Precision Aliphatic Polyesters via Segmer Assembly Polymerization
Abstract
:1. Introduction
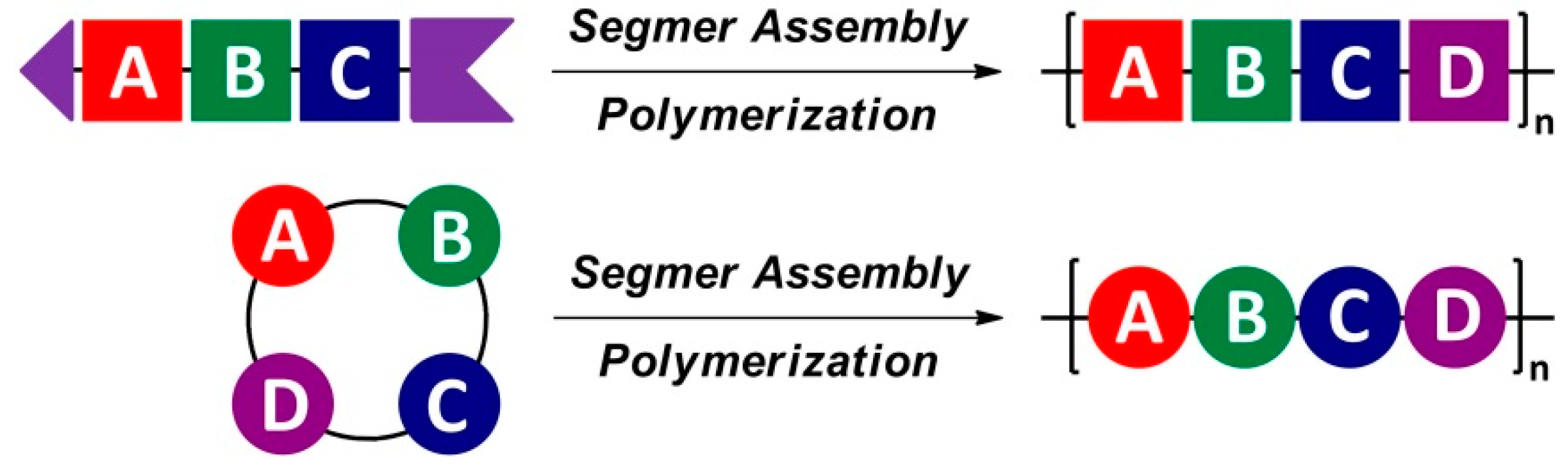
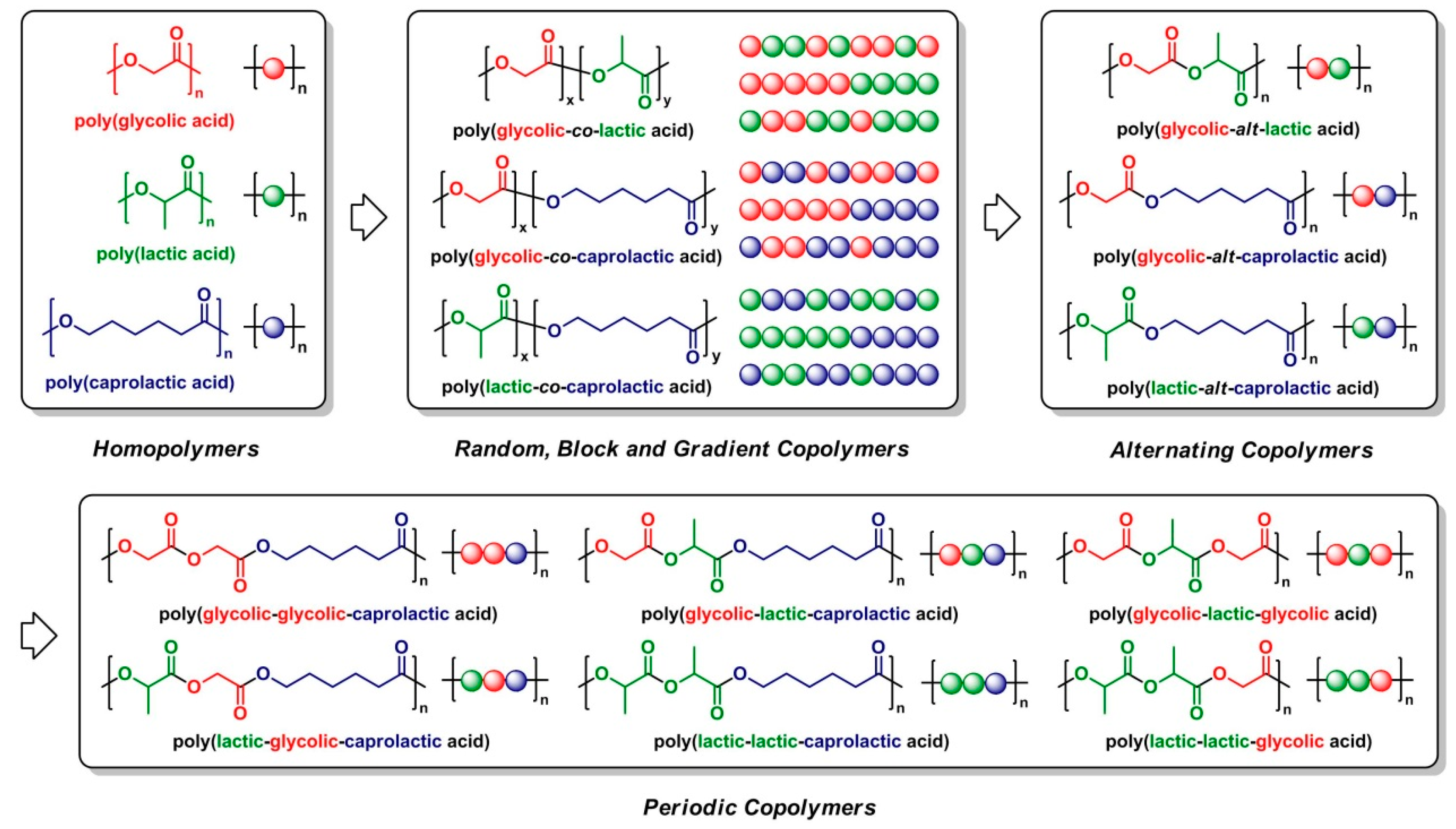
2. Segmer Assembly Polymerization (SAP)
3. Nucleophilic Displacement
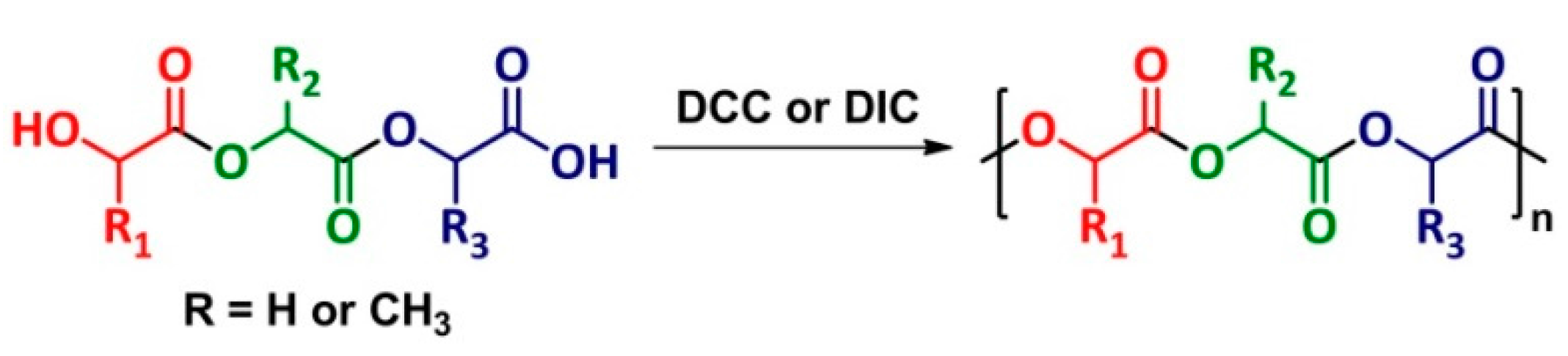
4. Polyesterification
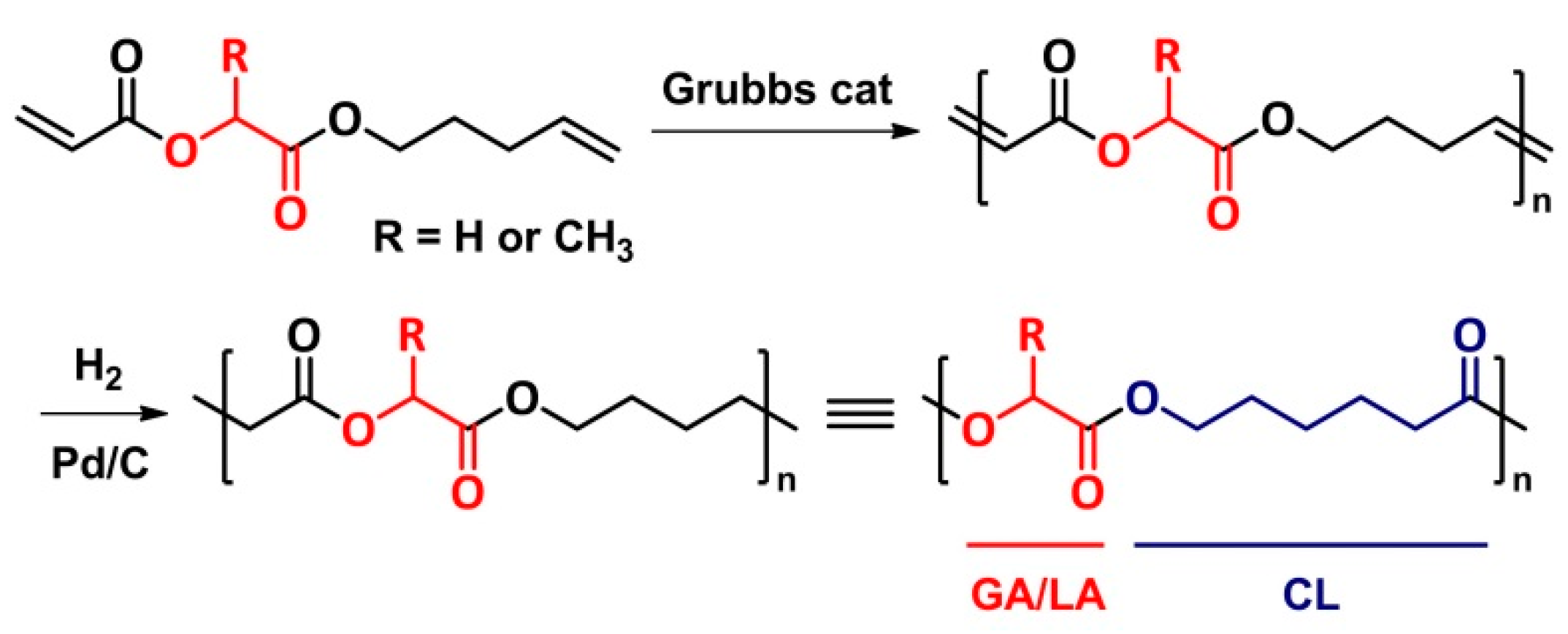
5. Cross-Metathesis Polymerization (CMP)
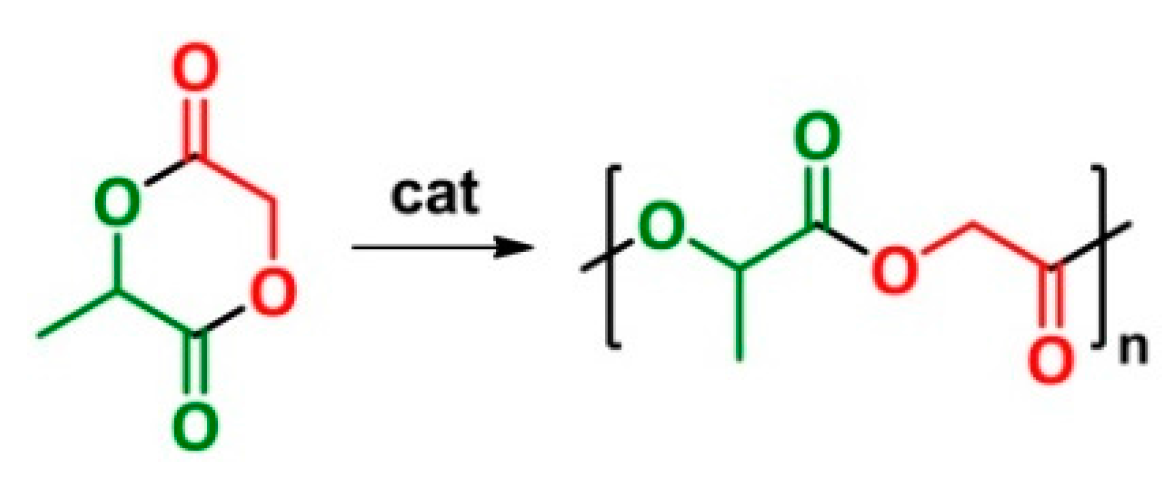
6. Ring-Opening Polymerization (ROP)
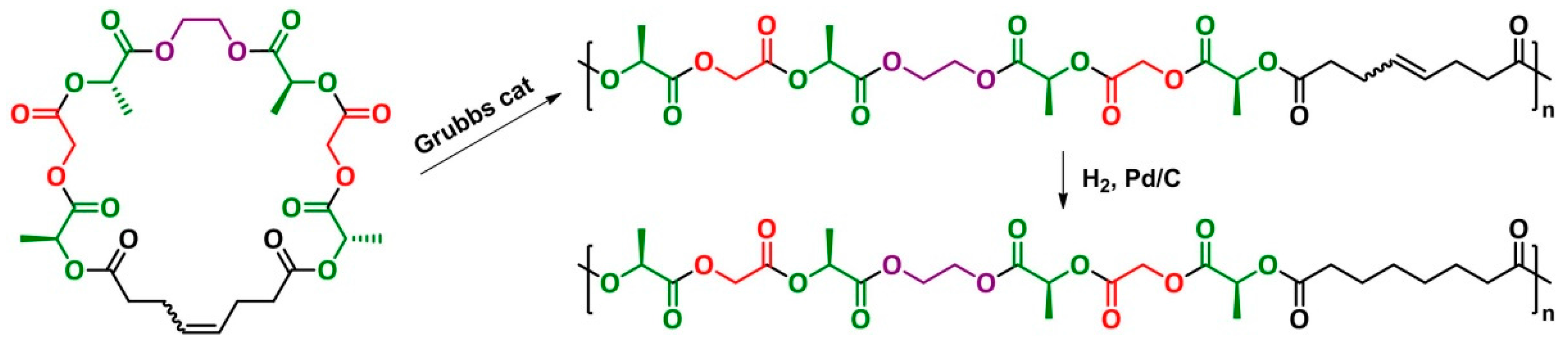
7. Ring-Opening Metathesis Polymerization (ROMP)
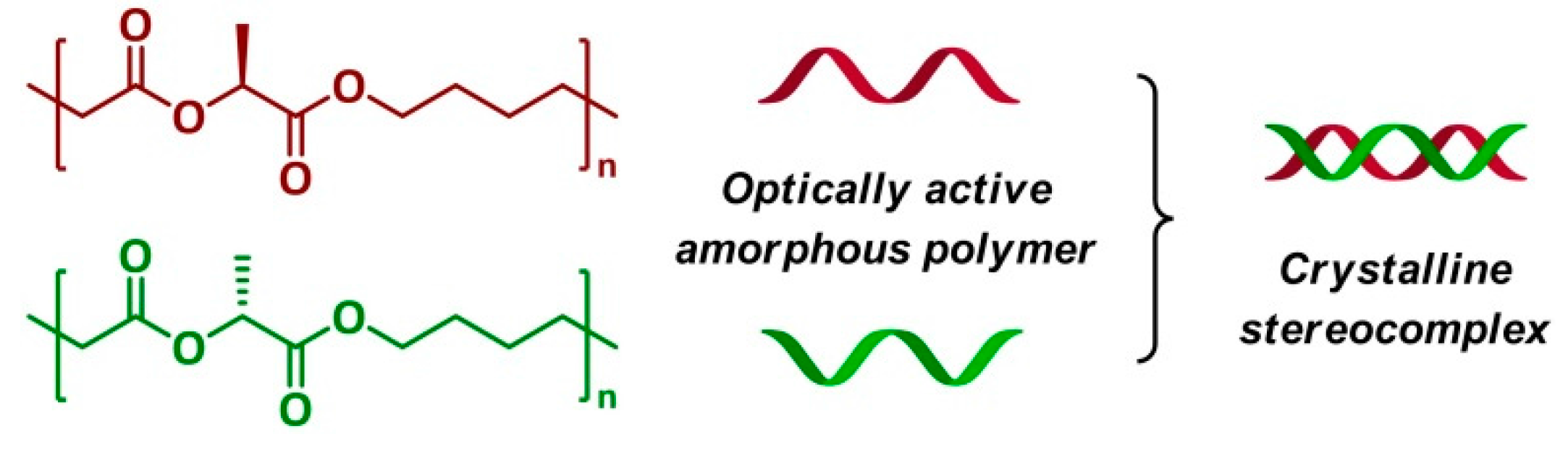
8. Entropy-Driven Ring-Opening Metathesis Polymerization (ED-ROMP)
9. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rieger, B.; Künkel, A.; Coates, G.W.; Reichardt, R.; Dinjus, E.; Zevaco, T.A. Synthetic Biodegradable Polymers; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Scholz, C. Polymers for Biomedicine: Synthesis, Characterization, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Odian, G. Step Polymerization. In Principles of Polymerization, 4th ed.; Odian, G., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 39–197. [Google Scholar]
- Odian, G. Ring-Opening Polymerization. In Principles of Polymerization, 4th ed.; Odian, G., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 544–618. [Google Scholar]
- Lecomte, P.; Jérôme, C. Recent Developments in Ring-Opening Polymerization of Lactones. In Synthetic Biodegradable Polymers; Rieger, B., Künkel, A., Coates, G.W., Reichardt, R., Dinjus, E., Zevaco, T.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 245, pp. 173–218. [Google Scholar]
- Albertsson, A.-C.; Varma, I.K. Recent Developments in Ring Opening Polymerization of Lactones for Biomedical Applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef] [PubMed]
- Kamber, N.E.; Jeong, W.; Waymouth, R.M.; Pratt, R.C.; Lohmeijer, B.G.G.; Hedrick, J.L. Organocatalytic Ring-Opening Polymerization. Chem. Rev. 2007, 107, 5813–5840. [Google Scholar] [CrossRef] [PubMed]
- Dove, A.P. Controlled Ring-Opening Polymerisation of Cyclic Esters: Polymer Blocks in Self-Assembled Nanostructures. Chem. Commun. 2008, 48, 6446–6470. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M. Stereocontrolled Ring-Opening Polymerization of Cyclic Esters: Synthesis of New Polyester Microstructures. Chem. Soc. Rev. 2010, 39, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Ajellal, N.; Carpentier, J.-F.; Guillaume, C.; Guillaume, S.M.; Helou, M.; Poirier, V.; Sarazin, Y.; Trifonov, A. Metal-Catalyzed Immortal Ring-Opening Polymerization of Lactones, Lactides and Cyclic Carbonates. Dalton Trans. 2010, 39, 8363–8376. [Google Scholar] [CrossRef] [PubMed]
- Kiesewetter, M.K.; Shin, E.J.; Hedrick, J.L.; Waymouth, R.M. Organocatalysis: Opportunities and Challenges for Polymer Synthesis. Macromolecules 2010, 43, 2093–2107. [Google Scholar] [CrossRef]
- Brown, H.A.; Waymouth, R.M. Zwitterionic Ring-Opening Polymerization for the Synthesis of High Molecular Weight Cyclic Polymers. Acc. Chem. Res. 2013, 46, 2585–2596. [Google Scholar] [CrossRef] [PubMed]
