Antibacterial Peptides in Dermatology–Strategies for Evaluation of Allergic Potential
Abstract
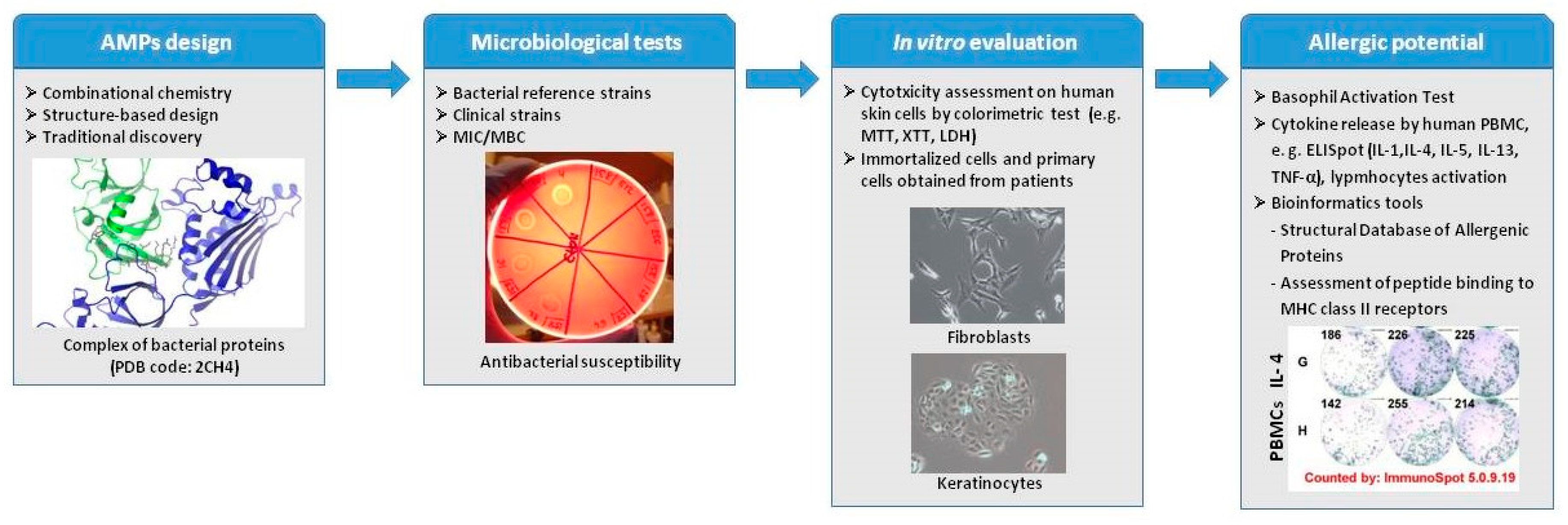
:1. Introduction
2. Adverse Reactions to Biological Drugs
3. Tests Useful in Prediction of Peptide Immunogenicity and Allergic Potential
3.1. Peptide Cytotoxicity
3.2. Basophil Activation Assay (BAT)
3.3. Cytokine Assays and Lymphocyte Activation Analysis
3.4. Bioinformatics Tools
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Komin, A.; Russell, L.M.; Hristova, K.A.; Searson, P.C. Peptide-based strategies for enhanced cell uptake, transcellular transport, and circulation: Mechanisms and challenges. Adv. Drug Deliv. Rev. 2017, 110–111, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Dennison, S.R.; Lea, B.; Snape, T.J.; Nicholl, I.D.; Radecka, I.; Harris, F. Anionic Antimicrobial and Anticancer Peptides from Plants. Crit. Rev. Plant Sci. 2013, 32, 303–320. [Google Scholar] [CrossRef]
- Brener, S.J.; Zeymer, U.; Adgey, A.A.J.; Vrobel, T.R.; Ellis, S.G.; Neuhaus, K.L.; Juran, N.; Ivanc, T.B.; Ohman, E.M.; Strony, J.; et al. Eptifibatide and low-dose tissue plasminogen activator in acute myocardial infarction: The Integrilin and Low-Dose Thrombolysis in Acute Myocardial Infarction (INTRO AMI) trial. J. Am. Coll. Cardiol. 2002, 39, 377–386. [Google Scholar] [CrossRef]
- Garcia-Calvo, M.; Peterson, E.P.; Rasper, D.M.; Vaillancourt, J.P.; Zamboni, R.; Nicholson, D.W.; Thornberry, N.A. Purification and catalytic properties of human caspase family members. Cell Death Differ. 1999, 6, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Rizzuti, M.; Nizzardo, M.; Zanetta, C.; Ramirez, A.; Corti, S. Therapeutic applications of the cell-penetrating HIV-1 Tat peptide. Drug Discov. Today 2015, 20, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Vlieghe, P.; Lisowski, V.; Martinez, J.; Khrestchatisky, M. Synthetic therapeutic peptides: Science and market. Drug Discov. Today 2010, 15, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, D.; Simerska, P.; Toth, I. Peptides as Therapeutics with Enhanced Bioactivity. Curr. Med. Chem. 2012, 19, 4451–4461. [Google Scholar] [CrossRef] [PubMed]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The Future of Peptide-based Drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Benore-Parsons, M.; Seidah, N.G.; Wennogle, L.P. Substrate phosphorylation can inhibit proteolysis by trypsin-like enzymes. Arch. Biochem. Biophys. 1989, 272, 274–280. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, S.; Yu, S.Y.; Na, D.H.; Su, Y.C.; Byun, Y.; Lee, K.C. Synthesis, characterization, and pharmacokinetic studies of PEGylated glucagon-like peptide-1. Bioconjug. Chem. 2005, 16, 377–382. [Google Scholar] [CrossRef] [PubMed]
- He, X.-H.; Shaw, P.-C.; Tam, S.-C. Reducing the immunogenicity and improving the in vivo activity of trichosanthin by site-directed pegylation. Life Sci. 1999, 65, 355–368. [Google Scholar] [CrossRef]
