The Multivariate Regression Statistics Strategy to Investigate Content-Effect Correlation of Multiple Components in Traditional Chinese Medicine Based on a Partial Least Squares Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals
2.3. Cell Cultureand Hypoxia
2.4. Instruments
2.5. Assay of Individual Saponin Combinations (Example 1)
2.5.1. The Preparation of the Panax ginseng Saponins Extract
2.5.2. The Preparation of Plasma Dilutions
2.5.3. The Preparation of Phosphate Buffer Solutions
2.5.4. The Preparation of ADP-2Na
2.5.5. Determination of Anti-Platelet Aggregation
2.5.6. The Comparison of Anti-Platelet Aggregation between the Panax ginseng Saponins Extract and Individual Saponin Combinations
2.5.7. The Anti-Platelet Aggregation of Different Individual Saponin Combinations
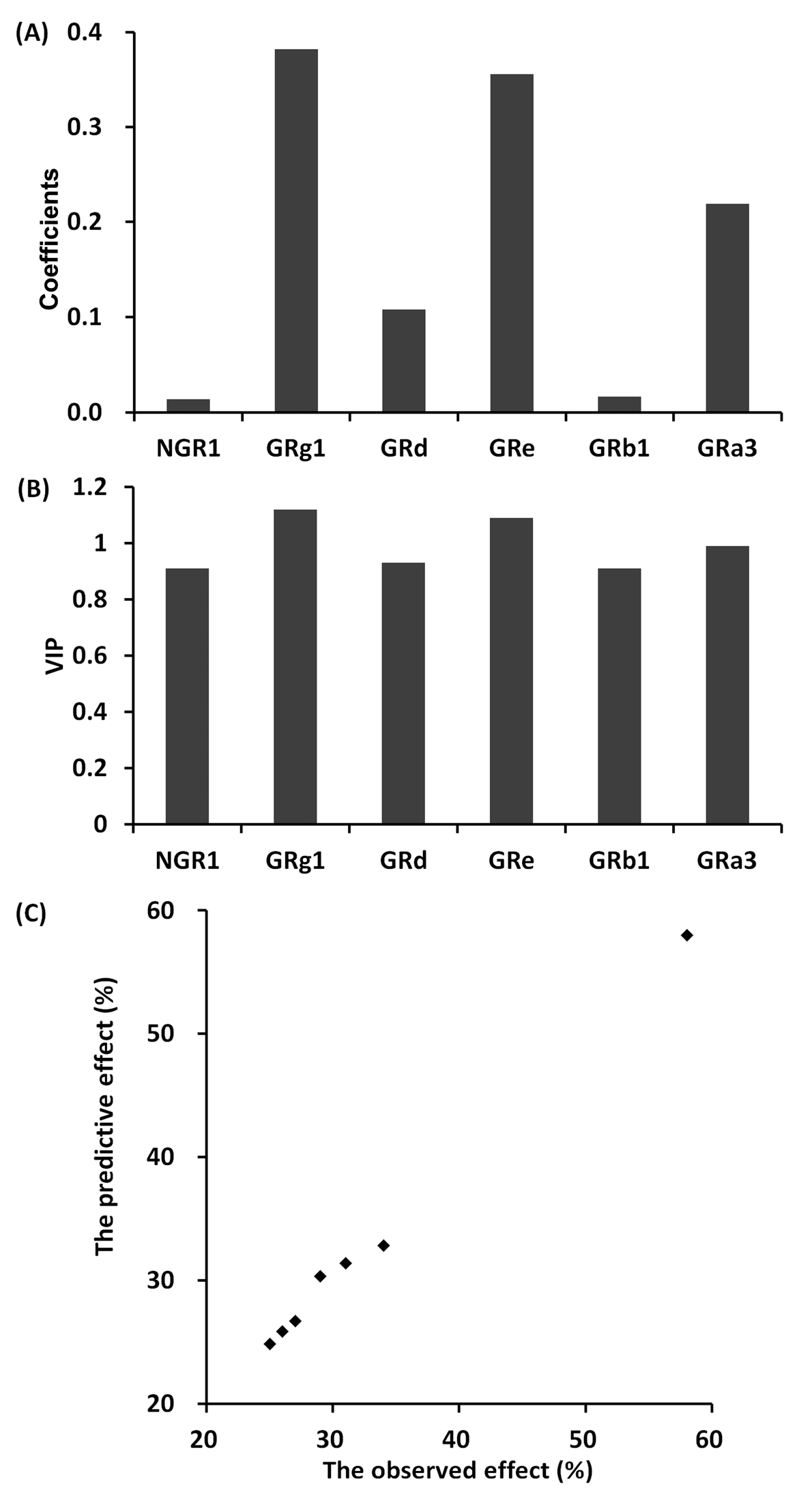
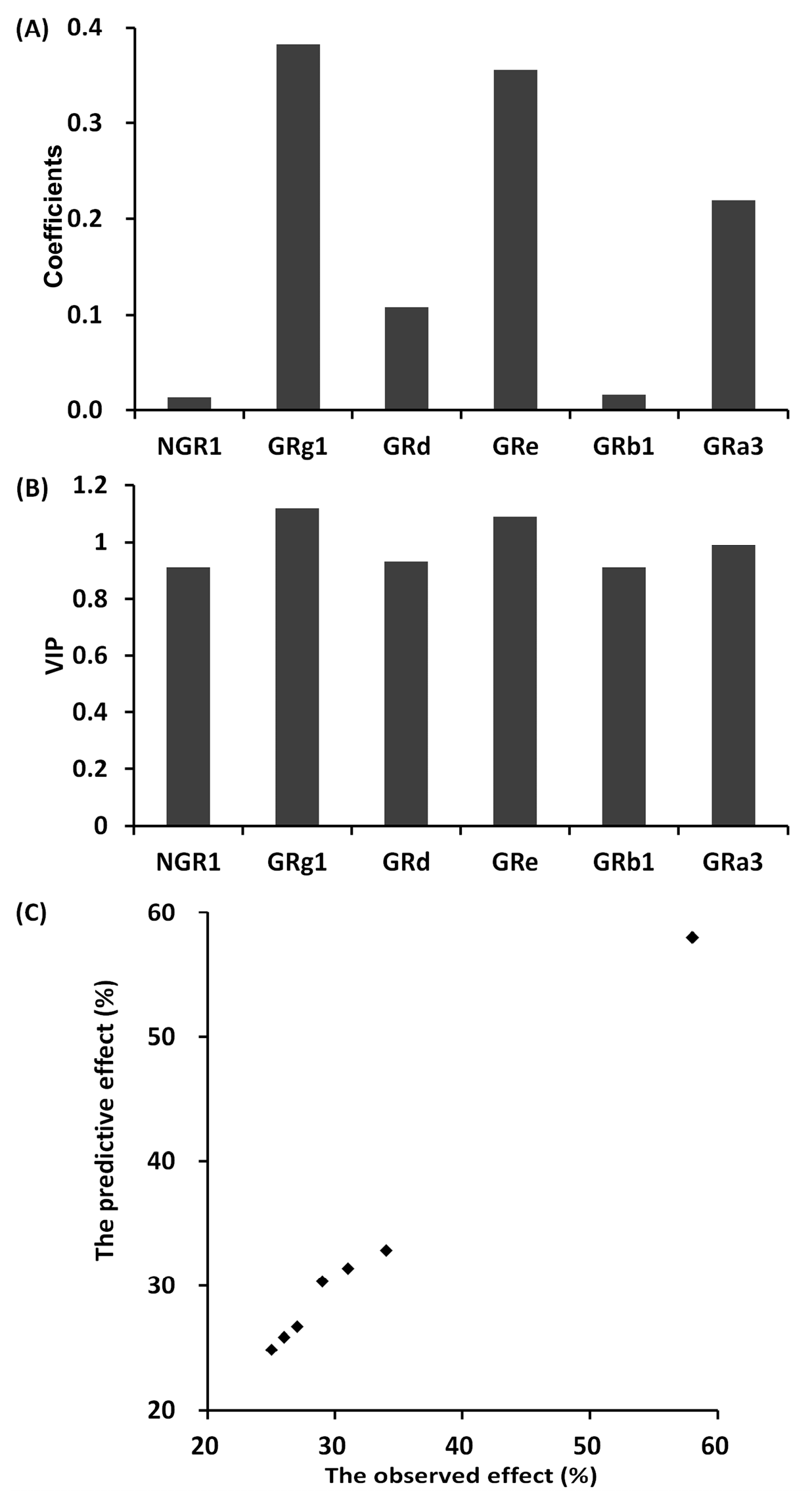
2.5.8. The Partial Least Squares Method between Different Individual Saponin Combinations and Anti-Platelet Aggregation Effect
2.6. Assay of Fingerprint (Example 2)
2.6.1. The Fingerprint Studies of Panax ginseng Saponins Extract from Different Origins
2.6.2. Cell Treatment
2.6.3. MTT Assay
2.6.4. Animal Studies
2.6.5. Determination of cTnI, CK and LDH
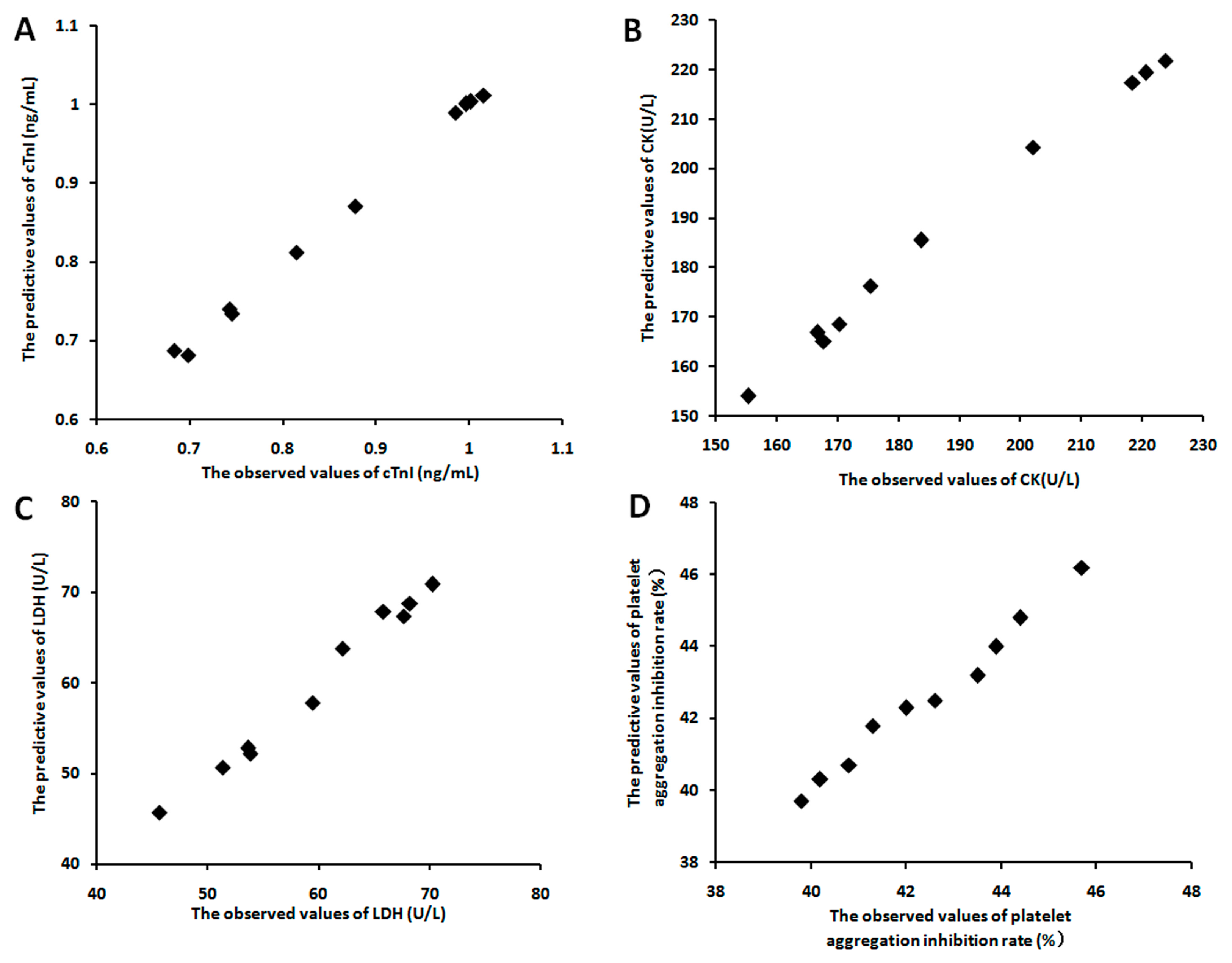
2.6.6. The Relevance Analysis between Fingerprint and Drug Effects
3. Results
3.1. Results of Individual Saponins Combination (Example 1)
3.1.1. The Comparison of Anti-Platelet Aggregation between Panax ginseng Saponins Extract and Individual Saponins Combination
3.1.2. The Anti-Platelet Aggregation Effect of Different Individual Saponin Combinations
3.2. Results of Fingerprint Studies (Example 2)
3.2.1. The Fingerprint Studies of Panax ginseng Saponins Extract from Different Origins
3.2.2. The Effects of Panax ginseng Saponins Extract from Different Origins
3.2.3. The Relevance between the Fingerprints and the Effects
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cui, X.; Wang, Y.; Kokudo, N.; Fang, D.; Tang, W. Traditional Chinese Medicine and related active compounds against hepatitis b virus infection. Biosci. Trends 2010, 4, 39–47. [Google Scholar] [PubMed]
