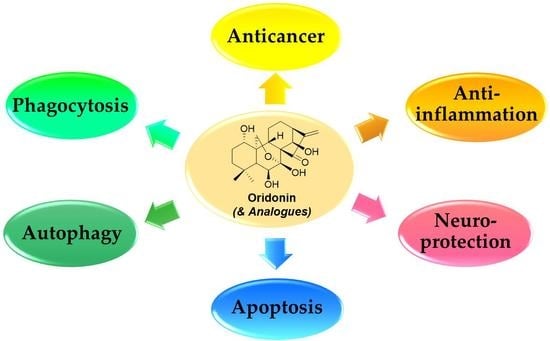
Therapeutic Potential of Oridonin and Its Analogs: From Anticancer and Antiinflammation to Neuroprotection
Abstract
:1. Introduction
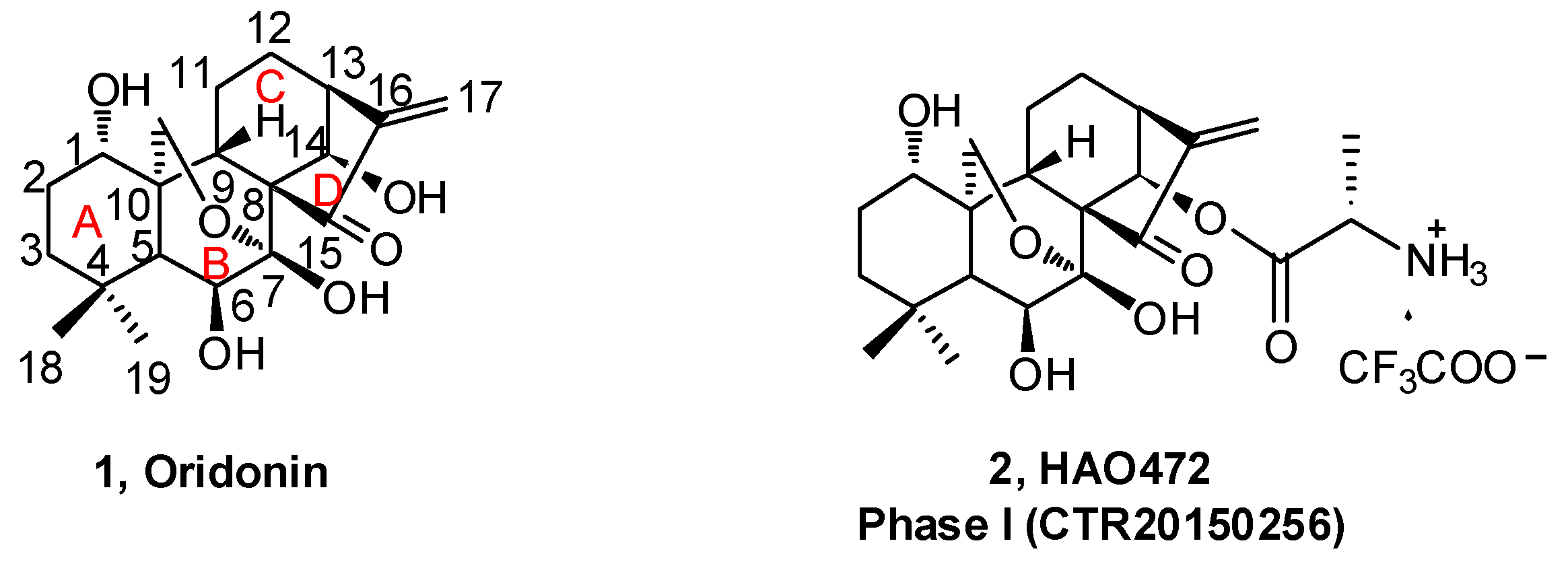
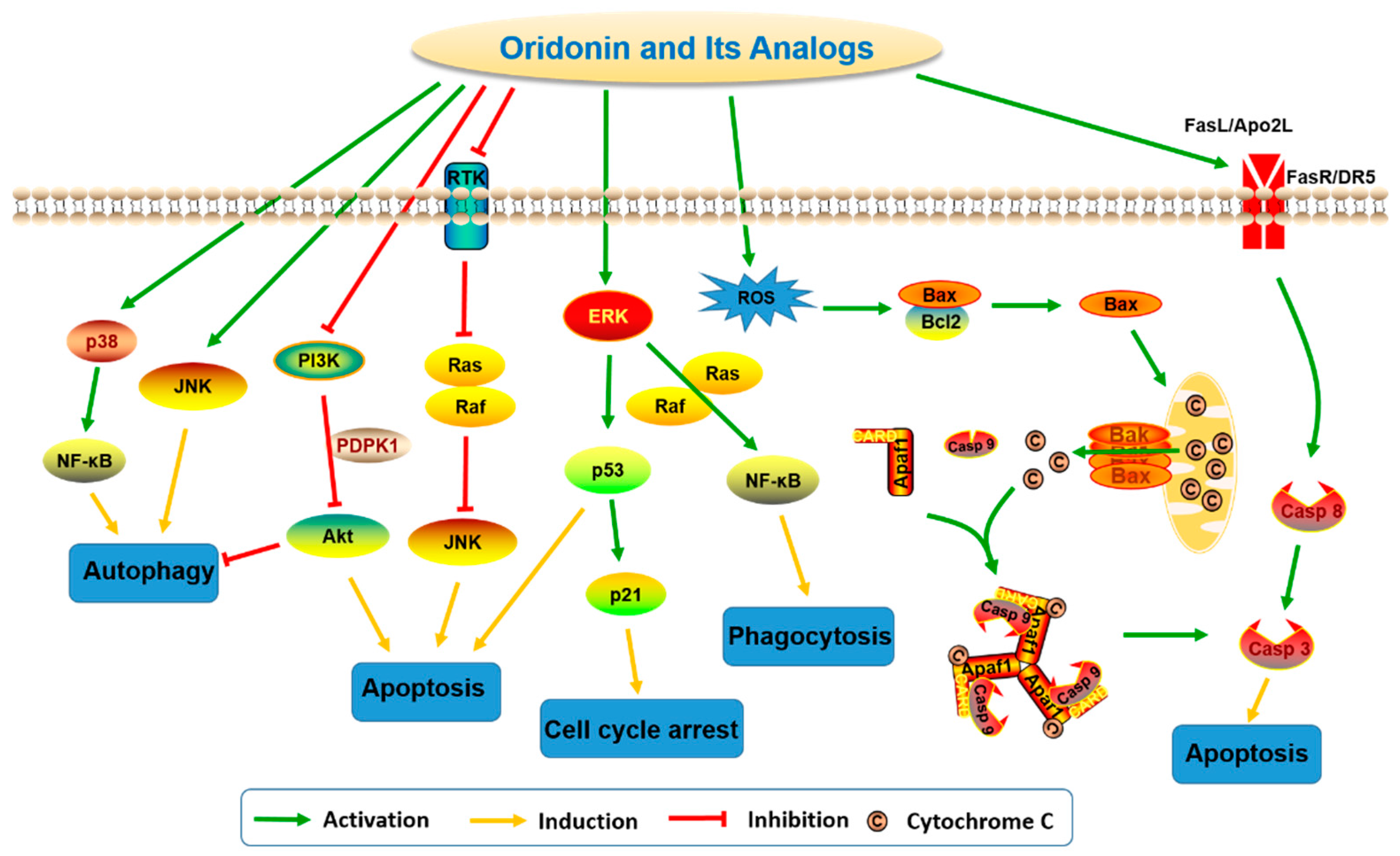
2. Oridonin and Its Analogs for Cancer Drug Discovery
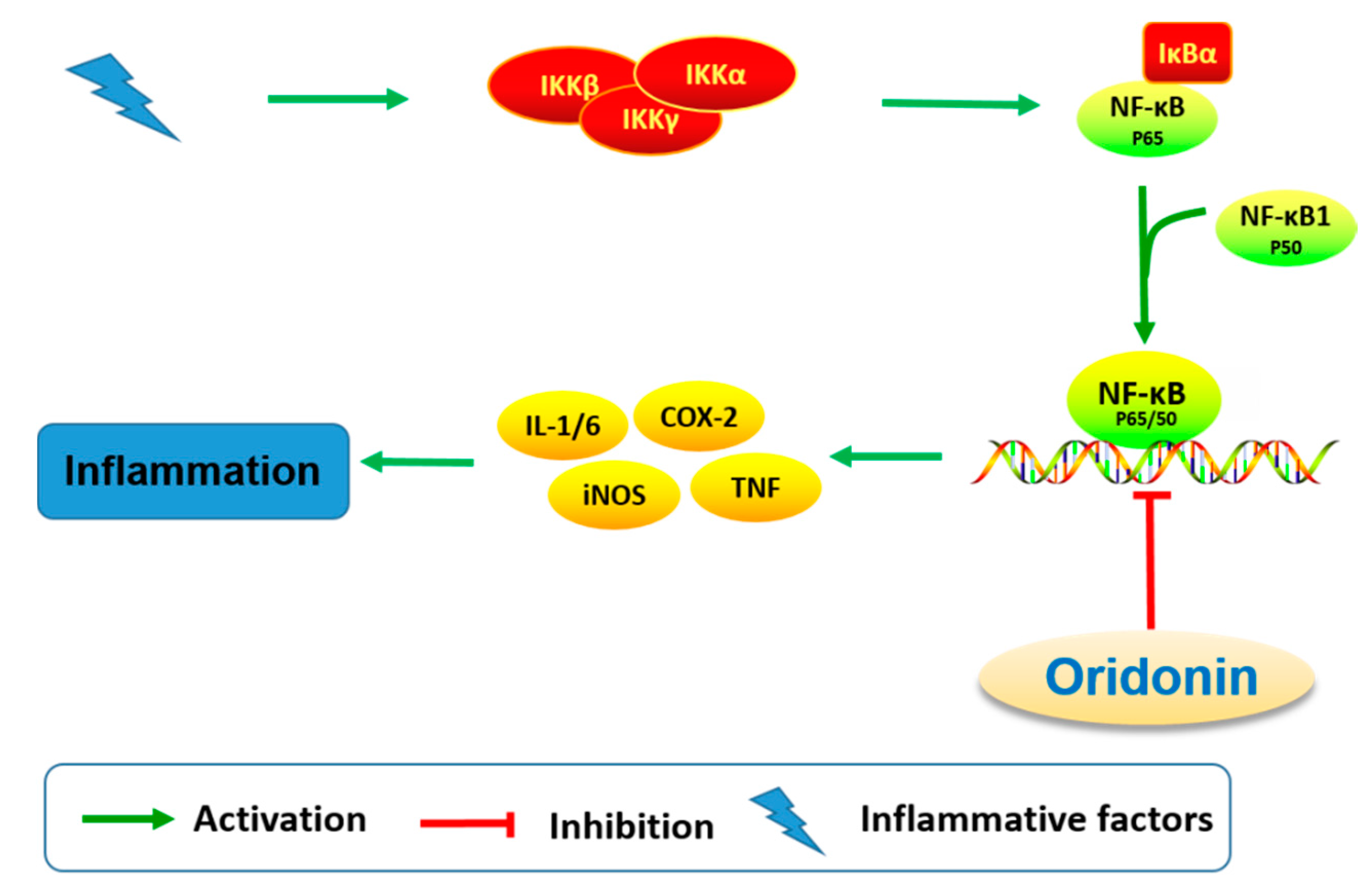
3. Antiinflammation Effects of Oridonin and Its Analogs
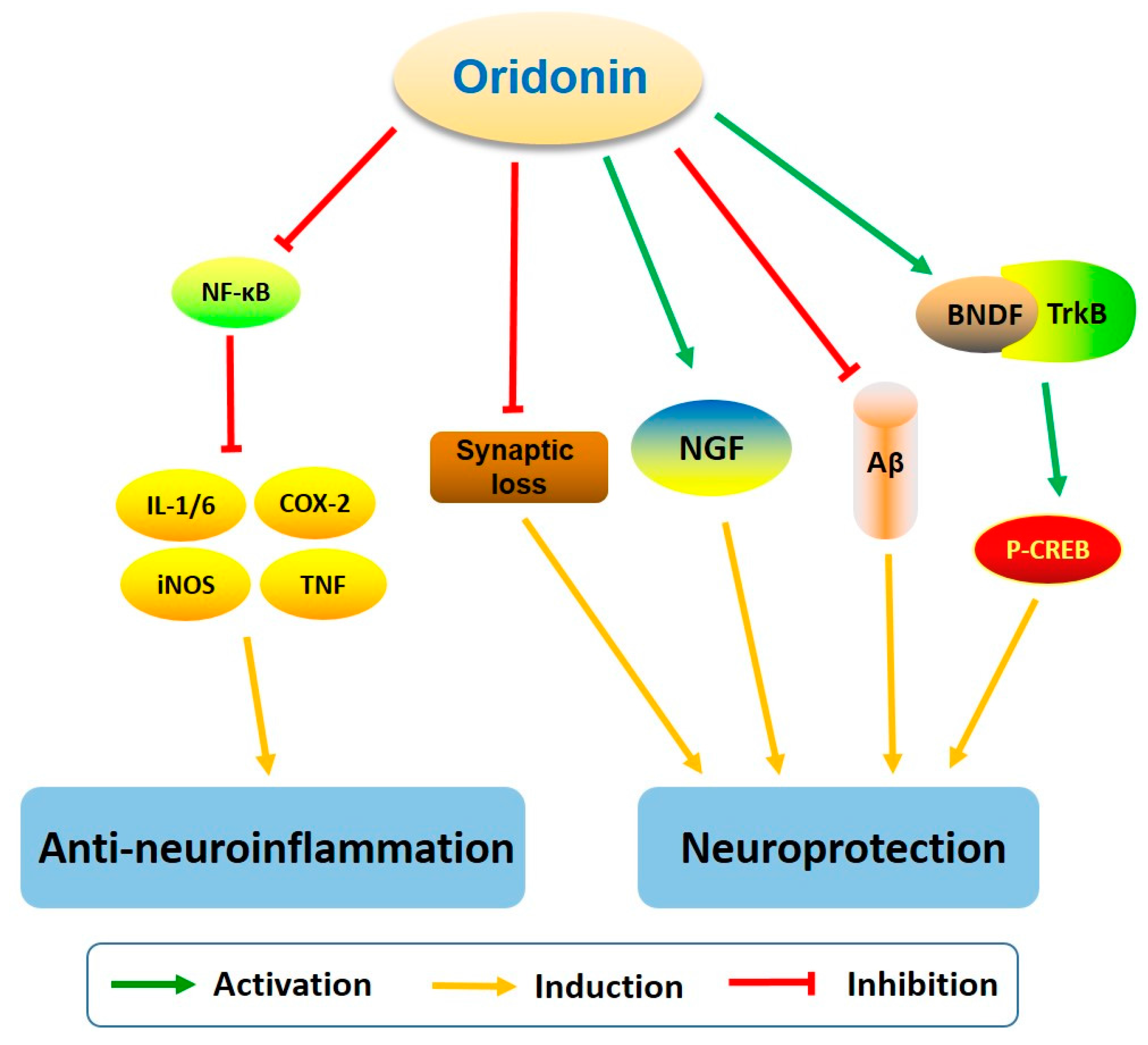
4. Neuroinflammation and Neuroprotection Activities
5. Oridonin for Neurodegenerative Diseases
6. Conclusions and Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| α-SMA | α-Smooth muscle actin |
| APP | Amyloid precursor protein |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma 2 |
| BDNF | Brain-derived neurotrophic factor |
| CREB | cAMP response element-binding protein |
| ECM | Extracellular matrix |
| HSC | Hepatic stellate cell |
| ip | Intraperitoneal |
| iv | Intravenous |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemotactic protein 1 |
| NMDA | N-Methyl-d-aspartic acid |
| Nrf2 | Nuclear factor erythroid-derived 2 (NFE2) related factor 2 |
| p21 | p21Cip1 protein |
| p53 | Tumor protein p53 |
| PI3K | Phosphatidylinositide 3-kinases |
| SAR | Structure-activity relationship |
| STAT | Signal transducer and activator of transcription |
| TLR-4 | Toll-like receptor 4 |
| TNBS | 2,4,6-Trinitrobenzenesulfonic acid |
| TrkB | Tropomysin receptor kinase B |
References
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, B.B.; Tiwari, V.K. Natural products: An evolving role in future drug discovery. Eur. J. Med. Chem. 2011, 46, 4769–4807. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Eder, J.; Sedrani, R.; Wiesmann, C. The discovery of first-in-class drugs: Origins and evolution. Nat. Rev. Drug Discov. 2014, 13, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Lu, J.; Huang, M.; Li, Y.; Chen, M.; Wu, G.; Gong, J.; Zhong, Z.; Xu, Z.; Dang, Y.; et al. Anti-cancer natural products isolated from Chinese medicinal herbs. Chin. Med. 2011, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Fujita, E.; Nagao, Y.; Kaneko, K.; Nakazawa, S.; Kuroda, H. The antitumor and antibacterial activity of the Isodon diterpenoids. Chem. Pharm. Bull. (Tokyo) 1976, 24, 2118–2127. [Google Scholar] [CrossRef] [PubMed]
- Fujita, E.; Fujita, T.; Katayama, H.; Shibuya, M. Oridonin, a new diterpenoid from Isodon species. Chem. Commun. (London) 1967, 0, 252–254. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wang, S.; Gao, Y.; Zhang, X.; Lu, C. Oridonin phosphate-induced autophagy effectively enhances cell apoptosis of human breast cancer cells. Med. Oncol. 2015, 32, 365. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lu, X.J.; Zhang, J.; Diao, H.; Li, G.; Xu, L.; Wang, T.; Wei, J.; Meng, W.; Ma, J.L.; et al. Oridonin, a novel lysine acetyltransferases inhibitor, inhibits proliferation and induces apoptosis in gastric cancer cells through p53- and caspase-3-mediated mechanisms. Oncotarget 2016, 7, 22623–22631. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.B.; Kang, H.; Wang, L.; Gao, L.; Liu, P.; Xie, J.; Zhang, F.X.; Weng, X.Q.; Shen, Z.X.; Chen, J.; et al. Oridonin, a diterpenoid extracted from medicinal herbs, targets AML1-ETO fusion protein and shows potent antitumor activity with low adverse effects on t(8;21) leukemia in vitro and in vivo. Blood 2007, 109, 3441–3450. [Google Scholar] [CrossRef] [PubMed]