- Hillmyer, M.A.; Tolman, W.B. Aliphatic Polyester Block Polymers: Renewable, Degradable, and Sustainable. Acc. Chem. Res. 2014, 47, 2390–2396. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Bibal, B. Hydrogen-Bonding Organocatalysts for Ring-Opening Polymerization. Green Chem. 2014, 16, 1687–1699. [Google Scholar] [CrossRef]
- Penczek, S.; Cypryk, M.; Duda, A.; Kubisa, P.; Słomkowski, S. Living Ring-Opening Polymerizations of Heterocyclic Monomers. Prog. Polym. Sci. 2007, 32, 247–282. [Google Scholar] [CrossRef]
- Jérôme, C.; Lecomte, P. Recent Advances in the Synthesis of Aliphatic Polyesters by Ring-Opening Polymerization. Adv. Drug Deliv. Rev. 2008, 60, 1056–1076. [Google Scholar] [CrossRef] [PubMed]
- Martin Vaca, B.; Bourissou, D. O-Carboxyanhydrides: Useful Tools for the Preparation of Well-Defined Functionalized Polyesters. ACS Macro Lett. 2015, 4, 792–798. [Google Scholar] [CrossRef]
- Anderson, J.M.; Shive, M.S. Biodegradation and Biocompatibility of PLA and PLGA Microspheres. Adv. Drug Deliv. Rev. 1997, 28, 5–24. [Google Scholar] [CrossRef]
- Langer, R. Biomaterials in Drug Delivery and Tissue Engineering: One Laboratory's Experience. Acc. Chem. Res. 2000, 33, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.C.; Tipton, A.J. Synthetic Biodegradable Polymers as Orthopedic Devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Ikada, Y.; Tsuji, H. Biodegradable Polyesters for Medical and Ecological Applications. Macromol. Rapid Commun. 2000, 21, 117–132. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, Structure and Properties of Polyhydroxyalkanoates: Biological Polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Ma, P.X.; Choi, J.-W. Biodegradable Polymer Scaffolds with Well-Defined Interconnected Spherical Pore Network. Tissue Eng. 2001, 7, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Hatefi, A.; Amsden, B. Biodegradable Injectable in situ Forming Drug Delivery Systems. J. Control. Release 2002, 80, 9–28. [Google Scholar] [CrossRef]
- Panyam, J.; Labhasetwar, V. Biodegradable Nanoparticles for Drug and Gene Delivery to Cells and Tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable Polymers as Biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable Polymers from Renewable Resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Polymers as Biomaterials for Tissue Engineering and Controlled Drug Delivery. In Tissue Engineering I; Lee, K., Kaplan, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 102, pp. 47–90. [Google Scholar]
- Jain, R.A. The Manufacturing Techniques of Various Drug Loaded Biodegradable Poly(lactide-co-glycolide) (PLGA) Devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Jagur-Grodzinski, J. Polymers for Tissue Engineering, Medical Devices, and Regenerative Medicine. Concise General Review of Recent Studies. Polym. Adv. Technol. 2006, 17, 395–418. [Google Scholar] [CrossRef]
- Puranik, A.S.; Dawson, E.R.; Peppas, N.A. Recent Advances in Drug Eluting Stents. Int. J. Pharm. 2013, 441, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.M.; Dove, A.P. Poly(lactide)s as Robust Renewable Materials. In Green Polymerization Methods: Renewable Starting Materials, Catalysis and Waste Reduction; Mathers, R.T., Meier, M.A.R., Eds.; WILEY-VCH: Weinheim, Germany, 2011; pp. 201–220. [Google Scholar]