- Zhang, L.; Schuppan, D. Traditional Chinese Medicine (TCM) for fibrotic liver disease: Hope and hype. J. Hepatol. 2014, 61, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.E.; Hung, Y.C.; Chang, K.C.; Shih, C.C.; Hung, J.W.; Liu, C.W.; Tan, T.Y.; Huang, C.C. Favorable circulatory system outcomes as adjuvant Traditional Chinese Medicine (TCM) treatment for cerebrovascular diseases in taiwan. PLoS ONE 2014, 9, e86351. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Santangelo, R. Panax ginseng and Panax quinquefolius: From pharmacology to toxicology. Food Chem. Toxicol. 2017, 107, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; Zhang, S.C.; Wen, L.; Mu, L.; Yang, F.; Chen, G. In vivo distribution and pharmacokinetics of multiple active components from danshen and sanqi and their combination via inner ear administration. J. Ethnopharmacol. 2014, 156, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Li, Y.; Wang, L.; Xue, M. Simultaneous determination of danshensu, rosmarinic acid, cryptotanshinone, tanshinone IIA, tanshinone I and dihydrotanshinone I by liquid chromatographic–mass spectrometry and the application to pharmacokinetics in rats. J. Pharm. Biomed. Anal. 2010, 53, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liu, Q.; Dou, Y.; Hsieh, Y.; Liu, Y.; Tao, R.; Zhu, D.; Lou, Y. Activating glucocorticoid receptor-erk signaling pathway contributes to ginsenoside rg1 protection against beta-amyloid peptide-induced human endothelial cells apoptosis. J. Ethnopharmacol. 2013, 147, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Jo, I.H.; Kim, Y.C.; Dong, H.K.; Kim, K.H.; Hyun, T.K.; Ryu, H.; Bang, K.H. Applications of molecular markers in the discrimination of panax species and korean ginseng cultivars (panax ginseng). J. Ginseng Res. 2017, 41, 444. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.J.; Wang, Z.; Hu, W.P.; Xu, S. Fast determination of trace dimethyl fumarate in milk with near infrared spectroscopy following fluidized bed enrichment. Anal. Bioanal. Chem. 2012, 404, 3189–3194. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Otsuka, M. Modeling of feed-forward control using the partial least squares regression method in the tablet compression process. Int. J. Pharm. 2017, 524, 407–413. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhou, J.; Cheng, H.; Wang, L.; Wei, K.; Wang, W.; Li, X. Validation of origins of tea samples using partial least squares analysis and euclidean distance method with near-infrared spectroscopy data. Spectrochim. Acta. Part A, Mol. Biomol. Spectrosc. 2012, 86, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Riou, J.; Guyon, H.; Falissard, B. An introduction to the partial least squares approach to structural equation modelling: A method for exploratory psychiatric research. Int. J. Methods Psychiatry Res. 2016, 25, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Lorber, A.; Wangen, L.E.; Kowalski, B.R. A theoretical foundation for the PLS algorithm. J. Chemom. 1987, 1, 19–31. [Google Scholar] [CrossRef]
- Qiao, Z.; Ma, J.; Liu, H. Evaluation of the antioxidant potential of salvia miltiorrhiza ethanol extract in a rat model of ischemia-reperfusion injury. Molecules 2011, 16, 10002–10012. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Liao, Y.; Tian, G.; Li, H.; Jia, Y.; Zhang, H.; Tan, Z.; Li, X.; Deng, W.; Liu, K.; et al. Panax notoginseng saponins inhibit Zymosan A induced atherosclerosis by suppressing integrin expression, FAK activation and NF-κB translocation. J. Ethnopharmacol. 2011, 138, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.R.; Yu, J.Y.; Lee, J.J.; You, S.H.; Chung, J.H.; Noh, J.Y.; Im, J.H.; Han, X.H.; Kim, T.J.; Shin, K.S.; et al. Antithrombotic and antiplatelet activities of Korean red ginseng extract. Basic Clin. Pharm. Toxicol. 2007, 100, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; He, Z.M.; Zhu, H.Y.; Gao, Y.G.; Zhao, Y.; Yang, H.; Zhang, L.X. Involvement of serotonergic, noradrenergic and dopaminergic systems in the antidepressant-like effect of ginsenoside rb1, a major active ingredient of panax ginseng CA Meyer. J. Ethnopharmacol. 