- Bao, R.; Shu, Y.; Wu, X.; Weng, H.; Ding, Q.; Cao, Y.; Li, M.; Mu, J.; Wu, W.; Ding, Q.; et al. Oridonin induces apoptosis and cell cycle arrest of gallbladder cancer cells via the mitochondrial pathway. BMC Cancer 2014, 14, 217. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Tashiro, S.; Onodera, S.; Minami, M.; Ikejima, T. Oridonin induced autophagy in human cervical carcinoma HeLa cells through Ras, JNK, and P38 regulation. J. Pharmacol. Sci. 2007, 105, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Liu, F.; Li, M. Inhibition of nuclear factor kappaB transcription activity drives a synergistic effect of cisplatin and oridonin on HepG2 human hepatocellular carcinoma cells. Anticancer Drugs 2016, 27, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yun, E.J.; Chen, W.; Ding, Y.; Wu, K.; Wang, B.; Ding, C.; Hernandez, E.; Santoyo, J.; Pong, R.C.; et al. Targeting 3-phosphoinositide-dependent protein kinase 1 associated with drug-resistant renal cell carcinoma using new oridonin analogs. Cell Death Dis. 2017, 8, e2701. [Google Scholar] [CrossRef] [PubMed]
- Ikezoe, T.; Chen, S.S.; Tong, X.J.; Heber, D.; Taguchi, H.; Koeffler, H.P. Oridonin induces growth inhibition and apoptosis of a variety of human cancer cells. Int. J. Oncol. 2003, 23, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhou, J.; Wu, K.; Huang, J.; Ding, Y.; Yun, E.J.; Wang, B.; Ding, C.; Hernandez, E.; Santoyo, J.; et al. Targeting XBP1-mediated β-catenin expression associated with bladder cancer with newly synthetic oridonin analogues. Oncotarget 2016, 7, 56842–56854. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.-M.; Lin, J.-Y. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013, 141, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.P.; Du, J.M.; Li, J.Y.; Liu, J.W. Oridonin promotes CD4+/CD25+ Treg differentiation, modulates Th1/Th2 balance and induces HO-1 in rat splenic lymphocytes. Inflamm. Res. 2008, 57, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, F.; Zhang, Y.; Li, J. Studies on the cell-immunosuppressive mechanism of Oridonin from Isodon serra. Int. Immunopharmacol. 2007, 7, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Pei, L.; Li, D.; Yao, H.; Cai, H.; Yao, H.; Wu, X.; Xu, J. Synthesis and antimycobacterial evaluation of natural oridonin and its enmein-type derivatives. Fitoterapia 2014, 99, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Bohanon, F.J.; Wang, X.; Graham, B.M.; Prasai, A.; Vasudevan, S.J.; Ding, C.; Ding, Y.; Radhakrishnan, G.L.; Rastellini, C.; Zhou, J.; et al. Enhanced anti-fibrogenic effects of novel oridonin derivative CYD0692 in hepatic stellate cells. Mol. Cell. Biochem. 2015, 410, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Bohanon, F.J.; Wang, X.; Graham, B.M.; Ding, C.; Ding, Y.; Radhakrishnan, G.L.; Rastellini, C.; Zhou, J.; Radhakrishnan, R.S. Enhanced effects of novel oridonin analog CYD0682 for hepatic fibrosis. J. Surg. Res. 2015, 199, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.M.; Kuo, C.Y.; Lin, C.Y.; Hung, M.F.; Shen, J.J.; Hwang, T.L. Intracellular glutathione depletion by oridonin leads to apoptosis in hepatic stellate cells. Molecules 2014, 19, 3327–3344. [Google Scholar] [CrossRef] [PubMed]
- Bohanon, F.J.; Wang, X.; Ding, C.; Ding, Y.; Radhakrishnan, G.L.; Rastellini, C.; Zhou, J.; Radhakrishnan, R.S. Oridonin inhibits hepatic stellate cell proliferation and fibrogenesis. J. Surg. Res. 2014, 190, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, L.; Yang, H.; Li, C.; Hui, Z.; Xu, Y.; Zhu, X. Oridonin Attenuates synaptic loss and cognitive deficits in an Abeta1–42-induced mouse model of Alzheimer’s disease. PLoS ONE 2016, 11, e0151397. [Google Scholar]
