Bioactive Compounds in Cornelian Cherry Vinegars
Abstract
:1. Introduction
2. Results
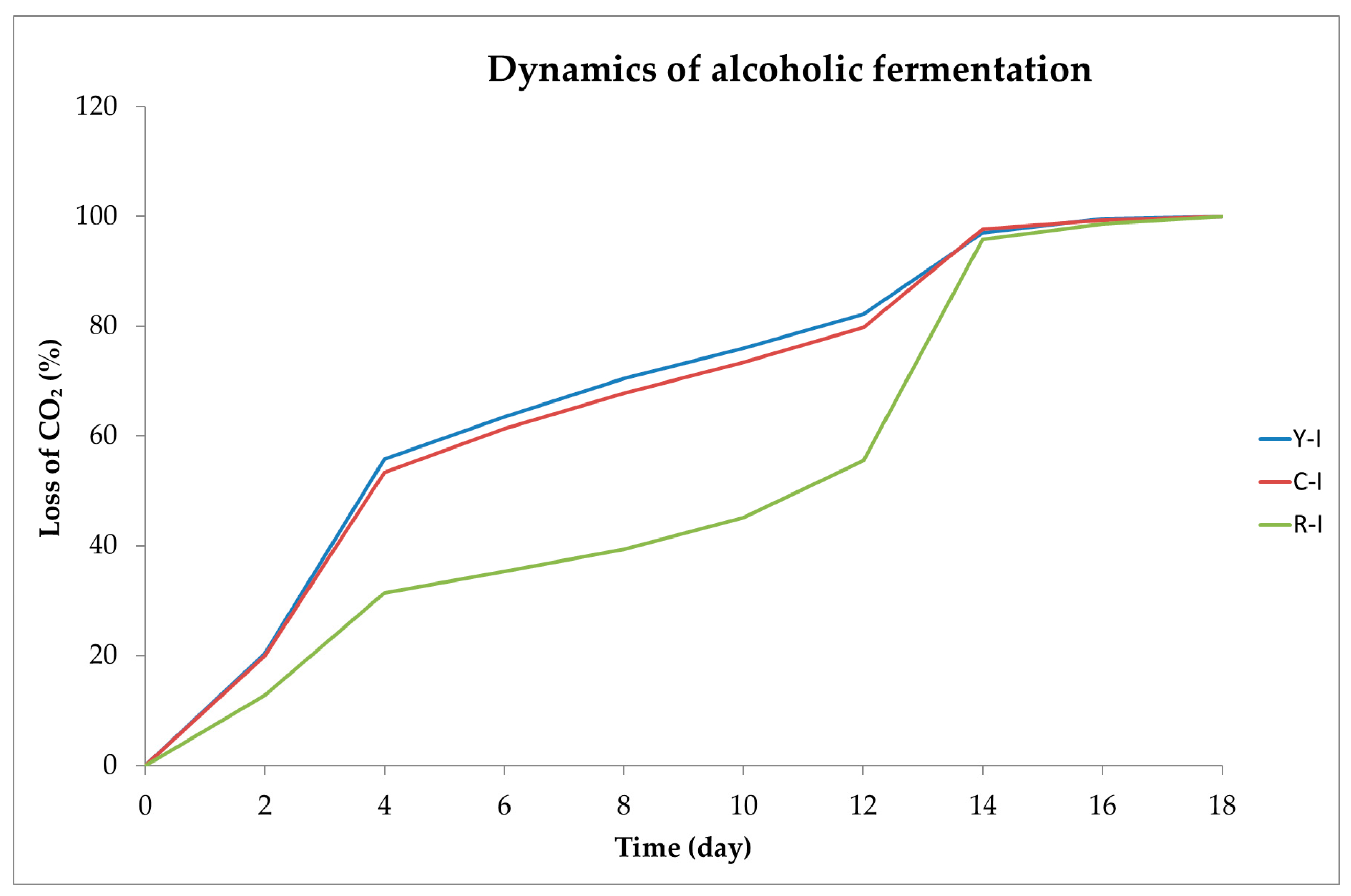
2.1. Dynamics of Alcoholic Fermentation
2.2. Extract Content, pH Value, and Concentrations of Acetic Acid, Alcohol and Glycerol
2.3. Color
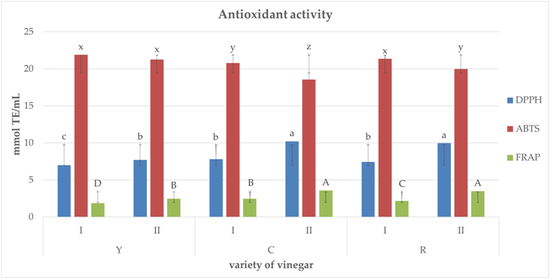
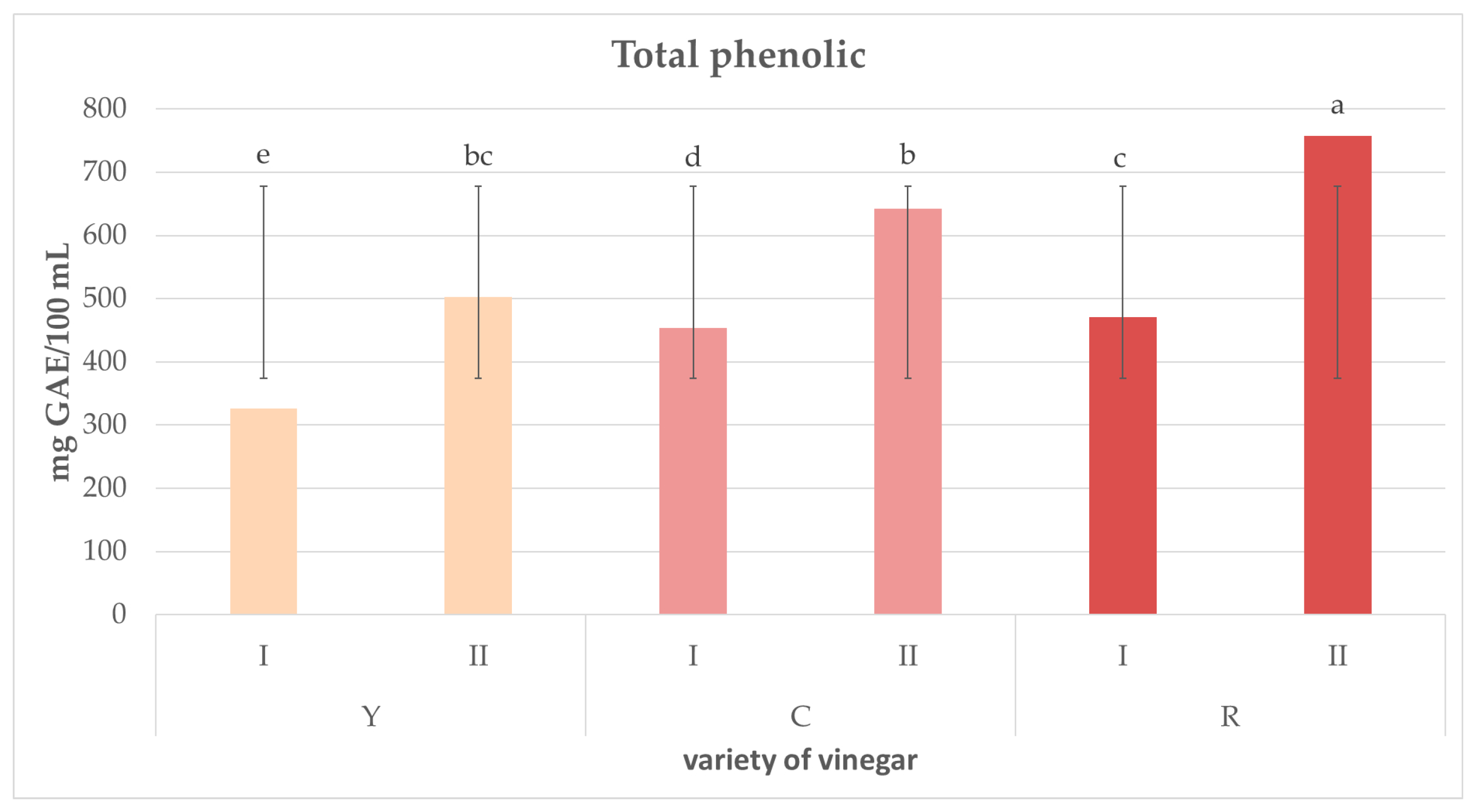