- Södergård, A.; Stolt, M. Properties of Lactic Acid Based Polymers and Their Correlation with Composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Wu, J.; Yu, T.-L.; Chen, C.-T.; Lin, C.-C. Recent Developments in Main Group Metal Complexes Catalyzed/Initiated Polymerization of Lactides and Related Cyclic Esters. Coord. Chem. Rev. 2006, 250, 602–626. [Google Scholar] [CrossRef]
- Stanford, M.J.; Dove, A.P. Stereocontrolled Ring-Opening Polymerisation of Lactide. Chem. Soc. Rev. 2010, 39, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Hung, W.-C.; Huang, B.-H.; Lin, C.-C. Recent Developments in Metal-Catalyzed Ring-Opening Polymerization of Lactides and Glycolides: Preparation of Polylactides, Polyglycolide, and Poly(lactide-co-glycolide). In Synthetic Biodegradable Polymers; Rieger, B., Künkel, A., Coates, G.W., Reichardt, R., Dinjus, E., Zevaco, T.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 245, pp. 219–284. [Google Scholar]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled Ring-Opening Polymerization of Lactide and Glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef] [PubMed]
- Labet, M.; Thielemans, W. Synthesis of Polycaprolactone: A Review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Matthew, H.W.T. Polymers for Tissue Engineering Scaffolds. In Polymeric Biomaterials, Second Edition, Revised and Expanded; Dumitriu, S., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2002; pp. 167–186. [Google Scholar]
- Williams, D.F. On the Nature of Biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef] [PubMed]
- Lunt, J. Large-Scale Production, Properties and Commercial Applications of Polylactic Acid Polymers. Polym. Degrad. Stab. 1998, 59, 145–152. [Google Scholar] [CrossRef]
- Shoichet, M.S. Polymer Scaffolds for Biomaterials Applications. Macromolecules 2010, 43, 581–591. [Google Scholar] [CrossRef]
- Anderson, J.M.; Shive, M.S. Biodegradation and Biocompatibility of PLA and PLGA Microspheres. Adv. Drug Deliv. Rev. 2012, 64, 72–82. [Google Scholar] [CrossRef]
- Ignatius, A.A.; Claes, L.E. In Vitro Biocompatibility of Bioresorbable Polymers: Poly(L, DL-lactide) and Poly(L-lactide-co-glycolide). Biomaterials 1996, 17, 631–639. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-Based Materials in Biomedical Applications. J. Biomater. Sci., Polym. Ed. 2017. [Google Scholar] [CrossRef] [PubMed]
- Douglas Baumann, M.; Kang, C.E.; Tator, C.H.; Shoichet, M.S. Intrathecal Delivery of a Polymeric Nanocomposite Hydrogel after Spinal Cord Injury. Biomaterials 2010, 31, 7631–7639. [Google Scholar] [CrossRef] [PubMed]
- Gilding, D.K.; Reed, A.M. Biodegradable Polymers for Use in Surgery—Polyglycolic/Poly(lactic acid) Homo- and Copolymers: 1. Polymer 1979, 20, 1459–1464. [Google Scholar] [CrossRef]
- Reed, A.M.; Gilding, D.K. Biodegradable Polymers for Use in Surgery—Poly(glycolic)/Poly(lactic acid) Homo and Copolymers: 2. In Vitro Degradation. Polymer 1981, 22, 494–498. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Putnam, D. The Heart of the Matter. Nat. Mater. 2008, 7, 836–837. [Google Scholar] [CrossRef] [PubMed]
