylosing spondylitis and other diseases. Environ. Int. 1993, 19, 467–473. [Google Scholar] [CrossRef]
- Nedrow, J.R.; Josefsson, A.; Park, S.; Back, T.; Hobbs, R.F.; Brayton, C.; Bruchertseifer, F.; Morgenstern, A.; Sgouros, G. Pharmacokinetics, microscale distribution, and dosimetry of alpha-emitter-labeled anti-PD-L1 antibodies in an immune competent transgenic breast cancer model. EJNMI Res. 2017, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Al Darwish, R.; Staudacher, A.H.; Li, Y.; Brown, M.P.; Bezak, E. Development of a transmission alpha particle dosimetry technique using A549 cells and a Ra-223 source for targeted alpha therapy. Med. Phys. 2016, 43, 6145–6153. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, N.L.; Graves, E.E. The Potential for Cerenkov luminescence imaging of alpha emitting isotopes. Phys. Med. Biol. 2012, 57, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Jaggi, J.S.; Seshan, S.V.; McDevitt, M.R.; Sgouros, G.; Hyjek, E.; Scheinberg, D.A. Mitigation of radiation nephropathy after internal α-particle irradiation of kidneys. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Dekempeneer, Y.; Keyaerts, M.; Krasniqi, A.; Puttemans, J.; Muyldermans, S.; Lahoutte, T.; D’huyvetter, M.; Devoogdt, N. Targeted alpha therapy using short-lived alpha-particles and the promise of nanobodies as targeting vehicle. Expert Opin. Biol. Ther. 2016, 16, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Kozempel, J.; Vlk, M.; Malková, E.; Bajzíková, A.; Bárta, J.; Santos-Oliveira, R.; Malta Rossi, A. Prospective carriers of 223Ra for targeted alpha particle therapy. J. Radioanal. Nucl. Chem. 2015, 304, 443–447. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Holzwarth, U.; Haberl, N.; Kozempel, J.; Hirn, S.; Wenk, A.; Schleh, C.; Schäffler, M.; Lipka, J.; Semmler-Behnke, M.; et al. Quantitative biokinetics of titanium dioxide nanoparticles after intravenous injection in rats: Part 1. Nanotoxicology 2017, 11, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Jekunen, A.; Kairemo, K.; Karnani, P. In Vivo Modulators of Antibody Kinetics. Acta Oncol. 1996, 35, 267–271. [Google Scholar] [CrossRef] [PubMed]
- McAlister, D.R.; Horwitz, E.P. Chromatographic generator systems for the actinides and natural decay series elements. Radiochim. Acta 2017, 99, 151–159. [Google Scholar] [CrossRef]
- Sobolev, A.S.; Aliev, R.A.; Kalmykov, S.N. Radionuclides emitting short-range particles and modular nanotransporters for their delivery to target cancer cells. Russ. Chem. Rev. 2016, 85, 1011–1032. [Google Scholar] [CrossRef]
- Steinber, E.P.; Stehney, A.F.; Stearns, C.; Spaletto, I. Production of 149Tb in gold by high-energy protons and its use as an intensity monitor. Nucl. Phys. A 1968, 113, 265–271. [Google Scholar] [CrossRef]
- Beyer, G.J.; Čomor, J.J.; Daković, M.; Soloviev, D.; Tamburella, C.; Hagebo, E.; Allan, B.; Dmitriev, S.N.; Zaitseva, N.G.; Starodub, G.Y.; et al. Production routes of the alpha emitting 149Tb for medical application. Radiochim. Acta 2002, 90, 247–252. [Google Scholar] [CrossRef]