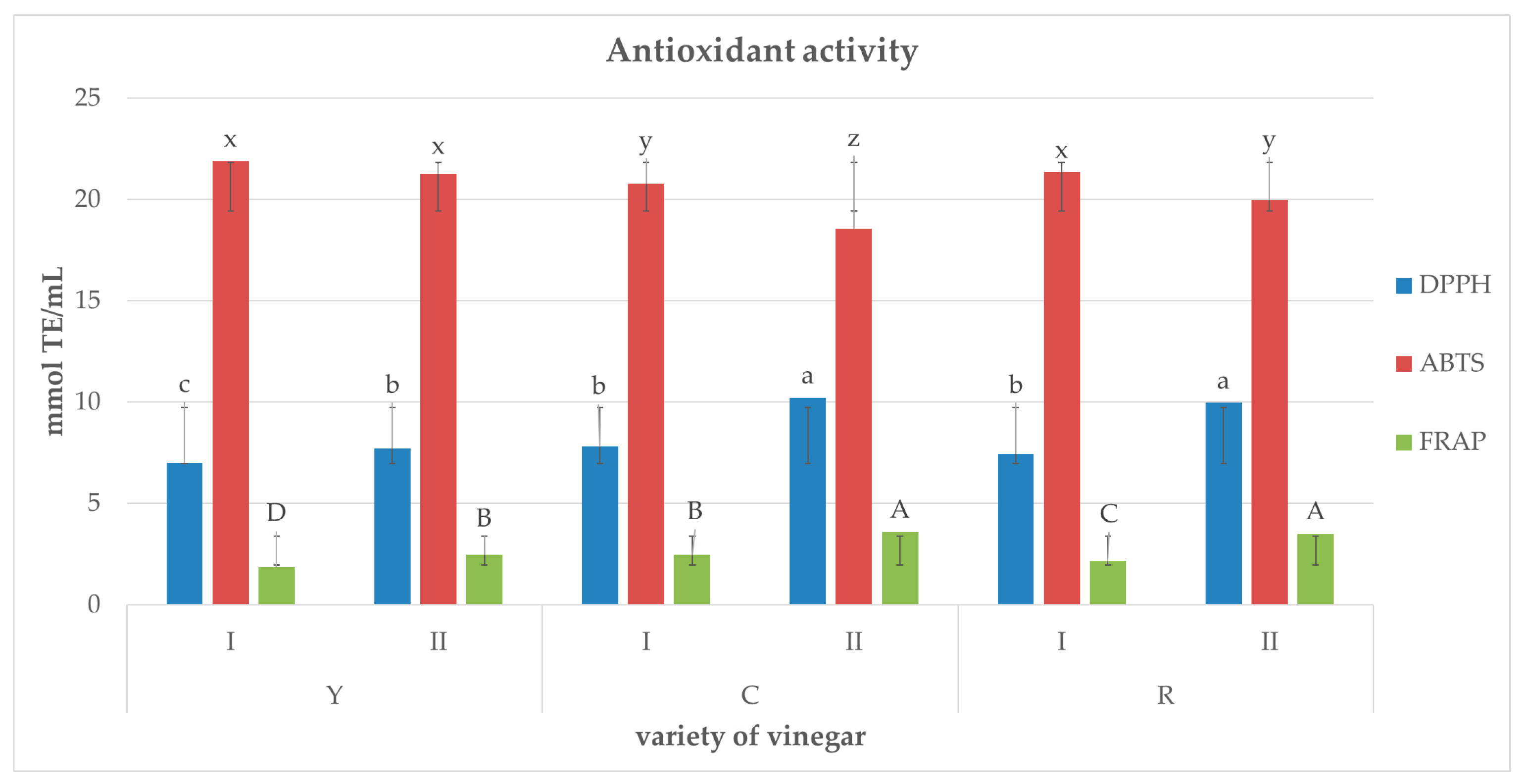
2.4. Concentration of Total Polyphenols and Antioxidative Activity
2.5. Quantitative Identification of Iridoids and Phenolic Compounds
3. Discussion
4. Materials and Methods
4.1. Reagents and Standards
4.2. Biological Material
4.3. Raw Material
4.4. Preparation of Fermentation Samples
4.5. Fermentation
4.6. Analytical Methods
4.6.1. Extract, pH, Acetic Acid, Ethanol and Glycerol Content
4.6.2. Instrumental Analysis of Color
4.6.3. Phenolic Compound Analysis
Determination of Total Polyphenols Content
Free-Radical-Scavenging Ability by the Use of a DPPH Radical
Free-Radical-Scavenging Ability by the Use of a ABTS Radical Cation.