2017, 204, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, P.; Beccaria, C.; Pereyra, E.A.L.; Renna, M.S.; Ortega, H.H.; Calvinho, L.F.; Dallard, B.E.; Baravalle, C. Intramammary inoculation of panax ginseng plays an immunoprotective role in staphylococcus aureus infection in a murine model. Res. Vet. Sci. 2017, 115, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, T.; Yang, L.; Wang, H.Y. Ginsenoside rb2 alleviates hepatic lipid accumulation by restoring autophagy via induction of sirt1 and activation of ampk. Int. J. Mol. Sci. 2017, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, J.; Ye, Z.; Han, X.; Zheng, X.; Hou, H.; Chen, W.; Li, X.; Zhao, L. 20(s)-Rg3 blocked epithelial-mesenchymal transition through dnmt3a/mir-145/fscn1 in ovarian cancer. Oncotarget 2017, 8, 53375. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Irfan, M.; Kim, S.D.; Kim, S.; Oh, J.H.; Park, C.K.; Kim, H.K.; Man, H.R. Ginsenoside rg3-enriched red ginseng extract inhibits platelet activation and in vivo thrombus formation. J. Ginseng Res. 2017, 41, 548. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main active components of panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2016, 41, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Cueto, G.; Lizarreta, J.; Ostra, M.; Ubide, C. Multicomponent determinations by partial least-squares regression analysis of fast-reaction multiwavelength profiles obtained by continuous addition of a reagent. Talanta 2002, 58, 569–578. [Google Scholar] [CrossRef]
- Sakudo, A.; Tsenkova, R.; Tei, K.; Onozuka, T.; Ikuta, K.; Yoshimura, E.; Onodera, T. Comparison of the vibration mode of metals in hno3 by a partial least-squares regression analysis of near-infrared spectra. Biosci. Biotechnol. Biochem. 2006, 70, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Sakudo, A.; Yoshimura, E.; Tsenkova, R.; Ikuta, K.; Onodera, T. Native state of metals in non-digested tissues by partial least squares regression analysis of visible and near-infrared spectra. J. Toxicol. Sci. 2007, 32, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Yuan, L.; Zhang, X.; Hayat, K.; Chen, H.; Liu, F.; Xiao, Z.; Niu, Y. Rapid measuring and modelling flavour quality changes of oxidised chicken fat by electronic nose profiles through the partial least squares regression analysis. Food Chem. 2013, 141, 4278–4288. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yuan, Y.Y.; Yu, Z.G.; Bi, K.S. Analysis methods of chromatographic fingerprints of panax gingseng CA Mey. Chin. Tradit. Pat. Med. 2006, 28, 473–477. [Google Scholar]
- Luo, S.Y.; Chen, S.; Qin, Y.D.; Chen, Z.W. Urotensin-receptor antagonist sb-710411 protects rat heart against ischemia-reperfusion injury via rhoa/rock pathway. PLoS ONE 2016, 11, e0146094. [Google Scholar]
- Tang, S.H.; Chen, J.X.; Wang, Y.P.; Wang, L.; Yang, H.J. Research ideas and strategies on the dose-effect relationship of traditional chinese medicine prescriptions and herbs. J. Tradit. Chin. Med. 2012, 32, 110–113. [Google Scholar] [CrossRef]

| No. (%) | NGR1 | GRg1 | GRd | GRe | GRb1 | GRa3 |
|---|---|---|---|---|---|---|
| 1 | 35.34 | 26.29 | 8.70 | 8.27 | 4.26 | 3.21 |
| 2 | 34.92 | 26.60 | 8.50 | 7.25 | 4.81 | 4.10 |
| 3 | 35.80 | 26.49 | 8.95 | 8.32 | 4.89 | 3.50 |
| 4 | 34.45 | 26.23 | 8.86 | 7.94 | 4.08 | 4.10 |
| 5 | 34.37 | 26.19 | 8.81 | 7.89 | 4.99 | 4.20 |
| MEAN | 34.98 | 26.36 | 8.76 | 7.93 | 4.61 | 3.82 |
| SD | 0.66 | 0.20 | 0.20 | 0.44 | 0.41 | 0.32 |
| No. (µg/mL) | NGR1 | GRg1 | GRd | GRe | GRb1 | GRa3 | Observed Effect (%) |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 5 | 0.5 | 40 | 4 | 4 | 30 |
| 2 | 1 | 40 | 2 | 20 | 0 | 1 | 33 |
| 3 | 2 | 2.5 | 8 | 10 | 8 | 0 | 26 |
| 4 | 4 | 20 | 0 | 5 | 1 | 8 | 28 |
| 5 | 8 | 0 | 1 | 2.5 | 16 | 2 | 24 |
| 6 | 16 | 10 | 4 | 0 | 2 | 0.5 | 25 |
| 7 | 32 | 80 | 16 | 80 | 32 | 16 | 59 |
| No. (mg/kg) | NGR1 | GRg1 | GRd | GRe | GRb1 | GRa3 | Observed Effect (%) |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 0.5 | 0.075 | 4 | 0.4 | 0.6 | 31 |
| 2 | 0.1 | 4 | 0.3 | 2 | 0 | 0.15 | 34 |
| 3 | 0.2 | 0.25 | 1.2 | 1 | 0.8 | 0 | 27 |
| 4 | 0.4 | 2 | 0 | 0.5 | 0.1 | 1.2 | 29 |