- Lebeda, O.; Jiran, R.; Ráliš, J.; Štursa, J. A new internal target system for production of At-211 on the cyclotron U-120M. Appl. Radiat. Isot. 2005, 63, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Zalutsky, M.R.; Pruszynski, M. Astatine-211: Production and Availability. Curr. Radiopharm. 2011, 4, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, A.; Apostolidis, C.; Molinet, R.; Luetzenkirchen, K. Method for Producing Actinium-225. EP1610346 A1. 28 December 2005. [Google Scholar]
- Apostolidis, C.; Molinet, R.; Rasmussen, G.; Morgenstern, A. Production of Ac-225 from Th-229 for targeted alpha therapy. Anal. Chem. 2005, 77, 6288–6291. [Google Scholar] [CrossRef] [PubMed]
- Griswold, J.R.; Medvedev, D.G.; Engle, J.W.; Copping, R.; Fitzsimmons, J.M.; Radchenko, V.; Cooley, J.C.; Fassbender, M.E.; Denton, D.L.; Murphy, K.E.; et al. Large scale accelerator production of 225Ac: Effective cross sections for 78-192 MeV protons incident on Th-232 targets. Appl. Radiat. Isot. 2016, 118, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.; Henriksen, G. The Preparation and Use of Radium-223 to Target Calcified Tissues for Pain Palliation, Bone Cancer Therapy, and Bone Surface Conditioning. WO 2000/40275. 13 July 2000. [Google Scholar]
- Henriksen, G.; Hoff, P.; Alstad, J.; Larsen, R.H. 223Ra for endoradiotherapeutic applications prepared from an immobilized 227Ac/227Th source. Radiochim. Acta 2001, 89, 661–666. [Google Scholar] [CrossRef]
- Guseva, L.I.; Tikhomirova, G.S.; Dogadkin, N.N. Anion-exchange separation of radium from alkaline-earth metals and actinides in aqueous-methanol solutions of HNO3. 227Ac-223Ra generator. Radiochemistry 2004, 46, 58–62. [Google Scholar] [CrossRef]
- Shishkin, D.N.; Kupitskii, S.V.; Kuznetsov, S.A. Extraction generator of 223Ra for nuclear medicine. Radiochemistry 2011, 53, 343–345. [Google Scholar] [CrossRef]
- Schwarz, U.; Daniels, R. Novel Radiotherapeutic Formulations Containing 224Ra and a Method for Their Production. WO 2002/015943. 28 February 2002. [Google Scholar]
- Šebesta, F.; Starý, J. A generator for preparation of carrier-free 224Ra. J. Radioanal. Chem. 1974, 21, 151–155. [Google Scholar] [CrossRef]
- Larsen, R.H. Radiopharmaceutical Solutions with Advantageous Properties. WO 2016/135200. 1 September 2016. [Google Scholar]
- Ziegler, J.F. SRIM-2013 Code. Available online: http://www.srim.org/ (accessed on 11 November 2017).
- Frenvik, J.O.; Dyrstad, K.; Kristensen, S.; Ryan, O.B. Development of separation technology for the removal of radium-223 from targeted thorium conjugate formulations. Part I: Purification of decayed thorium-227 on cation exchange columns. Drug Dev. Ind. Pharm. 2017, 43, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Frenvik, J.O.; Dyrstad, K.; Kristensen, S.; Ryan, O.B. Development of separation technology for the removal of radium-223 from targeted thorium conjugate formulations. Part II: Purification of targeted thorium conjugates on cation exchange columns. Drug Dev. Ind. Pharm. 2017, 43, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, K.P.; Kalinina, M.K.; Levkovich, Y.I. Blood flow velocity in capillaries of brain and muscles and its physiological significance. Microvasc. Res. 1981, 22, 143–155. [Google Scholar] [CrossRef]
- Maheshwari, V.; Dearling, J.L.J.; Treves, S.T.; Packard, A.B. Measurement of the rate of copper(II) exchange for 64Cu complexes of bifunctional chelators. Inorg. Chim. Acta 2012, 393, 318–323. [Google Scholar] [CrossRef]