Ferric Reducing/Antioxidant Power (FRAP) Assay
Quantification of Iridoids and Polyphenols by HPLC-PDA
4.7. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nazıroğlu, M.; Güler, M.; Özgül, C.; Saydam, G.; Küçükayaz, M.; Sözbir, E. Apple cider vinegar modulates serum lipid profile, erythrocyte, kidney, and liver membrane oxidative stress in ovariectomized mice fed high cholesterol. J. Membr. Biol. 2014, 247, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kim, J.Y.; Kim, J.; Kim, Y.J.; Kim, M.J.; Kwon, S.W.; Kwon, O. Pomegranate vinegar beverage reduces visceral fat accumulation in association with AMPK activation in overweight women: A double-blind, randomized, and placebo-controlled trial. J. Funct. Foods 2014, 8, 274–281. [Google Scholar] [CrossRef]
- Yang, J.F.; Yang, C.H.; Liang, M.T.; Gao, Z.J.; Wu, Y.W.; Chuang, L.Y. Chemical composition, antioxidant, and antibacterial activity of wood vinegar from Litchi chinensis. Molecules 2016, 21, 1150. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Kim, H.Y.; Singh, D.; Yeo, S.H.; Baek, S.Y.; Park, Y.K.; Lee, C.H. Metabolite profiling reveals the effect of dietary Rubuscoreanus vinegar on ovariectomy-induced osteoporosis in a rat model. Molecules 2016, 21, 149. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.L.; Zheng, F.J.; Sun, J.; Li, Z.C.; Lin, B.; Li, Y.R. Production and characteristics of high quality vinegar from sugarcane juice. Sugar Tech 2015, 17, 89–93. [Google Scholar] [CrossRef]
- Chou, C.H.; Liu, C.W.; Yang, D.J.; Wu, Y.H.S.; Chen, Y.C. Amino acid, mineral, and polyphenolic profiles of black vinegar, and its lipid lowering and antioxidant effects in vivo. Food Chem. 2015, 168, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Li, K.; Wang, P.; Cao, L. Effect of wine and vinegar processing of Rhizoma corydalis on the tissue distribution of tetrahydropalmatine, protopine and dehydrocorydaline in rats. Molecules 2012, 17, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Waldbauer, K.; Tang, L.; Xie, L.; McKinnon, R.; Zehl, M.; Yang, H.; Xu, H.; Kopp, B. Influence of vinegar and wine processing on the alkaloid content and composition of the traditional Chinese medicine Corydalis Rhizoma (Yanhusuo). Molecules 2014, 19, 11487–11504. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.K.H.H.; Lim, S.J. Varieties, production, composition and health benefits of vinegars: A review. Food Chem. 2017, 221, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Charoenkiatkul, S.; Thiyajai, P.; Judprasong, K. Nutrients and bioactive compounds in popular and indigenous durian (Duriozibethinusmurr.). Food Chem. 2016, 193, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Masino, F.; Chinnici, F.; Bendini, A.; Montevecchi, G.; Antonelli, A. A study on relationships among chemical, physical, and qualitative assessment in traditional balsamic vinegar. Food Chem. 2008, 106, 90–95. [Google Scholar] [CrossRef]
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm activity of plant polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, A.Z. Active Compounds of Cornelian Cherry Fruit (Cornus mas L.); Publishing House of University of Wroclaw: Wroclaw, Poland, 2012. [Google Scholar]
- Kucharska, A.Z.; Sokół-Łętowska, A.; Oszmiański, J.; Piórecki, N.; Fecka, I. Iridoids, Phenolic compounds and antioxidant activity of edible Honeysuckle berries (Loniceracaerulea var. Kamtschatica Sevast.). Molecules 2017, 22, 405. [Google Scholar] [CrossRef] [PubMed]