| 5 | 0.8 | 0 | 0.15 | 0.25 | 1.6 | 0.3 | 25 |
| 6 | 1.6 | 1 | 0.6 | 0 | 0.2 | 0.075 | 26 |
| 7 | 3.2 | 8 | 2.4 | 8 | 3.2 | 2.4 | 58 |
| Batch | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 | P13 | P14 | P15 | P16 | P17 | P18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 0.543 | 0.430 | 0.580 | 0.894 | 0.577 | 0.183 | 0.520 | 0.598 | 0.646 | 0.308 | 0.349 | 0.255 | 0.473 | 0.239 | 0.177 | 0.583 | 0.485 | 0.671 |
| S2 | 1.545 | 0.743 | 0.244 | 1.880 | 0.831 | 1.145 | 0.808 | 0.554 | 0.615 | 0.680 | 0.609 | 0.427 | 0.259 | 1.229 | 0.224 | 1.958 | 1.252 | 1.090 |
| S3 | 0.923 | 0.844 | 0.703 | 1.090 | 1.185 | 1.831 | 0.420 | 2.504 | 0.983 | 0.415 | 0.531 | 0.662 | 0.223 | 0.271 | 1.095 | 0.649 | 1.063 | 0.525 |
| S4 | 2.613 | 4.052 | 0.166 | 2.030 | 1.248 | 0.323 | 0.940 | 1.764 | 1.539 | 0.615 | 0.743 | 1.498 | 0.861 | 0.947 | 1.423 | 0.968 | 2.745 | 1.581 |
| S5 | 0.148 | 0.081 | 0.820 | 0.290 | 0.362 | 0.285 | 0.173 | 0.546 | 0.239 | 2.479 | 0.212 | 0.284 | 0.115 | 0.821 | 0.453 | 0.802 | 0.414 | 0.198 |
| S6 | 1.912 | 1.591 | 0.345 | 2.570 | 1.534 | 1.341 | 0.738 | 0.642 | 0.864 | 0.755 | 1.190 | 1.237 | 0.226 | 0.543 | 0.738 | 0.337 | 2.632 | 1.617 |
| S7 | 1.485 | 1.672 | 0.664 | 1.340 | 0.780 | 0.938 | 0.501 | 0.436 | 1.030 | 0.976 | 0.683 | 0.104 | 0.299 | 2.816 | 1.167 | 0.939 | 1.203 | 0.929 |
| S8 | 0.275 | 0.195 | 0.330 | 0.580 | 0.239 | 0.534 | 0.166 | 0.722 | 0.240 | 0.142 | 0.128 | 1.794 | 0.112 | 0.446 | 0.189 | 0.694 | 0.815 | 0.210 |
| S9 | 0.374 | 0.314 | 0.779 | 0.460 | 0.211 | 0.621 | 0.167 | 0.755 | 0.229 | 0.387 | 0.119 | 0.579 | 0.109 | 0.606 | 0.500 | 0.325 | 0.580 | 0.391 |
| S10 | 1.002 | 1.693 | 0.439 | 1.520 | 1.277 | 1.328 | 0.876 | 0.941 | 0.901 | 0.593 | 0.584 | 0.210 | 1.313 | 0.238 | 1.059 | 0.138 | 0.950 | 0.720 |
| Groups | In Vitro Study | In Vivo Study | ||||||
|---|---|---|---|---|---|---|---|---|
| E1 (ng/mL) | E2 (U/L) | E3 (U/L) | E4 (%) | E1 (ng/mL) | E2 (U/L) | E3 (U/L) | E4 (%) | |
| Sham | 0.265 | 121.5 | 24.7 | 35.6 | 3.32 | 221.6 | 95.1 | 45.8 |
| IR | 1.217 | 267.8 | 90.4 | 53.2 | 13.75 | 986.7 | 183.4 | 57.9 |