- Malyala, P.; O’Hagan, D.T.; Singh, M. Enhancing the Therapeutic Efficacy of CpG Oligonucleotides Using Biodegradable Microparticles. Adv. Drug Deliv. Rev. 2009, 61, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Campolongo, M.J.; Luo, D. Old Polymer Learns New Tracts. Nat. Mater. 2009, 8, 447–448. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S.S.; DeLuca, P.P. Methods to Assess in Vitro Drug Release from Injectable Polymeric Particulate Systems. Pharm. Res. 2006, 23, 460–474. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, K.A.; Niederauer, G.G.; Mauli Agrawal, C. Sterilization, Toxicity, Biocompatibility and Clinical Applications of Polylactic Acid/Polyglycolic Acid Copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Rose, F.R.A.J.; Oreffo, R.O.C. Bone Tissue Engineering: Hope vs Hype. Biochem. Biophys. Res. Commun. 2002, 292, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Seal, B.L.; Otero, T.C.; Panitch, A. Polymeric Biomaterials for Tissue and Organ Regeneration. Mater. Sci. Eng. R 2001, 34, 147–230. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in Tissue Engineering Bone and Cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Cai, Q.; Bei, J.; Wang, S. Synthesis and Degradation of a Tri-Component Copolymer Derived from Glycolide, L-Lactide, and ε-Caprolactone. J. Biomater. Sci., Polym. Ed. 2000, 11, 273–288. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer - Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Lamberti, M.; Pappalardo, D.; Pellecchia, C. Random Copolymerization of ε-Caprolactone and Lactides Promoted by Pyrrolylpyridylamido Aluminum Complexes. Macromolecules 2012, 45, 8614–8620. [Google Scholar] [CrossRef]
- Chile, L.-E.; Mehrkhodavandi, P.; Hatzikiriakos, S.G. A Comparison of the Rheological and Mechanical Properties of Isotactic, Syndiotactic, and Heterotactic Poly(lactide). Macromolecules 2016, 49, 909–919. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, K.J.; Shen, Z.; Yao, K.-M. Synthesis and Characterization of Highly Random Copolymer of ε-caprolactone and D,L-Lactide Using Rare Earth Catalyst. J. Polym. Sci., Part A: Polym. Chem. 1996, 34, 1799–1805. [Google Scholar] [CrossRef]
- Dean Allison, S. Effect of Structural Relaxation on the Preparation and Drug Release Behavior of Poly(lactic-co-glycolic) Acid Microparticle Drug Delivery Systems. J. Pharm. Sci. 2008, 97, 2022–2035. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, D.; Entrialgo-Castaño, M.; Kratz, K.; Lendlein, A. Knowledge-Based Approach towards Hydrolytic Degradation of Polymer-Based Biomaterials. Adv. Mater. 2009, 21, 3237–3245. [Google Scholar] [CrossRef] [PubMed]
- Zolnik, B.S.; Burgess, D.J. Effect of Acidic pH on PLGA Microsphere Degradation and Release. J. Control. Release 2007, 122, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Dean Allison, S. Analysis of Initial Burst in PLGA Microparticles. Expert Opin. Drug Deliv. 2008, 5, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, M.M.; Blanco, D.; Cruz, M.E.M.; Alonso, M.J. Formulation of L-Asparaginase-Loaded Poly(lactide-co-glycolide) Nanoparticles: Influence of Polymer Properties on Enzyme Loading, Activity and in Vitro Release. J. Control. Release 1998, 52, 53–62. [Google Scholar] [CrossRef]