- Chakravarty, R.; Chakraborty, S.; Ram, R.; Vatsa, R.; Bhusari, P.; Shukla, J.; Mittal, B.R.; Dash, A. Detailed evaluation of different 68Ge/68Ga generators: An attempt toward achieving efficient 68Ga radiopharmacy. J. Label. Compd. Radiopharm. 2016, 59, 87. [Google Scholar] [CrossRef] [PubMed]
- Notni, J.; Plutnar, J.; Wester, H.J. Bone-seeking TRAP conjugates: Surprising observations and their implications on the development of gallium-68-labeled bisphosphonates. EJNMMI Res. 2012, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Holub, J.; Meckel, M.; Kubíček, V.; Rösch, F.; Hermann, P. Gallium(III) complexes of NOTA-bis (phosphonate) conjugates as PET radiotracers for bone imaging. Contrast Media Mol. Imaging 2015, 10, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.A.; Liu, Y.L.; Chen, C.Y.; Chou, X.M. Ligand Preorganization in Metal Ion Complexation: Molecular Mechanics/Dynamics, Kinetics, and Laser-Excited Luminescence Studies of Trivalent Lanthanide Complex Formation with Macrocyclic Ligands TETA and DOTA. Inorg. Chem. 2001, 40, 3448–3455. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.S.; de Blois, E.; Konijnenberg, M.; Morgenstern, A.; Bruchertseifer, F.; Breeman, W.; de Jong, M. Optimizing labeling conditions of 213Bi-somatostatin analogs for receptor-mediated processes in preclinical models. J. Nucl. Med. 2014, 55 (Suppl. 1), 1179. [Google Scholar]
- Ryan, O.B.; Cuthbertson, A.; Herstad, G.; Grant, D.; Bjerke, R.M. Development of effective chelators for Th-227 to be used in targeted thorium conjugates. In Proceedings of the 10th International Symposium on Targeted Alpha Therapy, Kanazawa, Japan, 30 May–1 June 2017; p. 57. [Google Scholar]
- Notni, J.; Pohle, K.; Wester, H.J. Comparative gallium-68 labeling of TRAP-, NOTA-, and DOTA-peptides: Practical consequences for the future of gallium68-PET. EJNMMI Res. 2012, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Simeček, J.; Hermann, P.; Wester, H.J.; Notni, J. How is 68Ga-labeling of macrocyclic chelators influenced by metal ion contaminants in 68Ge/68Ga generator eluates? ChemMedChem 2013, 8, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkom, U.; Morgenstern, A. 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: A first-in-human experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathekge, M.; Knoesen, O.; Meckel, M.; Modiselle, M.; Vorster, M.; Marx, S. 213Bi-PSMA-617 targeted alpha-radionuclide therapy in metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1099–1100. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Reber, J.; Haller, S.; Dorrer, H.; Köster, U.; Johnston, K.; Zhernosekov, K.; Türler, A.; Schibli, R. Folate Receptor Targeted Alpha-Therapy Using Terbium-149. Pharmaceuticals 2014, 7, 353–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, C.; Vermeulen, C.; Köster, U.; Johnston, K.; Türler, A.; Schibli, R.; Van der Meulen, N.P. Alpha-PET with terbium-149: Evidence and perspectives for radiotheragnostics. EJNMMI Radiopharm. Chem. 2016, 1, 5. [Google Scholar] [CrossRef]
- Beyer, G.J.; Miederer, M.; Vranješ-Durić, S.; Čomor, J.J.; Künzi, G.; Hartley, O.; Senekowitsch-Schmidtke, R.; Soloviev, D.; Buchegger, F. The ISOLDE Collaboration. Targeted alpha therapy in vivo: Direct evidence for single cancer cell kill using 149Tb-rituximab. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Hartman, K.B.; Hamlin, D.K.; Wilbur, D.S.; Wilson, L.J. 211AtCl@US-Tube Nanocapsules: A New Concept in Radiotherapeutic-Agent Design. Small 2007, 3, 1496–1499. [Google Scholar] [CrossRef] [PubMed]