- Zulkawi, N.; Ng, K.H.; Zamberi, R.; Yeap, S.K.; Satharasinghe, D.; Jaganath, I.B.; Jamaluddin, A.B.; Tan, S.W.; Ho, W.Y.; Alitheen, N.B.; Long, K. In vitro characterization and in vivo toxicity, antioxidant and immunomodulatory effect of fermented foods; Xeniji™. BMC Complement. Altern. Med. 2017, 17, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, B.; David, L. Influence of temperature and preserving agents on the stability of cornelian cherries anthocyanins. Molecules 2014, 19, 8177–8188. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Lachowicz, S. Effect of the production of dried fruits and juice from chokeberry (Aroniamelanocarpa L.) on the content and antioxidative activity of bioactive compounds. Molecules 2016, 21, 1098. [Google Scholar] [CrossRef] [PubMed]
- Cosmulescu, S.; Trandafir, I.; Nour, V. Phenolic acids and flavonoids profiles of extracts from edible wild fruits and their antioxidant properties. Int. J. Food Prop. 2017, 20, 3124–3134. [Google Scholar] [CrossRef]
- Bozdogan, A. Viscosity and physicochemical properties of cornelian cherry (Cornus mas L.) concentrate. J. Food Meas. Charact. 2017, 11, 1326–1332. [Google Scholar] [CrossRef]
- Dinda, B.; Kyriakopoulos, A.M.; Dinda, S.; Zoumpourlis, V.; Thomaidis, N.S.; Velegraki, A.; Markopoulos, C.; Dinda, M. Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: Ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J. Ethnopharmacol. 2016, 193, 670–690. [Google Scholar] [CrossRef] [PubMed]
- Sozański, T.; Kucharska, A.Z.; Rapak, A.; Szumny, D.; Trocha, M.; Merwid-Ląd, A.; Dzimira, S.; Piasecki, T.; Piórecki, N.; Magdalan, J.; et al. Iridoid-loganic acid versus anthocyanins from the Cornus mas fruits (cornelian cherry): Common and different effects on diet-induced atherosclerosis, PPARs expression and inflammation. Atherosclerosis 2016, 254, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Sozański, T.; Kucharska, A.Z.; Szumny, A.; Magdalan, J.; Bielska, K.; Merwid-Ląd, A.; Woźniak, A.; Dzimira, S.; Piórecki, N.; Trocha, M. The protective effect of the Cornus mas fruits (cornelian cherry) on hypertriglyceridemia and atherosclerosis through PPARα activation in hypercholesterolemic rabbits. Phytomedicine 2014, 21, 1774–1784. [Google Scholar] [CrossRef] [PubMed]
- Sozański, T.; Kucharska, A.Z.; Szumny, A.; Magdalan, J.; Merwid-Ląd, A.; Nowak, B.; Piórecki, N.; Dzimira, S.; Jodkowska, A.; Szeląg, A.; et al. Cornelian cherry consumption increases the L-arginine/ADMA ratio, lowers ADMA and SDMA levels in the plasma, and enhances the aorta glutathione level in rabbits fed a high-cholesterol diet. J. Funct. Foods 2017, 34, 189–196. [Google Scholar] [CrossRef]
- Sáiz-Abajo, M.J.; Gonzáles-Sáiz, J.M.; Pizarro, C. Classification of wine and alcohol vinegar samples based on near-infrared spectroscopy. Feasibility study on the detection of adulterated vinegar samples. J. Agric. Food Chem. 2004, 52, 7711–7719. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, I.; Caliskan, O.Z.N.U.R.; Tornuk, F.; Ozcan, N.; Yalcin, H.; Baslar, M.; Sagdic, O. Antioxidant, antimicrobial, mineral, volatile, physicochemical and microbiological characteristics of traditional home-made Turkish vinegars. LWT-Food Sci. Technol. 2015, 63, 144–151. [Google Scholar] [CrossRef]
- Bakir, S.; Devecioglu, D.; Kayacan, S.; Toydemir, G.; Karbancioglu-Guler, F.; Capanoglu, E. Investigating the antioxidant and antimicrobial activities of different vinegars. Eur. Food Res. Technol. 2017, 243, 2083–2094. [Google Scholar] [CrossRef]
- Budak, N.H. Bioactive components of Prunus avium L. black gold (red cherry) and Prunus avium L. stark gold (white cherry) juices, wines and vinegars. J. Food Sci. Technol. 2017, 54, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Lea, A.G.H. Cider Vinegar. Processed Apple Products; Van Nostrand Reinhold: New York, NY, USA, 1989; pp. 279–301. [Google Scholar]
- Compliance Policy Guides CPG, Sec. 525.825 Vinegar, Definitions: Adulteration with Vinegar Eels. Silver Spring, MD, USA: Food and Drug Administration (FDA). 2015. Available online: http://www.fda.gov/ICECI/ComplianceManuals/CompliancePolicyGuidanceManual/ucm074471.htm (accessed on 9 September 2017).