- Witschi, C.; Doelker, E. Influence of the Microencapsulation Method and Peptide Loading on Poly(lactic acid) and Poly(lactic-co-glycolic acid) Degradation during in Vitro Testing. J. Control. Release 1998, 51, 327–341. [Google Scholar] [CrossRef]
- Tracy, M.A.; Ward, K.L.; Firouzabadian, L.; Wang, Y.; Dong, N.; Qian, R.; Zhang, Y. Factors Affecting the Degradation Rate of Poly(lactide-co-glycolide) Microspheres in Vivo and in Vitro. Biomaterials 1999, 20, 1057–1062. [Google Scholar] [CrossRef]
- Alexis, F. Factors Affecting the Degradation and Drug-Release Mechanism of Poly(lactic acid) and Poly[(lactic acid)-co-(glycolic acid)]. Polym. Int. 2005, 54, 36–46. [Google Scholar] [CrossRef]
- Bigg, D.M. Polylactide Copolymers: Effect of Copolymer Ratio and End Capping on Their Properties. Adv. Polym. Technol. 2005, 24, 69–82. [Google Scholar] [CrossRef]
- Leemhuis, M.; Kruijtzer, J.A.W.; van Nostrum, C.F.; Hennink, W.E. In Vitro Hydrolytic Degradation of Hydroxyl-Functionalized Poly(α-hydroxy acid)s. Biomacromolecules 2007, 8, 2943–2949. [Google Scholar] [CrossRef] [PubMed]
- Giteau, A.; Venier-Julienne, M.C.; Aubert-Pouëssel, A.; Benoit, J.P. How to Achieve Sustained and Complete Protein Release from PLGA-Based Microparticles? Int. J. Pharm. 2008, 350, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Dailey, L.A.; Kissel, T. New Poly(lactic-co-glycolic acid) Derivatives: Modular Polymers with Tailored Properties. Drug Discov. Today: Technol. 2005, 2, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chan, J.M.; Gu, F.X.; Rhee, J.-W.; Wang, A.Z.; Radovic-Moreno, A.F.; Alexis, F.; Langer, R.; Farokhzad, O.C. Self-Assembled Lipid Polymer Hybrid Nanoparticles: A Robust Drug Delivery Platform. ACS Nano 2008, 2, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Kokai, L.E.; Ghaznavi, A.M.; Marra, K.G. Incorporation of Double-Walled Microspheres into Polymer Nerve Guides for the Sustained Delivery of Glial Cell Line-Derived Neurotrophic Factor. Biomaterials 2010, 31, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Longo, J.M.; Sanford, M.J.; Coates, G.W. Ring-Opening Copolymerization of Epoxides and Cyclic Anhydrides with Discrete Metal Complexes: Structure-Property Relationships. Chem. Rev. 2016, 116, 15167–15197. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Ren, W.-M.; Lu, X.-B. Asymmetric Alternating Copolymerization of Meso-epoxides and Cyclic Anhydrides: Efficient Access to Enantiopure Polyesters. J. Am. Chem. Soc. 2016, 138, 11493–11496. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.W.; Treitler, D.S.; Dunn, E.W.; Castro, P.M.; Roisnel, T.; Thomas, C.M.; Coates, G.W. Polymerization of Enantiopure Monomers Using Syndiospecific Catalysts: A New Approach To Sequence Control in Polymer Synthesis. J. Am. Chem. Soc. 2009, 131, 16042–16044. [Google Scholar] [CrossRef] [PubMed]