- Kučka, J.; Hrubý, M.; Koňák, Č.; Kozempel, J.; Lebeda, O. Astatination of nanoparticles containing silver as possible carriers of 211At. Appl. Radiat. Isot. 2006, 64, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Leszczuk, E.; Piotrowska, A.; Bilewicz, A. Modified TiO2 nanoparticles as carries for At-211. J. Label. Compd. Radiopharm. 2013, 56, S242. [Google Scholar]
- Dziawer, L.; Koźmiński, P.; Męczyńska-Wielgosz, S.; Pruszyński, M.; Łyczko, M.; Wąs, B.; Celichowski, G.; Grobeny, J.; Jastrzębsky, J.; Bilewicz, A. Gold nanoparticle bioconjugates labelled with 211At for targeted alpha therapy. RSC Adv. 2017, 7, 41024–41032. [Google Scholar] [CrossRef]
- Chang, M.-Y.; Seideman, J.; Sofou, S. Enhanced Loading Efficiency and Retention of 225Ac in Rigid Liposomes for Potential Targeted Therapy of Micrometastases. Bioconj. Chem. 2008, 19, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Bharate, G.Y.; Daruwalla, J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur. J. Pharm. Biopharm. 2009, 71, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.H.; Park, K. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control. Release 2011, 153, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Baidoo, K.E.; Yong, K.; Brechbiel, M.W. Molecular Pathways: Targeted α-Particle Radiation Therapy. Clin. Cancer Res. 2013, 19, 530–537. [Google Scholar] [CrossRef] [PubMed]
- De Kruijff, R.M.; Wolterbeek, H.T.; Denkova, A.G. A Critical Review of Alpha Radionuclide Therapy—How to Deal with Recoiling Daughters? Pharmaceuticals 2015, 8, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.S.; Konijnenberg, M.W.; de Blois, E.; Koelewijn, S.; Baum, R.P.; Morgenstern, A.; Bruchertseifer, F.; Breeman, W.A.; de Jong, M. Influence of tumour size on the efficacy of targeted alpha therapy with 213Bi-[DOTA0,Tyr3]-octreotate. EJNMMI Res. 2016, 6, 6–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, E.Y.; Abbas Rizvi, S.M.; Qu, C.F.; Raja, C.; Brechbiel, M.W.; Morgenstern, A.; Apostolidis, C.; Allen, B.J. Pharmacokinetics and toxicity of 213Bi-labeled PAI2 in preclinical targeted alpha therapy for cancer. Cancer Biol. Ther. 2007, 6, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Nedrow, J.R.; Josefsson, A.; Park, S.; Hobbs, R.F.; Bruchertseifer, F.; Morgenstern, A.; Sgouros, G. Reducing renal uptake of free 213Bi associated with the decay of 225Ac-labeled radiopharmaceuticals. In Proceedings of the 10th International Symposium on Targeted Alpha Therapy, Kanazawa, Japan, 30 May–1 June 2017; p. 67. [Google Scholar]
- Hanadate, S.; Washiyama, K.; Yoshimoto, M.; Matsumoto, H.; Tsuji, A.; Higashi, T.; Yoshii, Y. Oral administration of barium sulfate reduces radiation exposure to the large intestine during alpha therapy with radium-223 dichloride. J. Nucl. Med. 2017, 58 (Suppl. l), 1030. [Google Scholar]
- Edem, P.E.; Fonslet, J.; Kjaer, A.; Herth, M.; Severin, G. In Vivo Radionuclide Generators for Diagnostics and Therapy. Bioinorg. Chem. Appl. 2016, 6148357. [Google Scholar] [CrossRef] [PubMed]