- Wang, S.; Yang, H.; Yu, L.; Jin, J.; Qian, L.; Zhao, H.; Xu, Y.; Zhu, X. Oridonin attenuates Abeta1–42-induced neuroinflammation and inhibits NF-kappaB pathway. PLoS ONE 2014, 9, e104745. [Google Scholar]
- Zhang, Z.Y.; Daniels, R.; Schluesener, H.J. Oridonin ameliorates neuropathological changes and behavioural deficits in a mouse model of cerebral amyloidosis. J. Cell. Mol. Med. 2013, 17, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Han, T.; Liao, J.; Hu, X.; Xu, S.; Tian, K.; Gu, X.; Cheng, K.; Li, Z.; Hua, H.; et al. Oridonin, a Promising ent-Kaurane Diterpenoid Lead Compound. Int. J. Mol. Sci. 2016, 17, 1395. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ding, C.; Ye, N.; Liu, Z.; Wold, E.A.; Chen, H.; Wild, C.; Shen, Q.; Zhou, J. Discovery and development of natural product oridonin-inspired anticancer agents. Eur. J. Med. Chem. 2016, 122, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Owona, B.A.; Schluesener, H.J. Molecular Insight in the Multifunctional Effects of oridonin. Drugs R&D 2015, 15, 233–244. [Google Scholar]
- Sun, P.; Wu, G.; Qiu, Z.; Chen, Y. Preparation of l-Alanine-(14-oridonin) Ester Trifluoroacetate for Treatment of Cancer. Patent Application CN104017000A, 3 September 2014. [Google Scholar]
- Li, C.-Y.; Wang, E.-Q.; Cheng, Y.; Bao, J.-K. Oridonin: An active diterpenoid targeting cell cycle arrest, apoptotic and autophagic pathways for cancer therapeutics. Int. J. Biochem. Cell Biol. 2011, 43, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gao, J.; Halicka, H.D.; Huang, X.; Traganos, F.; Darzynkiewicz, Z. The cytostatic and cytotoxic effects of oridonin (rubescenin), a diterpenoid from Rabdosia rubescens, on tumor cells of different lineage. Int. J. Oncol. 2005, 26, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ye, Y.; Chui, J.H.; Zhu, G.Y.; Li, Y.W.; Fong, D.W.; Yu, Z.L. Oridonin induces G2/M cell cycle arrest and apoptosis through MAPK and p53 signaling pathways in HepG2 cells. Oncol. Rep. 2010, 24, 647–651. [Google Scholar] [PubMed]
- Cheng, Y.; Qiu, F.; Ye, Y.-C.; Tashiro, S.-i.; Onodera, S.; Ikejima, T. Oridonin induces G2/M arrest and apoptosis via activating ERK–p53 apoptotic pathway and inhibiting PTK–Ras–Raf–JNK survival pathway in murine fibrosarcoma L929 cells. Arch. Biochem. Biophys. 2009, 490, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Z.; Yang, Y.B.; Xu, X.D.; Shen, H.W.; Shu, Y.M.; Ren, Z.; Li, X.M.; Shen, H.M.; Zeng, H.T. Oridonin induces apoptosis via PI3K/Akt pathway in cervical carcinoma HeLa cell line. Acta Pharmacol. Sin. 2007, 28, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Q.; Mu, Z.-Q.; You, S.; Tashiro, S.-I.; Onodera, S.; Ikejima, T. Fas/FasL Signaling Allows Extracelluar-Signal Regulated Kinase to Regulate Cytochrome c Release in Oridonin-Induced Apoptotic U937 Cells. Biol. Pharm. Bull. 2006, 29, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ding, Y.; Chen, C.H.; Zhou, Z.; Ding, C.; Chen, H.; Zhou, J.; Chen, C. A new oridonin analog suppresses triple-negative breast cancer cells and tumor growth via the induction of death receptor 5. Cancer Lett. 2016, 380, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-L.; Wu, L.-J.; Tashiro, S.-I.; Onodera, S.; Ikejima, T. Oridonin induced A375-S2 cell apoptosis via BAX-regulated caspase pathway activation, dependent on the cytochrome C/CASPASE-9 apoptosome. J. Asian Nat. Prod. Res. 2004, 6, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-L.; Wu, L.-J.; Zuo, H.-J.; Tashiro, S.