| IR + S1 | 0.997 | 218.4 | 67.6 | 45.7 | 11.97 | 766.9 | 169.2 | 54.1 |
| IR + S2 | 0.743 | 170.4 | 53.8 | 39.8 | 9.06 | 536.3 | 143 | 48.8 |
| IR + S3 | 0.815 | 175.5 | 59.4 | 42 | 9.75 | 587.1 | 152.8 | 52.4 |
| IR + S4 | 0.699 | 155.4 | 45.6 | 41.3 | 7.39 | 308.7 | 120.5 | 50.1 |
| IR + S5 | 0.986 | 202.1 | 65.8 | 43.5 | 10.54 | 726.5 | 166.3 | 52.8 |
| IR + S6 | 0.684 | 166.8 | 51.3 | 40.2 | 6.84 | 401.3 | 135.1 | 49.9 |
| IR + S7 | 0.746 | 167.8 | 53.6 | 40.8 | 8.05 | 467 | 140.1 | 49.9 |
| IR + S8 | 1.016 | 220.5 | 68.2 | 44.4 | 11.64 | 775.1 | 170.9 | 53.2 |
| IR + S9 | 1.002 | 223.7 | 70.2 | 43.9 | 11.2 | 814.4 | 174.9 | 53.8 |
| IR + S10 | 0.878 | 183.8 | 62.1 | 42.6 | 8.81 | 529.3 | 156.7 | 52.7 |
| Type | Effect | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 | P13 | P14 | P15 | P16 | P17 | P18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In Vitro Study | E1 | 0.27 | 0.39 | 0.13 | 0.04 | 0.13 | 0.32 | 0.15 | 0.37 | 0.33 | 0.28 | 0.33 | 0.36 | 0.24 | 0.24 | 0.23 | 0.26 | 0.06 | 0.38 |
| E2 | 0.12 | 0.27 | 0.04 | 0.10 | 0.18 | 0.02 | 0.05 | 0.24 | 0.20 | 0.27 | 0.26 | 0.18 | 0.37 | 0.02 | 0.16 | 0.05 | 0.33 | 0.34 | |
| E3 | 0.18 | 0.03 | 0.04 | 0.01 | 0.15 | 0.05 | 0.25 | 0.32 | 0.34 | 0.27 | 0.29 | 0.38 | 0.24 | 0.2 | 0.22 | 0.23 | 0.35 | 0.16 | |
| E4 | 0.35 | 0.12 | 0.26 | 0.32 | 0.29 | 0.12 | 0.29 | 0.34 | 0.38 | 0.33 | 0.33 | 0.19 | 0.28 | 0.18 | 0.03 | 0.19 | 0.38 | 0.3 | |
| In Vivo Study | E1 | 0.26 | 0.37 | 0.16 | 0.03 | 0.17 | 0.33 | 0.17 | 0.39 | 0.34 | 0.21 | 0.33 | 0.38 | 0.21 | 0.23 | 0.28 | 0.29 | 0.05 | 0.39 |
| E2 | 0.13 | 0.27 | 0.02 | 0.11 | 0.17 | 0.04 | 0.04 | 0.22 | 0.19 | 0.25 | 0.26 | 0.2 | 0.36 | 0.04 | 0.18 | 0.04 | 0.37 | 0.36 | |
| E3 | 0.19 | 0.04 | 0.03 | 0.02 | 0.11 | 0.06 | 0.24 | 0.33 | 0.30 | 0.31 | 0.36 | 0.31 | 0.22 | 0.16 | 0.21 | 0.25 | 0.31 | 0.21 | |
| E4 | 0.33 | 0.18 | 0.23 | 0.30 | 0.25 | 0.13 | 0.28 | 0.37 | 0.35 | 0.29 | 0.30 | 0.22 | 0.19 | 0.19 | 0.05 | 0.19 | 0.37 | 0.29 |