- Hindorf, C.; Chittenden, S.; Aksnes, A.K.; Parker, C.; Flux, G.D. Quantitative imaging of 223Ra-chloride (Alpharadin) for targeted alpha-emitting radionuclide therapy of bone metastases. Nucl. Med. Commun. 2012, 33, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.K.H.; Ramogida, C.F.; Rodriguez-Rodriguez, C.; Blinder, S.; Kunz, P.; Sossi, V.; Schaffer, P. Multi-isotope SPECT imaging of the Ac-225 decay chain: Feasibility studies. Phys. Med. Biol. 2017, 62, 4406–4420. [Google Scholar] [CrossRef] [PubMed]
- Bäck, T.; Jacobsson, L. The α-Camera: A Quantitative Digital Autoradiography Technique Using a Charge-Coupled Device for Ex Vivo High-Resolution Bioimaging of α-Particles. J. Nucl. Med. 2010, 51, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Altman, M.B.; Wang, S.J.; Whitlock, J.L.; Roeske, J.C. Cell detection in phase-contrast images used for alpha-particle track-etch dosimetry: A semi-automated approach. Phys. Med. Biol. 2005, 50, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Muggiolu, G.; Pomorski, M.; Claverie, G.; Berthet, G.; Mer-Calfati, C.; Saada, S.; Devès, G.; Simon, M.; Seznec, H.; Barberet, P. Single α-particle irradiation permits real-time visualization of RNF8 accumulation at DNA damaged sites. Sci. Rep. 2017, 7, 41764. [Google Scholar] [CrossRef] [PubMed]
- Gholami, Y.; Zhu, X.; Fulton, R.; Meikle, S.; El-Fakhri, G.; Kuncic, Z. Stochastic simulation of radium-223 dichloride therapy at the sub-cellular level. Phys. Med. Biol. 2015, 60, 6087–6096. [Google Scholar] [CrossRef] [PubMed]
- Roeske, J.C.; Stinchcomb, T.G. The average number of alpha-particle hits to the cell nucleus required to eradicate a tumour cell population. Phys. Med. Biol. 2006, 51, N179–N186. [Google Scholar] [CrossRef] [PubMed]
- Lazarov, E.; Arazi, L.; Efrati, M.; Cooks, T.; Schmidt, M.; Keisari, Y.; Kelson, I. Comparative in vitro microdosimetric study of murine- and human-derived cancer cells exposed to alpha particles. Radiat. Res. 2012, 177, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Batra, V.; Ranieri, P.; Makvandi, M.; Tsang, M.; Hou, C.; Li, Y.; Vaidyanathan, G.; Pryma, D.A.; Maris, J.M. Development of meta-[211At]astatobenzylguanidine ([211At]MABG) as an alpha particle emitting systemic targeted radiotherapeutic for neuroblastoma. Cancer Res. 2015, 75 (Suppl. 15), 1610. [Google Scholar] [CrossRef]
- Ohshima, Y.; Watanabe, S.; Tsuji, A.; Nagatsu, K.; Sakashima, T.; Sugiyama, A.; Harada, Y.; Waki, A.; Yoshinaga, K.; Ishioka, N. Therapeutic efficacy of α-emitter meta-211At-astato-benzylguanidine (MABG) in a pheochromocytoma model. J. Nucl. Med. 2016, 57 (Suppl. 2), 468. [Google Scholar]
- Vaidyanathan, G.; Affleck, D.J.; Alston, K.L.; Zhao, X.-G.; Hens, M.; Hunter, D.H.; Babich, J.; Zalutsky, M.R. A Kit Method for the High Level Synthesis of [211At]MABG. Bioorg. Med. Chem. 2007, 15, 3430–3436. [Google Scholar] [CrossRef] [PubMed]
- Nonnekens, J.; Chatalic, K.L.S.; Molkenboer-Kuenen, J.D.M.; Beerens, C.E.M.T.; Bruchertseifer, F.; Morgenstern, A.; Veldhoven-Zweistra, J.; Schottelius, M.; Wester, H.-J.; van Gent, D.C.; et al. 213Bi-Labeled Prostate-Specific Membrane Antigen-Targeting Agents Induce DNA Double-Strand Breaks in Prostate Cancer Xenografts. Cancer Biother. Radiopharm. 2017, 32, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Krolicki, L.; Bruchertseifer, F.; Kunikowska, J.; Koziara, H.; Królicki, B.; Jakuciński, M.; Pawlak, D.; Apostolidis, C.; Rola, R.; Merlo, A.; et al. Targeted alpha therapy of glioblastoma multiforme: Clinical experience with 213Bi- and 225Ac-Substance P. In Proceedings of the 10th International Symposium on Targeted Alpha Therapy, Kanazawa, Japan, 30 May–1 June 2017; p. 24. [Google Scholar]