-I.; Onodera, S.; Ikejima, T. Cytochrome c release from oridonin-treated apoptotic A375-S2 Cells is dependent on p53 and Extracellular signal-regulated kinase activation. J. Pharmacol. Sci. 2004, 96, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Q.; You, S.; Tashiro, S.-I.; Onodera, S.; Ikejima, T. Activation of Phosphoinositide 3-kinase, protein kinase C, and extracellular signal-regulated kinase is required for oridonin-enhanced phagocytosis of apoptotic bodies in human macrophage-like U937 Cells. J. Pharmacol. Sci. 2005, 98, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.H.; Liu, F.; Wei, W.; Liu, L.B.; Xu, M.H.; Guo, Z.Y.; Li, W.; Jiang, B.; Wu, Y.L. Oridonin induces apoptosis and senescence by increasing hydrogen peroxide and glutathione depletion in colorectal cancer cells. Int. J. Mol. Med. 2012, 29, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.N.; Huang, J.; Yang, J.; Tashiro, S.; Onodera, S.; Ikejima, T. Caspase inhibition augmented oridonin-induced cell death in murine fibrosarcoma l929 by enhancing reactive oxygen species generation. J. Pharmacol. Sci. 2008, 108, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhang, Y.; Chen, H.; Yang, Z.; Wild, C.; Chu, L.; Liu, H.; Shen, Q.; Zhou, J. Novel nitrogen-enriched oridonin analogues with thiazole-fused A-ring: Protecting group-free synthesis, enhanced anticancer profile, and improved aqueous solubility. J. Med. Chem. 2013, 56, 5048–5058. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Wang, L.; Chen, H.; Wild, C.; Ye, N.; Ding, Y.; Wang, T.; White, M.A.; Shen, Q.; Zhou, J. ent-Kaurane-based regio- and stereoselective inverse electron demand hetero-Diels-Alder reactions: synthesis of dihydropyran-fused diterpenoids. Org. Biomol. Chem. 2014, 12, 8442–8452. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhang, Y.; Chen, H.; Yang, Z.; Wild, C.; Ye, N.; Ester, C.D.; Xiong, A.; White, M.A.; Shen, Q.; et al. Oridonin ring A-based diverse constructions of enone functionality: Identification of novel dienone analogues effective for highly aggressive breast cancer by inducing apoptosis. J. Med. Chem. 2013, 56, 8814–8825. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhang, Y.; Chen, H.; Wild, C.; Wang, T.; White, M.A.; Shen, Q.; Zhou, J. Overcoming synthetic challenges of oridonin A-ring structural diversification: Regio- and stereoselective installation of azides and 1,2,3-triazoles at the C-1, C-2, or C-3 position. Org. Lett. 2013, 15, 3718–3721. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Luo, S.; Yao, H.; Cai, H.; Miao, X.; Wu, F.; Yang, D.H.; Wu, X.; Xie, W.; Yao, H.; et al. Probing the anticancer action of oridonin with fluorescent analogues: visualizing subcellular localization to mitochondria. J. Med. Chem. 2016, 59, 5022–5034. [Google Scholar] [CrossRef] [PubMed]
- Nan, F.; Zhang, Y.; Liu, H.; Ding, J.; Meng, L.; Xu, C. Preparation of ent-Kaurane Type Diterpenoid Derivatives as Antitumor Agents. Patent Application CN102584760A, 18 July 2012. [Google Scholar]
- Huang, S.X.; Xiao, W.L.; Li, L.M.; Li, S.H.; Zhou, Y.; Ding, L.S.; Lou, L.G.; Sun, H.D. Bisrubescensins A-C: Three new dimeric ent-kauranoids isolated from Isodon rubescens. Org. Lett. 2006, 8, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Q.; Wang, H.L.; Chen, K.; Wang, S.B.; Xu, Y.; Ye, Q.; Sun, Y.W. Oridonin derivative ameliorates experimental colitis by inhibiting activated T-cells and translocation of nuclear factor-kappa B. J. Dig. Dis. 2016, 17, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yao, H.; Luo, S.; Zhang, Y.K.; Yang, D.H.; Li, D.; Wang, G.; Hu, M.; Qiu, Y.; Wu, X.; et al. A Novel Potent Anticancer Compound Optimized from a Natural Oridonin Scaffold Induces Apoptosis and Cell Cycle Arrest through the Mitochondrial Pathway. J. Med. Chem. 2017, 60, 1449–1468. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, D.; Xu, S.; Cai, H.; Yao, H.; Zhang, Y.; Jiang, J.; Xu, J. The conversion of oridonin to spirolactone-type or enmein-type diterpenoid: Synthesis and biological evaluation of ent-6,7-seco-oridonin derivatives as novel potential anticancer agents. Eur. J. Med. Chem. 2012, 52, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cai, H.; Jiang, B.; Liu, G.; Wang, Y.; Wang, L.; Yao, H.; Wu, X.; Sun, Y.; Xu, J. Synthesis of spirolactone-type diterpenoid derivatives from kaurene-type oridonin with improved antiproliferative effects and their apoptosis-inducing activity in human hepatoma Bel-7402 cells. Eur. J. Med. Chem. 2013, 59, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, S.; Cai, H.; Pei, L.; Zhang, H.; Wang, L.; Yao, H.; Wu, X.; Jiang, J.; Sun, Y.; et al. Enmein-type diterpenoid analogs from natural kaurene-type oridonin: Synthesis and their antitumor biological evaluation. Eur. J. Med. Chem. 2013, 64, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, S.; Cai, H.; Pei, L.; Wang, L.; Wu, X.; Yao, H.; Jiang, J.; Sun, Y.; Xu, J. Library construction and biological evaluation of enmein-type diterpenoid analogues as potential anticancer agents. ChemMedChem 2013, 8, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Lu, W.; Nan, F.J. Synthesis and revision of stereochemistry of rubescensin S. Org. Biomol. Chem. 2011, 9, 4436–4439. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, Y.; Li, Q.; Xiao, Q.; Jia, Y.; Tu, P. Formal synthesis of semiaquilegin A. Tetrahedron Lett. 2010, 51, 1121–1123. [Google Scholar] [CrossRef]
- Huang, J.; Wu, L.; Tashiro, S.; Onodera, S.; Ikejima, T. A comparison of the signal pathways between the TNF alpha- and oridonin-induced murine L929 fibrosarcoma cell death. Acta Med. Okayama 2005, 59, 261–270. [Google Scholar] [PubMed]
- Leung, C.H.; Grill, S.P.; Lam, W.; Han, Q.B.; Sun, H.D.; Cheng, Y.C. Novel mechanism of inhibition of nuclear factor-kappa B DNA-binding activity by diterpenoids isolated from Isodon rubescens. Mol. Pharmacol. 2005, 68, 286–297. [Google Scholar] [PubMed]
- Ikezoe, T.; Yang, Y.; Bandobashi, K.; Saito, T.; Takemoto, S.; Machida, H.; Togitani, K.; Koeffler, H.P.; Taguchi, H. Oridonin, a diterpenoid purified from Rabdosia rubescens, inhibits the proliferation of cells from lymphoid malignancies in association with blockade of the NF-kappa B signal pathways. Mol. Cancer Ther. 2005, 4, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Saas, P.; Wang, H.; Xu, Y.; Chen, K.; Zhong, J.; Yuan, Y.; Wang, Y.; Sun, Y. Oridonin’s therapeutic effect: suppressing Th1/Th17 simultaneously in a mouse model of Crohn’s disease. J. Gastroenterol. Hepatol. 2015, 30, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xue, Y.; Wang, Y.; Feng, D.; Lin, S.; Xu, L. Multiple-modulation effects of Oridonin on the production of proinflammatory cytokines and neurotrophic factors in LPS-activated microglia. Int. Immunopharmacol. 2009, 9, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Minagar, A.; Shapshak, P.; Fujimura, R.; Ownby, R.; Heyes, M.; Eisdorfer, C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J. Neurol. Sci. 2002, 202, 13–23. [Google Scholar] [CrossRef]