- Saichana, N.; Matsushita, K.; Adachi, O.; Frebort, I.; Frebortova, J. Acetic acid bacteria: A group of bacteria with versatile biotechnological applications. Biotechnol. Adv. 2015, 33, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Wu, J.; Yu, Y.; Xiao, G.; Xu, Y. Evolution of the antioxidant capacity and phenolic contents of persimmon during fermentation. Food Sci. Biotechnol. 2017, 26, 563–571. [Google Scholar] [CrossRef]
- Dias, D.R.; Silva, M.S.; de Souza, A.C.; Magalhães-Guedes, K.T.; de Rezende Ribeiro, F.S.; Schwan, R.F. Vinegar production from Jabuticaba (Myrciaria jaboticaba) fruit using immobilized acetic acid bacteria. Food Technol. Biotechnol. 2016, 54, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Lucini, L.; Torchio, F.; Dordoni, R.; De Faveri, D.M.; Lambri, M. Metabolite profiling and volatiles of pineapple wine and vinegar obtained from pineapple waste. Food Chem. 2017, 229, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, S.; Pellati, F.; Melegari, M.; Bertelli, D. Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of Rubus, Ribes, and Aronia. J. Food Sci. 2004, 69, 164–169. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Bai, Y.; Fu, C.; Zhou, M.; Gao, B.; Donsheng, L.; Hu, Y.; Xu, N. Effects of mixed cultures of Saccharomyces cerevisiae and Lactobacillus plantarum in alcoholic fermentation on the physicochemical and sensory properties of citrus vinegar. LWT-Food Sci. Technol. 2017, 84, 753–763. [Google Scholar] [CrossRef]
- Natera, R.; Castro, R.; Valme-Garcia-Moreno, M.D.; Hernandez, M.J.; Garcia-Barroso, C. Chemometric studies of vinegars from different raw materials and processes of production. J. Agric. Food Chem. 2003, 51, 3345–3351. [Google Scholar] [CrossRef] [PubMed]
- Păltinean, R.; Mocan, A.; Vlase, L.; Gheldiu, A.M.; Crișan, G.; Ielciu, I.; Voștinaru, O.; Crișan, O. Evaluation of polyphenolic ontent, antioxidant and diuretic activities of six fumaria species. Molecules 2017, 22, 639. [Google Scholar] [CrossRef] [PubMed]
- Dragović-Uzelac, V.; Levaj, B.; Bursać, D.; Pedisić, S.; Radojčić, I.; Biško, A. Total phenolics and antioxidant capacity assays of selected fruits. Agric. Conspec. Sci. 2007, 72, 279–284. [Google Scholar]
- Tural, S.; Koca, I. Physico-chemical and antioxidant properties of cornelian cherry fruits (Cornus mas L.) grow in Turkey. Sci. Hortic. 2008, 116, 362–366. [Google Scholar] [CrossRef]
- Rop, O.; Mlcek, J.; Kramarova, D.; Jurikova, T. Selected cultivars of cornelian cherry (Cornus mas L.) as a new food source for human nutrition. Afr. J. Biotechnol. 2010, 9, 1205–1210. [Google Scholar]
- Özcan, M.M.; Al Juhaimi, F.; Gülcü, M.; Uslu, N.; Geçgel, Ü.; Ghafoor, K.; Dursun, N. Effect of harvest time on physico-chemical properties and bioactive compounds of pulp and seeds of grape varieties. J. Food Sci. Technol. 2017, 54, 2230–2240. [Google Scholar] [CrossRef] [PubMed]
- Obreque-Slier, E.; Pena-Neira, A.; Lopez-Solis, R.; Zamora-Marin, F.; Ricardo-da Silva, J.M.; Laureano, O. Comparative study of the phenolic composition of seeds and skins from Carménère and Cabernet Sauvignon grape varieties (Vitis vinifera L.) during ripening. J. Agric. Food Chem. 2010, 58, 3591–3599. [Google Scholar] [CrossRef] [PubMed]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Ren, M.; Wang, X.; Tian, C.; Li, X.; Zhang, B.; Song, X.; Zhang, J. Characterization of organic acids and phenolic compounds of cereal vinegars and fruit vinegars in China. J. Food Process. Preserv. 2017, 41, 1–8. [Google Scholar] [CrossRef]