- Cordier, D.; Krolicki, L.; Morgenstern, A.; Merlo, A. Targeted Radiolabeled Compounds in Glioma Therapy. Semin. Nucl. Med. 2016, 46, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Marcu, L.; Bezak, E.; Allen, B.J. Global comparison of targeted alpha vs targeted beta therapy for cancer: In vitro, in vivo and clinical trials. Crit. Rev. Oncol. Hematol. 2018, 123, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Aghevlian, S.; Boyle, A.J.; Reilly, R.M. Radioimmunotherapy of cancer with high linear energy transfer (LET) radiation delivered by radionuclides emitting α-particles or Auger electrons. Adv. Drug Deliv. Rev. 2017, 109, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Jurcic, J.; Scheinberg, D. Efficacy of Ac-225-labeled anti-CD33 antibody in acute myeloid leukemia (AML) correlates with peripheral blast count. In Proceedings of the 10th International Symposium on Targeted Alpha Therapy, Kanazawa, Japan, 30 May–1 June 2017; p. 22. [Google Scholar]
- Actinium Pharmaceuticals Provides Update on Actimab-A Phase 2 Clinical Trial for Patients with Acute Myeloid Leukemia. Available online: https://ir.actiniumpharma.com/press-releases/detail/247 (accessed on 31 December 2017).
- Autenrieth, M.E.; Horn, T.; Kurtz, F.; Nguyen, K.; Morgenstern, A.; Bruchertseifer, F.; Schwaiger, M.; Blechert, M.; Seidl, C.; Senekowitsch-Schmidtke, R.; et al. Intravesical radioimmunotherapy of carcinoma in situ of the urinary bladder after BCG failure. Urol. A 2017, 56, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Tworowska, I.; Stallons, T.; Saidi, A.; Wagh, N.; Rojas-Quijano, F.; Jurek, P.; Kiefer, G.; Torgue, J.; Delpassand, E. Pb203-AR-RMX conjugates for image-guided TAT of neuroendocrine tumors (NETs). In Proceedings of the American Association for Cancer Research Annual Meeting 2017, Washington, DC, USA, 1–5 April 2017. [Google Scholar] [CrossRef]
- RadioMedix and AREVA Med Announce Initiation of Phase 1 Clinical Trial of AlphaMedixTM, a Targeted Alpha Therapy for Patients with Neuroendocrine Tumors. Available online: http://radiomedix.com/news/radiomedix-and-areva-med-announce-initiation-of-phase-1-clinical-trial-of-alphamedixtm-a-targeted-alpha-therapy-for-patients-with-neuroendocrine-tumors (accessed on 31 December 2017).
- Dadachova, E.; Morgenstern, A.; Bruchertseifer, F.; Rickles., D.J. Radioimmunotherapy with novel IgG to melanin and its comparison with immunotherapy. J. Nucl. Med. 2017, 58 (Suppl. 1), 1036. [Google Scholar]
- Boudousq, V.; Bobyk, L.; Busson, M.; Garambois, V.; Jarlier, M.; Charalambatou, P.; Pèlegrin, A.; Paillas, S.; Chouin, N.; Quenet, F.; et al. Comparison between Internalizing Anti-HER2 mAbs and Non-Internalizing Anti-CEA mAbs in Alpha-Radioimmunotherapy of Small Volume Peritoneal Carcinomatosis Using 212Pb. PLoS ONE 2013, 8, e69613. [Google Scholar] [CrossRef] [PubMed]