- Streit, W.J. Microglial response to brain injury: A brief synopsis. Toxicol. Pathol. 2000, 28, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Scarano, F.; Baltuch, G. Microglia as mediators of inflammatory and degenerative diseases. Annu. Rev. Neurosci. 1999, 22, 219–240. [Google Scholar] [CrossRef] [PubMed]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Minghetti, L.; Levi, G. Microglia as effector cells in brain damage and repair: focus on prostanoids and nitric oxide. Prog. Neurobiol. 1998, 54, 99–125. [Google Scholar] [CrossRef]
- McGeer, P.L.; Yasojima, K.; McGeer, E.G. Association of interleukin-1 beta polymorphisms with idiopathic Parkinson’s disease. Neurosci. Lett. 2002, 326, 67–69. [Google Scholar] [CrossRef]
- Le, W.; Rowe, D.; Xie, W.; Ortiz, I.; He, Y.; Appel, S.H. Microglial activation and dopaminergic cell injury: An in vitro model relevant to Parkinson’s disease. J. Neurosci. 2001, 21, 8447–8455. [Google Scholar] [PubMed]
- Masliah, E.; Mallory, M.; Alford, M.; DeTeresa, R.; Hansen, L.A.; McKeel, D.W. Jr.; Morris, J.C. Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology 2001, 56, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Sze, C.I.; Troncoso, J.C.; Kawas, C.; Mouton, P.; Price, D.L.; Martin, L.J. Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. J. Neuropathol. Exp. Neurol. 1997, 56, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W.; Crystal, H.A.; Bevona, C.; Honer, W.; Vincent, I.; Davies, P. Correlations of synaptic and pathological markers with cognition of the elderly. Neurobiol. Aging 1995, 16, 285–298. [Google Scholar] [CrossRef]
- Davies, C.A.; Mann, D.M.; Sumpter, P.Q.; Yates, P.O. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer’s disease. J. Neurol. Sci. 1987, 78, 151–164. [Google Scholar] [CrossRef]
- Chen, K.; Henry, R.A.; Hughes, S.M.; Connor, B. Creating a neurogenic environment: The role of BDNF and FGF2. Mol. Cell. Neurosci. 2007, 36, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.; Li, H.; Thomas-Crusells, J.; Lahtinen, H.; Viitanen, T.; Nanobashvili, A.; Kokaia, Z.; Airaksinen, M.S.; Voipio, J.; Kaila, K.; et al. BDNF-induced TrkB activation down-regulates the K+–Cl− cotransporter KCC2 and impairs neuronal Cl− extrusion. J. Cell Biol. 2002, 159, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Nabeshima, T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J. Pharmacol. Sci. 2003, 91, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Hardiman, O.; Doherty, C.P.; Elamin, M.; Bede, P. Neurodegenerative Disorders: A Clinical Guide; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Chang, R.C.-C. Advanced Understanding of Neurodegenerative Diseases; InTech: Rijeka, Croatia, 2011; p. 1. [Google Scholar]
- Chang, R.C.-C. Neurodegenerative Diseases: Processes, Prevention, Protection and Monitoring; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Losada-Barreiro, S.; Bravo-Diaz, C. Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. Eur. J. Med. Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.L.; Yang, C.M. Role of redox signaling in neuroinflammation and neurodegenerative diseases. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Sowndhararajan, K.; Kim, S. Neuroprotective and cognitive enhancement potentials of Angelica gigas Nakai root: A review. Sci. Pharm. 2017, 85, 21. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, C.Z.; Ding, C.; Wild, C.; Copits, B.; Swanson, G.T.; Johnson, K.M.; Zhou, J. A combined bioinformatics and chemoinformatics approach for developing asymmetric bivalent AMPA receptor positive allosteric modulators as neuroprotective agents. ChemMedChem 2013, 8, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Ansari, N.; Khodagholi, F. Natural products as promising drug candidates for the treatment of Alzheimer’s disease: Molecular mechanism aspect. Curr. Neuropharmacol. 2013, 11, 414–429. [Google Scholar] [CrossRef] [PubMed]