- Milton Harris, J.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Kosikowska, P.; Pikula, M.; Langa, P.; Trzonkowski, P.; Obuchowski, M.; Lesner, A. Synthesis and evaluation of biological activity of antimicrobial pro-proliferative peptide conjugates. PLoS ONE 2015, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Laverty, G.; McLaughlin, M.; Shaw, C.; Gorman, S.P.; Gilmore, B.F. Antimicrobial activity of short, synthetic cationic lipopeptides. Chem. Biol. Drug Des. 2010, 75, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Selsted, M.E.; Szklarek, D.; Harwig, S.S.; Daher, K.; Bainton, D.F.; Lehrer, R.I. Defensins. Natural peptide antibiotics of human neutrophils. J. Clin. Investig. 1985, 76, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Tani, K.; Murphy, W.J.; Chertov, O.; Salcedo, R.; Koh, C.Y.; Utsunomiya, I.; Funakoshi, S.; Asai, O.; Herrmann, S.H.; Wang, J.M.; et al. Defensins act as potent adjuvant taht promote cellular and humoral immune response in mice to a lymphhoma idiotype and carrier antigents. Int. Immunol. 2000, 12, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Koczulla, R.; Von Degenfeld, G.; Kupatt, C.; Krötz, F.; Zahler, S.; Gloe, T.; Issbrücker, K.; Unterberger, P.; Zaiou, M.; Lebherz, C.; et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J. Clin. Investig. 2003, 111, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Steinstraesser, L.; Koehler, T. Host Defense Peptides in Wound Healing. Mol. Med. 2008, 14, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, J.J.; Biragyn, A.; Kwak, L.W.; Yang, D. Roles of antimicrobial peptides such as defensins in innate and adaptive immunity. Ann. Rheum. Dis. 2003, 62, ii17–ii21. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Nagaoka, I.; Yamataka, A.; Kobayashi, H.; Yanai, T.; Kato, Y.; Miyano, T. Effect of antibacterial cathelicidin peptide CAP18/LL-37 on sepsis in neonatal rats. Pediatr. Surg. Int. 2005, 21, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Padhi, A.; Sengupta, M.; Sengupta, S.; Roehm, K.H.; Sonawane, A. Antimicrobial peptides and proteins in mycobacterial therapy: Current status and future prospects. Tuberculosis 2014, 94, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.; Ren, D. Antimicrobial Peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [PubMed]
- Dębowski, D.; Łukajtis, R.; Łȩgowska, A.; Karna, N.; Pikuła, M.; Wysocka, M.; Maliszewska, I.; Sieńczyk, M.; Lesner, A.; Rolka, K. Inhibitory and antimicrobial activities of OGTI and HV-BBI peptides, fragments and analogs derived from amphibian skin. Peptides 2012, 35, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Abel, J.J. Crystaline insulin. Proc. Natl. Acad. Sci. USA 1926, 12, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Swartz, J.R. Advances in Escherichia coli production of therapeutic proteins. Curr. Opin. Biotechnol. 2001, 12, 195–201. [Google Scholar] [CrossRef]
- Du Vigneaud, V.; Ressler, C.; Swan, J.M.; Roberts, C.W.; Katsoyannis, P.G.; Gordon, S. The Synthesis of an Octapeptide Amide with the Hormonal Activity of Oxytocin. J. Am. Chem. Soc. 1953, 75, 4879–4880. [Google Scholar] [CrossRef]
- Tang, J.; Liu, H.; Gao, C.; Mu, L.; Yang, S.; Rong, M.; Zhang, Z.; Liu, J.; Ding, Q.; Lai, R. A small peptide with potential ability to promote wound healing. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Strzelecka, P.; Czaplinska, D.; Sadej, R.; Wardowska, A.; Pikula, M.; Lesner, A. Simplified, serine-rich theta-defensin analogues as antitumour peptides. Chem. Biol. Drug Des. 2017, 90, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, J.; Shi, H.; Yu, L. Isolation and characterization of anti-inflammatory peptides derived from whey protein. J. Dairy Sci. 2016, 99, 6902–6912. [Google Scholar] [CrossRef] [PubMed]