- Ballangrud, Å.M.; Yang, W.-H.; Palm, S.; Enmon, R.; Borchardt, P.E.; Pellegrini, V.A.; McDevitt, M.R.; Scheinberg, D.A.; Sgouros, G. Alpha-Particle Emitting Atomic Generator (Actinium-225)-Labeled Trastuzumab (Herceptin) Targeting of Breast Cancer Spheroids. Clin. Cancer Res. 2004, 10, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Boskovitz, A.; McLendon, R.E.; Okamura, T.; Sampson, J.H.; Bigner, D.D.; Zalutsky, M.R. Treatment of HER2-positive breast carcinomatous meningitis with intrathecal administration of α-particle-emitting 211At-labeled trastuzumab. Nucl. Med. Biol. 2009, 36, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Pruszyński, M.; D’Huyvetter, M.; Cędrowska, E.; Lahoutte, T.; Bruchertseifer, F.; Morgenstern, A. Preclinical evaluation of anti-HER2 2Rs15d nanobody labeled with 225Ac. In Proceedings of the 10th International Symposium on Targeted Alpha Therapy, Kanazawa, Japan, 30 May–1 June 2017; p. 34. [Google Scholar]
- Dekempeneer, Y.; D’Huyvetter, M.; Aneheim, E.; Xavier, C.; Lahoutte, T.; Bäck, T.; Jensen, H.; Caveliers, V.; Lindegren, S. Preclinical evaluation of astatinated nanobodies for targeted alpha therapy. In Proceedings of the 10th International Symposium on Targeted Alpha Therapy, Kanazawa, Japan, 30 May–1 June 2017; p. 35. [Google Scholar]
- Puentes, L.; Xu, K.; Hou, C.; Mach, R.H.; Maris, J.M.; Pryma, D.A.; Makvandi, M. Targeting PARP-1 to deliver alpha-particles to cancer chromatin. In Proceedings of the American Association for Cancer Research Annual Meeting 2017, Washington, DC, USA, 1–5 April 2017. [Google Scholar] [CrossRef]
- Carrasquillo, J.A. Alpha Radionuclide Therapy: Principles and Applications to NETs. In Diagnostic and Therapeutic Nuclear Medicine for Neuroendocrine Tumors; Pacak, K., Taïeb, D., Eds.; Humana Press: Cham, Switzerland, 2017; pp. 429–445. [Google Scholar]
- Norain, A.; Dadachova, E. Targeted Radionuclide Therapy of Melanoma. Semin. Nucl. Med. 2016, 46, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Banerjee, S. Envisaging an alpha therapy programme in the atomic energy establishments: The priorities and the nuances. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1244–1246. [Google Scholar] [CrossRef] [PubMed]
- Koziorowski, J.; Stanciu, A.E.; Gomez-Vallejo, V.; Llop, J. Radiolabeled nanoparticles for cancer diagnosis and therapy. Anticancer Agents Med. Chem. 2017, 17, 333–354. [Google Scholar] [CrossRef] [PubMed]
- Beeler, E.; Gabani, P.; Singh, O.M. Implementation of nanoparticles in therapeutic radiation oncology. J. Nanopart. Res. 2017, 19, 179. [Google Scholar] [CrossRef]
- Drude, N.; Tienken, L.; Mottaghy, F.M. Theranostic and nanotheranostic probes in nuclear medicine. Methods 2017, 130, 14–22. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.F.; Robertson, D.; Pevsner, P.H.; Wall, J.S.; Mirzadeh, S.; Kennel, S.J. LnPO4 Nanoparticles Doped with Ac-225 and Sequestered Daughters for Targeted Alpha Therapy. Cancer Biother. Radiopharm. 2014, 29, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.V.; Woodward, J.D.; Chen, N.; Rondinone, A.J.; Castano, C.H.; Mirzadeh, S. Synthesis and characterization of lanthanum phosphate nanoparticles as carriers for Ra-223 and Ra-225 for targeted alpha therapy. Nucl. Med. Biol. 2015, 42, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Salberg, G.; Larsen, R. Alpha-Emitting Hydroxyapatite Particles. WO 2005/079867. 1 September 2015. [Google Scholar]