- Okun, M.S. Deep-brain stimulation—Entering the era of human neural-network modulation. N. Engl. J. Med. 2014, 371, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y. Recent research progress in and future perspective on treatment of Parkinson’s disease. Integr. Med. Int. 2014, 1, 67–79. [Google Scholar] [CrossRef]
- Doody, R.; Ferris, S.; Salloway, S.; Sun, Y.; Goldman, R.; Watkins, W.; Xu, Y.; Murthy, A. Donepezil treatment of patients with MCI A 48-week randomized, placebo-controlled trial. Neurology 2009, 72, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, G.T. Diagnosis and treatment of Alzheimer’s disease. J. Clin. Psychiatry 2003, 64 (Suppl. 9), 3–6. [Google Scholar] [PubMed]
- Chen, X.; Pan, W. The treatment strategies for neurodegenerative diseases by integrative medicine. Integr. Med. Int. 2014, 1, 223–225. [Google Scholar] [CrossRef]
- Fang, J.; Pang, X.; Yan, R.; Lian, W.; Li, C.; Wang, Q.; Liu, A.-L.; Du, G.-H. Discovery of neuroprotective compounds by machine learning approaches. RSC Adv. 2016, 6, 9857–9871. [Google Scholar] [CrossRef]
- Fang, J.; Li, Y.; Liu, R.; Pang, X.; Li, C.; Yang, R.; He, Y.; Lian, W.; Liu, A.L.; Du, G.H. Discovery of multitarget-directed ligands against Alzheimer’s disease through systematic prediction of chemical-protein interactions. J. Chem. Inf. Model. 2015, 55, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Villeneuve, N.F.; Wang, X.J.; Sun, Z.; Chen, W.; Li, J.; Lou, H.; Wong, P.K.; Zhang, D.D. Oridonin confers protection against arsenic-induced toxicity through activation of the Nrf2-mediated defensive response. Environ. Health Perspect. 2008, 116, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Li, C.Y.; Lu, J.M.; Tian, R.B.; Wei, J. Allicin ameliorates cognitive deficits ageing-induced learning and memory deficits through enhancing of Nrf2 antioxidant signaling pathways. Neurosci. Lett. 2012, 514, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Sun, J.; Zhang, T.T.; Ma, B.; Cui, S.M.; Chen, D.W.; He, Z.G. Pharmacokinetic behaviors and oral bioavailability of oridonin in rat plasma. Acta Pharmacol. Sin. 2006, 27, 1642–1646. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, D.; Zhao, Z.; Jia, L.; Zheng, D.; Liu, G.; Hao, L.; Zhang, Q.; Tian, X.; Li, C.; et al. Synthesis, characterization, in vitro and in vivo evaluation of PEGylated oridonin conjugates. Int. J. Pharm. 2013, 456, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Pasut, G.; Veronese, F.M. PEG conjugates in clinical development or use as anticancer agents: An overview. Adv. Drug Del. Rev. 2009, 61, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Deshpande, D.; Korde, A.; Amiji, M. A review of multifunctional nanoemulsion systems to overcome oral and CNS drug delivery barriers. Mol. Membr. Biol. 2010, 27, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Duan, C.; Zhang, D.; Jia, L.; Liu, G.; Liu, Y.; Wang, F.; Li, C.; Guo, H.; Zhang, Q. Galactosylated chitosan nanoparticles for hepatocyte-targeted delivery of oridonin. Int. J. Pharm. 2012, 436, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Del. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhang, D.; Xu, X.; Feng, F.; Ren, G.; Chu, Q.; Zhang, Q.; Tian, K. Oridonin nanosuspension was more effective than free oridonin on G2/M cell cycle arrest and apoptosis in the human pancreatic cancer PANC-1 cell line. Int. J. Nanomed. 2012, 7, 1793–1804. [Google Scholar]