- Rahnamaeian, M. Antimicrobial peptides: Modes of mechanism, modulation of defense responses. Plant Signal. Behav. 2011, 6, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Usmani, S.S.; Bedi, G.; Samuel, J.S.; Singh, S.; Kalra, S.; Kumar, P.; Ahuja, A.A.; Sharma, M.; Gautam, A.; Raghava, G.P.S. THPdb: Database of FDA-approved peptide and protein therapeutics. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.; Arkin, S.; Cocea, L.; Devanarayan, V.; Kirshner, S.; Kromminga, A.; Quarmby, V.; Richards, S.; Schneider, C.K.; Subramanyam, M.; et al. Assessment and Reporting of the Clinical Immunogenicity of Therapeutic Proteins and Peptides—Harmonized Terminology and Tactical Recommendations. AAPS J. 2014, 16, 658–673. [Google Scholar] [CrossRef] [PubMed]
- Corominas, M.; Gastaminza, G.; Lobera, T. Hypersensitivity reactions to biological drugs. J. Investig. Allergol. Clin. Immunol. 2014, 24, 212–225. [Google Scholar] [PubMed]
- Moussa, E.M.; Panchal, J.P.; Moorthy, B.S.; Blum, J.S.; Joubert, M.K.; Narhi, L.O.; Topp, E.M. Immunogenicity of Therapeutic Protein Aggregates. J. Pharm. Sci. 2016, 105, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, M.; Bird, C.; Dilger, P.; Gaines-Das, R.; Thorpe, R. Strategies for detection, measurement and characterization of unwanted antibodies induced by therapeutic biologicals. J. Immunol. Methods 2003, 278, 1–17. [Google Scholar] [CrossRef]
- Fineberg, S.E.; Galloway, J.A.; Fineberg, N.S.; Rathbun, M.J.; Hufferd, S. Immunogenicity of recombinant DNA human insulin. Diabetologia 1983, 25, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, A.; Chirmule, N.; Nair, P. Immunogenicity of Biotherapeutics: Causes and Association with Posttranslational Modifications. J. Immunol. Res. 2016, 2016, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Fathallah, A.M.; Bankert, R.B.; Balu-Iyer, S.V. Immunogenicity of subcutaneously administered therapeutic proteins—A mechanistic perspective. AAPS J. 2013, 15, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Cousens, L.P.; Alvarez, D.; Mahajan, P.B. Determinants of immunogenic response to protein therapeutics. Biologicals 2012, 40, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.; Shores, E.; Wagner, C.; Mire-Sluis, A. Scientific and regulatory considerations on the immunogenicity of biologics. Trends Biotechnol. 2006, 24, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.F.; Phillips, E.J.; Wiese, M.D.; Heddle, R.J.; Brown, S.G.A. Immediate-type hypersensitivity drug reactions. Br. J. Clin. Pharmacol. 2014, 78, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, S.R.; Grummel, V.; Hracsko, Z.; Pongratz, V.; Pernpeintner, V.; Gasperi, C.; Buck, D.; Hemmer, B. Interferon-beta specific T cells are associated with the development of neutralizing antibodies in interferon-beta treated multiple sclerosis patients. J. Autoimmun. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, L.; Ron, Y.; Kivity, S.; Ben-Horin, S.; Israeli, E.; Fraser, G.M.; Dotan, I.; Chowers, Y.; Confino-Cohen, R.; Weiss, B. Infliximab-Related Infusion Reactions: Systematic Review. J. Crohn’s Colitis 2015, 9, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Vultaggio, A.; Matucci, A.; Nencini, F.; Pratesi, S.; Parronchi, P.; Rossi, O.; Romagnani, S.; Maggi, E. Anti-infliximab IgE and non-IgE antibodies and induction of infusion-related severe anaphylactic reactions. Allergy Eur. J. Allergy Clin. Immunol. 