- Westrøm, S.; Bønsdorff, T.B.; Bruland, Ø.; Larsen, R. Therapeutic Effect of α-Emitting Ra-Labeled Calcium Carbonate Microparticles in Mice with Intraperitoneal Ovarian Cancer. Transl. Oncol. 2018, 11, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, S.; Majkowska-Pilip, A.; Bilewicz, A.; Dobrowolski, J.C. On AunAt clusters as potential astatine carriers. RSC Adv. 2017, 7, 35854–35857. [Google Scholar] [CrossRef]
- Piotrowska, A.; Męczyńska-Wielgosz, S.; Majkowska-Pilip, A.; Koźmiński, P.; Wójciuk, G.; Cędrowska, E.; Bruchertseifer, F.; Morgenstern, A.; Kruszewski, M.; Bilewicz, A. Nanozeolite bioconjugates labeled with 223Ra for targeted alpha therapy. Nucl. Med. Biol. 2017, 47, 10–18. [Google Scholar] [CrossRef] [PubMed]
- De Kruijff, R.M.; Drost, K.; Thijssen, L.; Morgenstern, A.; Bruchertseifer, F.; Lathouwers, D.; Wolterbeek, H.T.; Denkova, A.G. Improved 225Ac daughter retention in LnPO4 containing polymersomes. Appl. Radiat. Isot. 2017, 128, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Bandekar, A.; Sempkowski, M.; Banerjee, S.R.; Pomper, M.G.; Bruchertseifer, F.; Morgenstern, A.; Sofou, S. Nanoconjugation of PSMA-targeting ligands enhances perinuclear localization and improves efficacy of delivered alpha-particle emitters against tumor endothelial analogues. Mol. Cancer Ther. 2016, 15, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Sempkowski, M.; Holleran, T.; Linz, T.; Bertalan, T.; Josefsson, A.; Bruchertseifer, F.; Morgenstern, A.; Sofou, S. Alpha-particle radiotherapy: For large solid tumors diffusion trumps targeting. Biomaterials 2017, 130, 67–75. [Google Scholar] [CrossRef] [PubMed]
| Radionuclide System * | Half-Life | Eαmax/Echain [MeV] | Production | Status | References |
|---|---|---|---|---|---|
| 149Tb | 4.12 h | 3.97 | 152Gd(p, 4n)149Tb | Research | [22,23] |
| 211At | 7.2 h | 5.87 | 209Bi(α, 3n)211At | Clinical trials | [24,25] |
| 229Th/ | 7340 years | 5.83/27.62 | 229Th decay | Clinical trials | [26,27,28] |
| 225Ac// | 10 days | 226Ra(p, 2n)225Ac | |||
| 213Bi | 46 min | 232Th(p, x)225Ac | |||
| 227Ac/ | 27 years | 5.87/26.70 | 227Ac/227Th/223Ra decay | Clinical praxis | [29,30,31,32] |
| 227Th/ | 18 days | ||||
| 223Ra | 11 days | ||||
| 228Th/ | 1.9 years | 5.69/27.54 | 228Th/224Ra decay | Formerly in c.p., Research | [33,34,35] |
| 224Ra/ | 3.7 days | ||||
| 212Pb | 10.6 h |
| Material | Range (nm) |
|---|---|
| Au | 11 |
| ZrO2 ICRU-712 | 26 |
| Al2O3 ICRU-106 | 27 |
| TiO2 ICRU-652 | 28 |
| SiO2 ICRU-245 | 46 |
| adult cortical bone | 53 |
| human blood | 85 |
| prostate tissue | 87 |
| water | 89 |
| nitrogen gas | 76,000 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozempel, J.; Mokhodoeva, O.; Vlk, M. Progress in Targeted Alpha-Particle Therapy. What We Learned about Recoils Release from In Vivo Generators. Molecules 2018, 23, 581. https://doi.org/10.3390/molecules23030581
Kozempel J, Mokhodoeva O, Vlk M. Progress in Targeted Alpha-Particle Therapy. What We Learned about Recoils Release from In Vivo Generators. Molecules. 2018; 23(3):581. https://doi.org/10.3390/molecules23030581
Chicago/Turabian StyleKozempel, Ján, Olga Mokhodoeva, and Martin Vlk. 2018. "Progress in Targeted Alpha-Particle Therapy. What We Learned about Recoils Release from In Vivo Generators" Molecules 23, no. 3: 581. https://doi.org/10.3390/molecules23030581