- Jaffredo, C.G.; Chapurina, Y.; Guillaume, S.M.; Carpentier, J.-F. From Syndiotactic Homopolymers to Chemically Tunable Alternating Copolymers: Highly Active Yttrium Complexes for Stereoselective Ring-Opening Polymerization of β-Malolactonates. Angew. Chem. Int. Ed. 2014, 53, 2687–2691. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jia, Z.; Chen, C.; Cong, Y.; Mao, X.; Wu, J. Alternating Sequence Controlled Copolymer Synthesis of α-Hydroxy Acids via Syndioselective Ring-Opening Polymerization of O-Carboxyanhydrides Using Zirconium/Hafnium Alkoxide Initiators. J. Am. Chem. Soc. 2017, 139, 10723–10732. [Google Scholar] [CrossRef] [PubMed]
- Hawker, C.J.; Wooley, K.L. The Convergence of Synthetic Organic and Polymer Chemistries. Science 2005, 309, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.-F.; Meyer, T.Y.; Ouchi, M.; Sawamoto, M. Sequence-Controlled Polymers: Synthesis, Self-Assembly, and Properties; American Chemical Society: Washington, DC, USA, 2014. [Google Scholar]
- Lutz, J.-F. Sequence-Controlled Polymers; Wiley-VCH: Weinheim, Germany, 2018. [Google Scholar]
- Badi, N.; Lutz, J.-F. Sequence Control in Polymer Synthesis. Chem. Soc. Rev. 2009, 38, 3383–3390. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.-F. Sequence-Controlled Polymerizations: The Next Holy Grail in Polymer Science? Polym. Chem. 2010, 1, 55–62. [Google Scholar] [CrossRef]
- Ouchi, M.; Badi, N.; Lutz, J.-F.; Sawamoto, M. Single-Chain Technology Using Discrete Synthetic Macromolecules. Nat. Chem. 2011, 3, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Badi, N.; Chan-Seng, D.; Lutz, J.-F. Microstructure Control: An Underestimated Parameter in Recent Polymer Design. Macromol. Chem. Phys. 2013, 214, 135–142. [Google Scholar] [CrossRef]
- Lutz, J.-F.; Ouchi, M.; Liu, D.R.; Sawamoto, M. Sequence-Controlled Polymers. Science 2013, 341, 1238149. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.-F.; Lehn, J.-M.; Meijer, E.W.; Matyjaszewski, K. From Precision Polymers to Complex Materials and Systems. Nat. Rev. Mater. 2016, 1, 16024. [Google Scholar] [CrossRef]
- Li, Z.-L.; Li, Z.-C. Periodic Copolymers by Step-Growth Polymerization. In Sequence-Controlled Polymers; Lutz, J.-F., Ed.; Wiley-VCH: Weinheim, Germany, 2018; pp. 349–378. [Google Scholar]
- Rebert, N.W. Synthesis of O-(2’-Bromopropionyl)glycolic Acid and Its Polymerization: Synthesis of an Alternating Lactic and Glycolic Acid Copolymer. Macromolecules 1994, 27, 5533–5535. [Google Scholar] [CrossRef]
- Li, J.; Washington, M.A.; Bell, K.L.; Weiss, R.M.; Rothstein, S.N.; Little, S.R.; Edenborn, H.M.; Meyer, T.Y. Engineering Hydrolytic Degradation Behavior of Poly(lactic-co-glycolic acid) through Precise Control of Monomer Sequence. In Sequence-Controlled Polymers: Synthesis, Self-Assembly, and Properties; Lutz, J.-F., Meyer, T.Y., Ouchi, M., Sawamoto, M., Eds.; American Chemical Society: Washington, DC, USA, 2014; Volume 1170, pp. 271–286. [Google Scholar]
- Stayshich, R.M.; Meyer, T.Y. Preparation and Microstructural Analysis of Poly(lactic-alt-glycolic acid). J. Polym. Sci., Part A: Polym. Chem. 2008, 46, 4704–4711. [Google Scholar] [CrossRef]