2010, 65, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Korswagen, L.A.; Bartelds, G.M.; Krieckaert, C.L.M.; Turkstra, F.; Nurmohamed, M.T.; Van Schaardenburg, D.; Wijbrandts, C.A.; Tak, P.P.; Lems, W.F.; Dijkmans, B.A.C.; et al. Venous and arterial thromboembolic events in adalimumab-treated patients with antiadalimumab antibodies: A case series and cohort study. Arthritis Rheum. 2011, 63, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Aster, R.H.; Curtis, B.R.; McFarland, J.G.; Bougie, D.W. Drug-induced immune thrombocytopenia: Pathogenesis, diagnosis, and management. J. Thromb. Haemost. 2009, 7, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; Rossert, J.; Casadevall, N.; Stead, R.B.; Duliege, A.M.; Froissart, M.; Eckardt, K.U. A peptide-based erythropoietin-receptor agonist for pure red-cell aplasia. N. Engl. J. Med. 2009, 361, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Vultaggio, A.; Maggi, E.; Matucci, A. Immediate adverse reactions to biologicals: From pathogenic mechanisms to prophylactic management. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, C.A.; Fineberg, S.E.; Kim, D.D. Immunogenicity of xenopeptide hormone therapies. Peptides 2006, 27, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, S.; Guerin, T.; Toth, I.; Stephenson, R.J. Recent advances in self-assembled peptides: Implications for targeted drug delivery and vaccine engineering. Adv. Drug Deliv. Rev. 2017, 110–111, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Ladner, R.C.; Sato, A.K.; Gorzelany, J.; De Souza, M. Synthetic therapeutic peptides: Science and market. Drug Discov. Today 2008, 8, 40–56. [Google Scholar] [CrossRef]
- Pikuła, M.; Zieliński, M.; Specjalski, K.; Barańska-Rybak, W.; Dawgul, M.; Langa, P.; Jassem, E.; Kamysz, W.; Trzonkowski, P. In Vitro Evaluation of the Allergic Potential of Antibacterial Peptides: Camel and Citropin. Chem. Biol. Drug Des. 2016, 87, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Jiskoot, W.; Kijanka, G.; Randolph, T.W.; Carpenter, J.F.; Koulov, A.V.; Mahler, H.C.; Joubert, M.K.; Jawa, V.; Narhi, L.O. Mouse Models for Assessing Protein Immunogenicity: Lessons and Challenges. J. Pharm. Sci. 2016, 105, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Barańska-Rybak, W.; Pikula, M.; Dawgul, M.; Kamysz, W.; Trzonkowski, P.; Roszkiewicz, J. Safety profile of antimicrobial peptides: Camel, citropin, protegrin, temporin a and lipopeptide on hacat keratinocytes. Acta Pol. Pharm. Drug Res. 2013, 70, 795–801. [Google Scholar]
- Ebo, D.G.; Bridts, C.H.; Hagendorens, M.M.; Aerts, N.E.; De Clerck, L.S.; Stevens, W.J. Basophil activation test by flow cytometry: Present and future applications in allergology. Cytom. Part B Clin. Cytom. 2008, 74B, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Leysen, J.; Sabato, V.; Verweij, M.M.; De Knop, K.J.; Bridts, C.H.; De Clerck, L.S.; Ebo, D.G. The basophil activation test in the diagnosis of immediate drug hypersensitivity. Expert Rev. Clin. Immunol. 2011, 7, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Song, W.-J.; Chang, Y.