| Type | Effect | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 | P13 | P14 | P15 | P16 | P17 | P18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In Vitro Study | E1 | 1.08 | 1.08 | 1.00 | 1.18 | 1.11 | 1.18 | 1.11 | 0.92 | 0.99 | 0.97 | 1.06 | 1.01 | 1.17 | 1.05 | 0.98 | 0.96 | 1.06 | 1.13 |
| E2 | 0.91 | 1.00 | 0.91 | 1.04 | 1.15 | 1.09 | 1.13 | 1.03 | 1.12 | 0.94 | 1.19 | 1.09 | 1.06 | 1.16 | 1.09 | 1.12 | 1.09 | 0.91 | |
| E3 | 1.08 | 0.92 | 0.99 | 1.17 | 1.01 | 1.01 | 0.93 | 1.05 | 0.99 | 0.93 | 1.15 | 0.92 | 1.1 | 1.1 | 0.99 | 0.94 | 1.13 | 0.98 | |
| E4 | 1.17 | 0.97 | 0.97 | 1.09 | 0.91 | 1.16 | 1.14 | 1.06 | 1.19 | 0.98 | 1.15 | 0.92 | 1.04 | 1.08 | 1.05 | 1.1 | 1.19 | 1.07 | |
| In Vivo Study | E1 | 1.03 | 1.05 | 1.01 | 1.12 | 1.06 | 1.05 | 1.10 | 0.91 | 0.97 | 0.95 | 1.02 | 1.02 | 1.15 | 1.06 | 0.98 | 0.99 | 1.01 | 1.12 |
| E2 | 0.91 | 1.02 | 0.92 | 0.99 | 1.13 | 1.10 | 1.12 | 1.01 | 1.14 | 0.93 | 1.15 | 1.07 | 1.15 | 1.18 | 1.07 | 1.13 | 1.08 | 0.93 | |
| E3 | 1.08 | 0.95 | 0.96 | 1.14 | 0.99 | 1.00 | 0.96 | 1.04 | 0.98 | 0.94 | 1.17 | 0.93 | 1.08 | 1.07 | 1.02 | 0.97 | 1.15 | 1 | |
| E4 | 1.18 | 0.99 | 0.97 | 1.10 | 0.93 | 1.14 | 1.14 | 1.07 | 1.18 | 0.97 | 1.11 | 0.93 | 1.02 | 1.06 | 1.04 | 1.08 | 1.13 | 1.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Li, S.-n.; Pei, X.; Hao, K. The Multivariate Regression Statistics Strategy to Investigate Content-Effect Correlation of Multiple Components in Traditional Chinese Medicine Based on a Partial Least Squares Method. Molecules 2018, 23, 545. https://doi.org/10.3390/molecules23030545
Peng Y, Li S-n, Pei X, Hao K. The Multivariate Regression Statistics Strategy to Investigate Content-Effect Correlation of Multiple Components in Traditional Chinese Medicine Based on a Partial Least Squares Method. Molecules. 2018; 23(3):545. https://doi.org/10.3390/molecules23030545
Chicago/Turabian StylePeng, Ying, Su-ning Li, Xuexue Pei, and Kun Hao. 2018. "The Multivariate Regression Statistics Strategy to Investigate Content-Effect Correlation of Multiple Components in Traditional Chinese Medicine Based on a Partial Least Squares Method" Molecules 23, no. 3: 545. https://doi.org/10.3390/molecules23030545