- Weiss, R.M.; Li, J.; Liu, H.H.; Washington, M.A.; Giesen, J.A.; Grayson, S.M.; Meyer, T.Y. Determining Sequence Fidelity in Repeating Sequence Poly(lactic-co-glycolic acid)s. Macromolecules 2017, 50, 550–560. [Google Scholar] [CrossRef]
- Stayshich, R.M.; Meyer, T.Y. New Insights into Poly(lactic-co-glycolic acid) Microstructure: Using Repeating Sequence Copolymers To Decipher Complex NMR and Thermal Behavior. J. Am. Chem. Soc. 2010, 132, 10920–10934. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.M.; Jones, E.M.; Shafer, D.E.; Stayshich, R.M.; Meyer, T.Y. Synthesis of Repeating Sequence Copolymers of Lactic, Glycolic, and Caprolactic Acids. J. Polym. Sci., Part A: Polym. Chem. 2011, 49, 1847–1855. [Google Scholar] [CrossRef]
- Stayshich, R.M.; Weiss, R.M.; Li, J.; Meyer, T.Y. Periodic Incorporation of Pendant Hydroxyl Groups in Repeating Sequence PLGA Copolymers. Macromol. Rapid Commun. 2011, 32, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Stayshich, R.M.; Meyer, T.Y. Exploiting Sequence To Control the Hydrolysis Behavior of Biodegradable PLGA Copolymers. J. Am. Chem. Soc. 2011, 133, 6910–6913. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rothstein, S.N.; Little, S.R.; Edenborn, H.M.; Meyer, T.Y. The Effect of Monomer Order on the Hydrolysis of Biodegradable Poly(lactic-co-glycolic acid) Repeating Sequence Copolymers. J. Am. Chem. Soc. 2012, 134, 16352–16359. [Google Scholar] [CrossRef] [PubMed]
- Washington, M.A.; Swiner, D.J.; Bell, K.R.; Fedorchak, M.V.; Little, S.R.; Meyer, T.Y. The Impact of Monomer Sequence and Stereochemistry on the Swelling and Erosion of Biodegradable Poly(lactic-co-glycolic acid) Matrices. Biomaterials 2017, 117, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Washington, M.A.; Balmert, S.C.; Fedorchak, M.V.; Little, S.R.; Watkins, S.C.; Meyer, T.Y. Monomer Sequence in PLGA Microparticles: Effects on Acidic Microclimates and in Vivo Inflammatory Response. Acta Biomater. 2018, 65, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Tabata, Y.; Abe, H. Synthesis and Properties of Alternating Copolymers of 3-Hydroxybutyrate and Lactate Units with Different Stereocompositions. Macromolecules 2014, 47, 7354–7361. [Google Scholar] [CrossRef]
- Bielawski, C.W.; Grubbs, R.H. Living Ring-Opening Metathesis Polymerization. Prog. Polym. Sci. 2007, 32, 1–29. [Google Scholar] [CrossRef]
- Da Silva, L.C.; Rojas, G.; Schulz, M.D.; Wagener, K.B. Acyclic Diene Metathesis Polymerization: History, Methods and Applications. Prog. Polym. Sci. 2017, 69, 79–107. [Google Scholar] [CrossRef]
- Li, Z.-L.; Zeng, F.-R.; Ma, J.-M.; Sun, L.-H.; Zeng, Z.; Jiang, H. Precision Aliphatic Polyesters with Alternating Microstructures via Cross-Metathesis Polymerization: An Event of Sequence Control. Macromol. Rapid Commun. 2017, 38, 1700050. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.K.; Choi, T.-L.; Sanders, D.P.; Grubbs, R.H. A General Model for Selectivity in Olefin Cross Metathesis. J. Am. Chem. Soc. 2003, 125, 11360–11370. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.-R.; Ma, J.-M.; Sun, L.-H.; Zeng, Z.; Jiang, H.; Li, Z.-L. Optically Active Precision Aliphatic Polyesters via Cross-Metathesis Polymerization. Macromolecular Chemistry and Physics under review.