- Jia, L.; Shen, J.; Zhang, D.; Duan, C.; Liu, G.; Zheng, D.; Tian, X.; Liu, Y.; Zhang, Q. In vitro and in vivo evaluation of oridonin-loaded long circulating nanostructured lipid carriers. Int. J. Biol. Macromol. 2012, 50, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, Y.; Feng, N.; Xu, J. Preparation and evaluation of self-microemulsifying drug delivery system of oridonin. Int. J. Pharm. 2008, 355, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, P.; Feng, N.; Zhang, X.; Wu, S.; Zhao, J. Optimization and in situ intestinal absorption of self-microemulsifying drug delivery system of oridonin. Int. J. Pharm. 2009, 365, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, D.; Chen, M.; Duan, C.; Dai, W.; Jia, L.; Zhao, W. Studies on pharmacokinetics and tissue distribution of oridonin nanosuspensions. Int. J. Pharm. 2008, 355, 321–327. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 3–10 and 12 are available from the authors. |
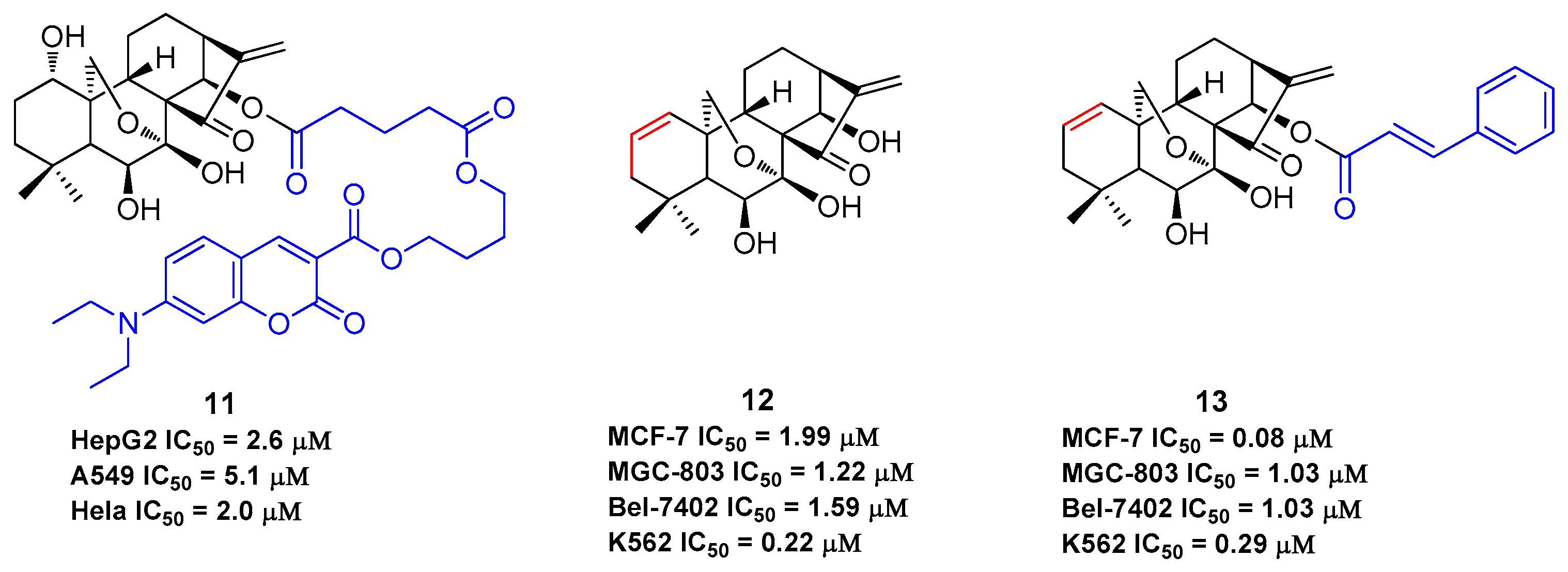
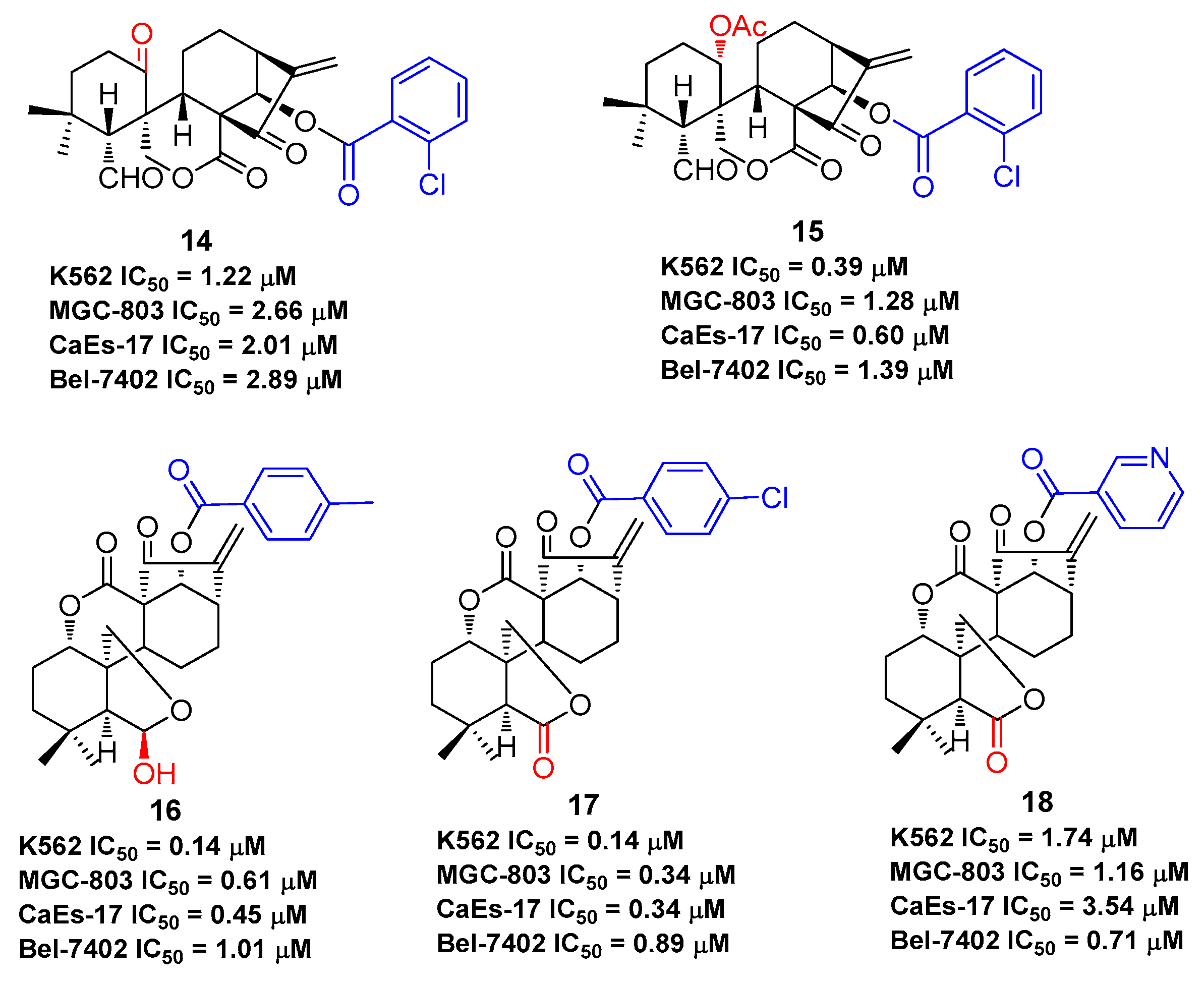

| Compd. | IC50 | Ref. |
|---|---|---|
| Oridonin (1) | MCF-7: 6.6 μM; MDA-MB-231: 29.4 μM; MDA-MB-468: 5.3 μM; MCF-7/ADR: 34.8 μM; HepG2: 15.2 μM; MGC-803: 9.06 μM; Bel-7402: 5.41 μM; K562: 4.33 μM | [45,46,47,48,49,53] |
| HAO472 (2) | Not disclosed Phase I (CTR20150246) in China: leukemia | * |
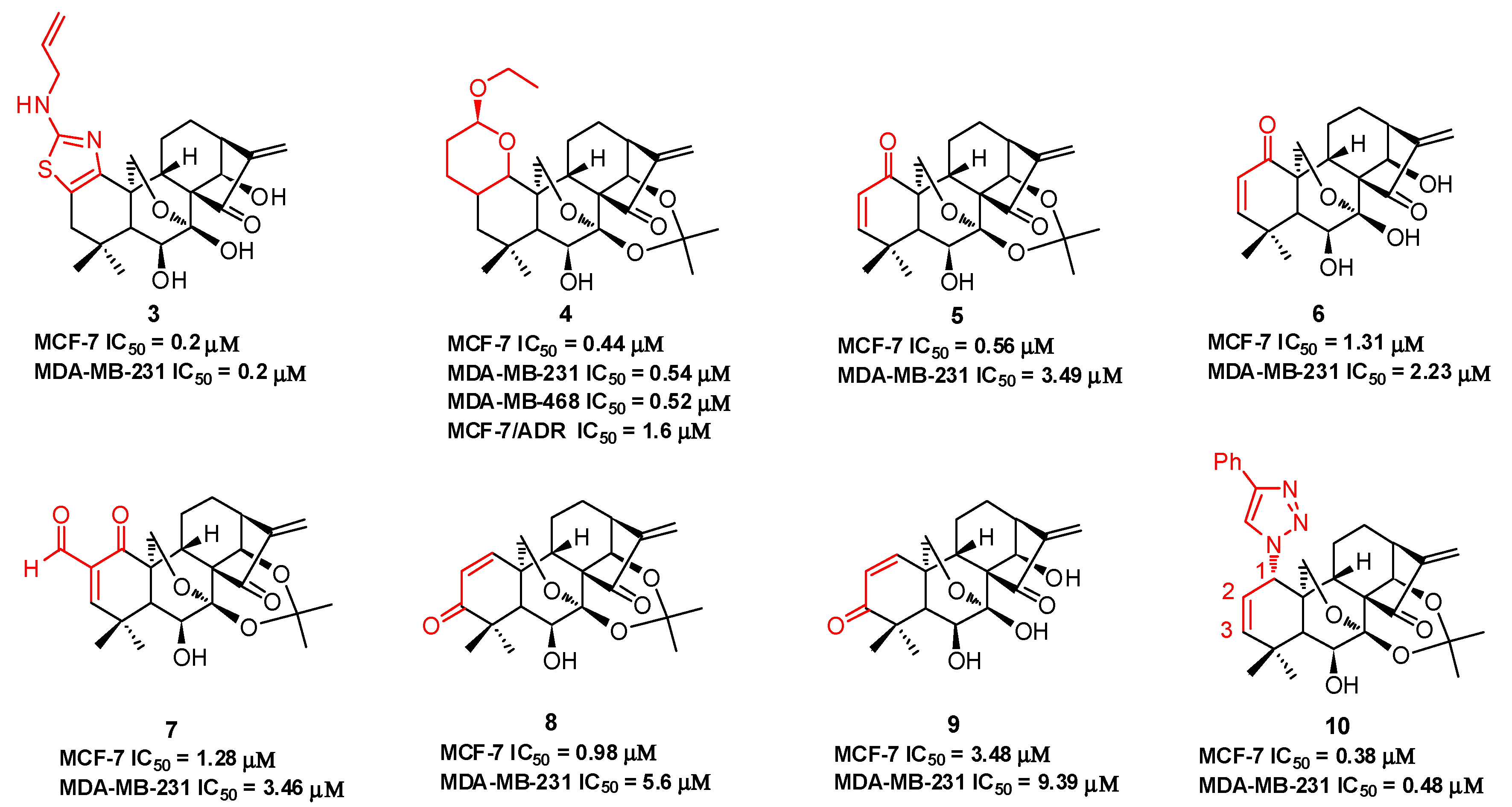
| 3 | MCF-7: 0.2 μM; MDA-MB-231: 0.2 μM; AsPC1: 1.1 μM; Panc-1: 1.1 μM; DU145: 1.2 μM | [45] |
| 4 | MCF-7: 0.44 μM; MDA-MB-231: 0.54 μM; MDA-MB-468: 0.52 μM; MCF-7/ADR: 1.6 μM | [46] |
| 5 | MCF-7: 0.56 μM; MDA-MB-231: 3.49 μM; MCF-7/ADR: 5.03 μM | [47] |
| 6 | MCF-7: 1.31 μM; MDA-MB-231: 2.23 μM; MCF-7/ADR: 5.82 μM | [47] |
| 7 | MCF-7: 1.28 μM; MDA-MB-231: 3.46 μM; MCF-7/ADR: 6.55 μM | [47] |
| 8 | MCF-7: 0.98 μM; MDA-MB-231: 5.60 μM; MCF-7/ADR: 6.02 μM | [47] |
| 9 | MCF-7: 3.48 μM; MDA-MB-231: 9.39 μM | [47] |
| 10 | MCF-7: 3.48 μM; MDA-MB-231: 9.39 μM | [48] |
| 11 | HepG2: 2.6 μM; A549: 5.1 μM; Hela: 2.0 μM | [49] |
| 12 | MCF-7: 1.99 μM; MGC-803: 1.22 μM; Bel-7402: 1.59 μM; K562: 0.22 μM | [53] |
| 13 | MCF-7: 0.08 μM; MGC-803: 1.03 μM; Bel-7402: 1.03 μM; K562: 0.29 μM | [53] |
| 14 | K562: 1.22 μM; MGC-803: 2.66 μM; CaEs-17: 2.01 μM; Bel-7402: 2.89 μM | [54] |
| 15 | K562: 0.39 μM; MGC-803: 1.28 μM; CaEs-17: 0.60 μM; Bel-7402: 1.39 μM | [55] |
| 16 | K562: 0.14 μM; MGC-803: 0.61 μM; CaEs-17: 0.45 μM; Bel-7402: 1.01 μM | [56] |
| 17 | K562: 0.14 μM; MGC-803: 0.34 μM; CaEs-17: 0.34 μM; Bel-7402: 0.89 μM | [57] |
| 18 | K562: 1.74 μM; MGC-803: 1.16 μM; CaEs-17: 3.54 μM; Bel-7402: 0.71 μM | [57] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Wold, E.A.; Ding, Y.; Shen, Q.; Zhou, J. Therapeutic Potential of Oridonin and Its Analogs: From Anticancer and Antiinflammation to Neuroprotection. Molecules 2018, 23, 474. https://doi.org/10.3390/molecules23020474
Xu J, Wold EA, Ding Y, Shen Q, Zhou J. Therapeutic Potential of Oridonin and Its Analogs: From Anticancer and Antiinflammation to Neuroprotection. Molecules. 2018; 23(2):474. https://doi.org/10.3390/molecules23020474
Chicago/Turabian StyleXu, Jimin, Eric A. Wold, Ye Ding, Qiang Shen, and Jia Zhou. 2018. "Therapeutic Potential of Oridonin and Its Analogs: From Anticancer and Antiinflammation to Neuroprotection" Molecules 23, no. 2: 474. https://doi.org/10.3390/molecules23020474