-S. Recent applications of basophil activation tests in the diagnosis of drug hypersensitivity. Asia Pac. Allergy 2013, 3, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Sturm, G.J.; Kranzelbinder, B.; Sturm, E.M.; Heinemann, A.; Groselj-Strele, A.; Aberer, W. The basophil activation test in the diagnosis of allergy: Technical issues and critical factors. Allergy 2009, 64, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Pikuła, M.; Smużyńska, M.; Krzystyniak, A.; Zieliński, M.; Langa, P.; Deptuła, M.; Schumacher, A.; Łata, J.; Cichorek, M.; Grubb, A.; et al. Cystatin C peptidomimetic derivative with antimicrobial properties as a potential compound against wound infections. Bioorg. Med. Chem. 2017, 25. [Google Scholar] [CrossRef] [PubMed]
- Maggi, E. T-cell responses induced by allergen-specific immunotherapy. Clin. Exp. Immunol. 2010, 161, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, C.H.; Hoogduijn, M.J.; Franquesa, M.; Wolvius, E.B.; Brama, P.A.J.; Farrell, E. Allogeneic chondrogenically differentiated human mesenchymal stromal cells do not induce immunogenic responses from T lymphocytes in vitro. Cytotherapy 2016, 18, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.; Licenziati, S.; Corulli, M.; Canaris, A.D.; De Francesco, M.A.; Fiorentini, S.; Peroni, L.; Fallacara, F.; Dima, F.; Balsari, A.; et al. Flow cytometric analysis of activation markers on stimulated T cells and their correlation with cell proliferation. Cytometry 1997, 27, 71–76. [Google Scholar] [CrossRef]
- Pichler, W.J.; Yawalkar, N. Allergic reactions to drugs: Involvement of T cells. Thorax 2000, 55, 61S–65S. [Google Scholar] [CrossRef]
- Schein, C.H.; Ivanciuc, O.; Braun, W. Bioinformatics Approaches to Classifying Allergens and Predicting Cross-Reactivity. Immunol. Allergy Clin. N. Am. 2007, 27, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Ivanciuc, O.; Midoro-Horiuti, T.; Schein, C.H.; Xie, L.; Hillman, G.R.; Goldblum, R.M.; Braun, W. The property distance index PD predicts peptides that cross-react with IgE antibodies. Mol. Immunol. 2009, 46, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Kolla, R.V.; Sidney, J.; Weiskopf, D.; Fleri, W.; Kim, Y.; Peters, B.; Sette, A. Evaluating the Immunogenicity of Protein Drugs by Applying In Vitro MHC Binding Data and the Immune Epitope Database and Analysis Resource. Clin. Dev. Immunol. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dhanda, S.K.; Grifoni, A.; Pham, J.; Vaughan, K.; Sidney, J.; Peters, B.; Sette, A. Development of a strategy and computational application to select candidate protein analogues with reduced HLA binding and immunogenicity. Immunology 2018, 153, 118–132. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deptuła, M.; Wardowska, A.; Dzierżyńska, M.; Rodziewicz-Motowidło, S.; Pikuła, M. Antibacterial Peptides in Dermatology–Strategies for Evaluation of Allergic Potential. Molecules 2018, 23, 414. https://doi.org/10.3390/molecules23020414
Deptuła M, Wardowska A, Dzierżyńska M, Rodziewicz-Motowidło S, Pikuła M. Antibacterial Peptides in Dermatology–Strategies for Evaluation of Allergic Potential. Molecules. 2018; 23(2):414. https://doi.org/10.3390/molecules23020414
Chicago/Turabian StyleDeptuła, Milena, Anna Wardowska, Maria Dzierżyńska, Sylwia Rodziewicz-Motowidło, and Michał Pikuła. 2018. "Antibacterial Peptides in Dermatology–Strategies for Evaluation of Allergic Potential" Molecules 23, no. 2: 414. https://doi.org/10.3390/molecules23020414