- Augurt, T.A.; Rosensaft, M.N.; Perciaccante, V.A. Polymers of Unsymmetrically Substituted 1,4-Dioxane-2,5-diones. U.S. Patent 4033938, 1977. [Google Scholar]
- Shen, Z.-R.; Zhu, J.-H.; Ma, Z. Synthesis and Characterization of Poly(DL-lactic acid/glycolic acid). Makromol. Chem., Rapid Commun. 1993, 14, 457–460. [Google Scholar] [CrossRef]
- Dong, C.-M.; Qiu, K.-Y.; Gu, Z.-W.; Feng, X.-D. Synthesis of Poly(D,L-lactic acid-alt-glycolic acid) from D,L-3-Methylglycolide. J. Polym. Sci., Part A: Polym. Chem. 2000, 38, 4179–4184. [Google Scholar] [CrossRef]
- Dong, C.-M.; Qiu, K.-Y.; Gu, Z.-W.; Feng, X.-D. Living Polymerization of D,L-3-Methylglycolide Initiated with Bimetallic (Al/Zn) μ-Oxo Alkoxide and Copolymers Thereof. J. Polym. Sci., Part A: Polym. Chem. 2001, 39, 357–367. [Google Scholar] [CrossRef]
- Dong, C.-M.; Qiu, K.-Y.; Gu, Z.-W.; Feng, X.-D. Synthesis of Star-Shaped Poly(D,L-Lactic Acid-alt-Glycolic Acid)-b-Poly(L-Lactic acid) with the Poly(D,L-Lactic Acid-alt-Glycolic Acid) Macroinitiator and Stannous Octoate Catalyst. J. Polym. Sci., Part A: Polym. Chem. 2002, 40, 409–415. [Google Scholar] [CrossRef]
- Dong, C.-M.; Guo, Y.-Z.; Qiu, K.-Y.; Gu, Z.-W.; Feng, X.-D. In Vitro Degradation and Controlled Release Behavior of D,L-PLGA50 and PCL-b-D,L-PLGA50 Copolymer Microspheres. J. Control. Release 2005, 107, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Gutekunst, W.R.; Hawker, C.J. A General Approach to Sequence-Controlled Polymers Using Macrocyclic Ring Opening Metathesis Polymerization. J. Am. Chem. Soc. 2015, 137, 8038–8041. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Choi, T.-L. Fast Tandem Ring-Opening/Ring-Closing Metathesis Polymerization from a Monomer Containing Cyclohexene and Terminal Alkyne. J. Am. Chem. Soc. 2012, 134, 7270–7273. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.M.; Short, A.L.; Meyer, T.Y. Sequence-Controlled Copolymers Prepared via Entropy-Driven Ring-Opening Metathesis Polymerization. ACS Macro Lett. 2015, 4, 1039–1043. [Google Scholar] [CrossRef]
- Hodge, P. Entropically Driven Ring-Opening Polymerization of Strainless Organic Macrocycles. Chem. Rev. 2014, 114, 2278–2312. [Google Scholar] [CrossRef] [PubMed]
- Tschan, M.J.-L.; Brulé, E.; Haquette, P.; Thomas, C.M. Synthesis of Biodegradable Polymers from Renewable Resources. Polym. Chem. 2012, 3, 836–851. [Google Scholar] [CrossRef]
- Fukushima, K.; Fujiwara, T. New Routes to Tailor-Made Polyesters. In Polymers for Biomedicine: Synthesis, Characterization, and Applications; Scholz, C., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 149–189. [Google Scholar]
- Tong, R. New Chemistry in Functional Aliphatic Polyesters. Ind. Eng. Chem. Res. 2017, 56, 4207–4219. [Google Scholar] [CrossRef]
- Thomas, C.M.; Lutz, J.-F. Precision Synthesis of Biodegradable Polymers. Angew. Chem. Int. Ed. 2011, 50, 9244–9246. [Google Scholar] [CrossRef] [PubMed]
- Solleder, S.C.; Schneider, R.V.; Wetzel, K.S.; Boukis, A.C.; Meier, M.A.R. Recent Progress in the Design of Monodisperse, Sequence-Defined Macromolecules. Macromol. Rapid Commun. 2017, 38, 201600711. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, F.-R.; Liang, Y.; Li, Z.-L. Precision Aliphatic Polyesters via Segmer Assembly Polymerization. Molecules 2018, 23, 452. https://doi.org/10.3390/molecules23020452
Zeng F-R, Liang Y, Li Z-L. Precision Aliphatic Polyesters via Segmer Assembly Polymerization. Molecules. 2018; 23(2):452. https://doi.org/10.3390/molecules23020452
Chicago/Turabian StyleZeng, Fu-Rong, Yang Liang, and Zi-Long Li. 2018. "Precision Aliphatic Polyesters via Segmer Assembly Polymerization" Molecules 23, no. 2: 452. https://doi.org/10.3390/molecules23020452