- Bunea, A.; Rugina, D.O.; Pintea, A.M.; Sconta, Z.; Bunea, C.I.; Socaciu, C. Comparative polyphenolic content and antioxidant activities of some wild and cultivated blueberries from Romania. Not. Bot. Horti Agrobot. Cluj Napoca 2011, 39, 70–76. [Google Scholar]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Cerutti, A.K.; Bounous, G. Goji berry fruit (Lycium spp.): Antioxidant compound fingerprint and bioactivity evaluation. J. Funct. Foods 2015, 18, 1070–1085. [Google Scholar] [CrossRef]
- Jatoi, M.A.; Jurić, S.; Vidrih, R.; Vinceković, M.; Vuković, M.; Jemrić, T. The effects of postharvest application of lecithin to improve storage potential and quality of fresh goji (Lycium barbarum L.) berries. Food Chem. 2017, 230, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Šeruga, M.; Medvidović-Kosanović, M.; Novak, I. Antioxidant activity and polyphenols of Aronia in comparison to other berry species. Agric. Conspec. Sci. 2007, 72, 301–306. [Google Scholar]
- Rop, O.; Mlcek, J.; Jurikova, T.; Valsikova, M.; Sochor, J.; Reznicek, V.; Kramarova, D. Phenolic content, antioxidant capacity, radical oxygen species scavenging and lipid peroxidation inhibiting activities of extracts of five black chokeberry (Aronia melanocarpa (michx.) elliot) cultivars. J. Med. Plants Res. 2010, 22, 2432–2437. [Google Scholar]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Bakir, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Capanoglu, E. Fruit antioxidants during vinegar processing: Changes in content and in vitro bio-accessibility. Int. J. Mol. Sci. 2016, 17, 1658. [Google Scholar] [CrossRef] [PubMed]
- Pastoriza, S.; Rufián-Henares, J.A. Contribution of melanoidins to the antioxidant capacity of the Spanish diet. Food Chem. 2004, 164, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Kalaycıoğlu, Z.; Erim, F.B. Total phenolic contents, antioxidant activities, and bioactive ingredients of juices from pomegranate cultivars worldwide. Food Chem. 2017, 221, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, C.; Callejón, R.M.; Hidalgo, C.; Torija, M.J.; Troncoso, A.M.; Morales, M.L. Employment of different processes for the production of strawberry vinegars: Effects on antioxidant activity, total phenols and monomeric anthocyanins. LWT-Food Sci. Technol. 2013, 52, 139–145. [Google Scholar] [CrossRef]
- Kelebek, H.; Kadiroğlu, P.; Demircan, N.B.; Selli, S. Screening of bioactive components in grape and apple vinegars: Antioxidant and antimicrobial potential. J. Inst. Brew. 2017, 123, 407–416. [Google Scholar] [CrossRef]
- Turner, A.; Chen, S.N.; Nikolic, D.; van Breemen, R.; Farnsworth, N.R.; Pauli, G.F. Coumaroyl iridoids and a depside from cranberry (Vaccinium macrocarpon). J. Nat. Prod. 2007, 70, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Jenen, H.D.; Krogfelt, K.A.; Cornett, C.; Hansen, S.H.; Christensen, S.B. Hydrophilic carboxylic acids and iridoid glycosides in the juice of American and European cranberries (Vaccinium macrocarpon and V. oxycoccos), lingonberries (V. Vitis-idaea), and blueberries (V. myrtillus). J. Agric. Food Chem. 2002, 50, 6871–6874. [Google Scholar] [CrossRef]
- Deng, S.; West, B.J.; Jensen, C.J. UPLC–TOF-MS characterization and identification of bioactive iridoids in Cornus mas fruit. J. Anal. Methods Chem. 2013, 2013, 710972. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Chi, H.; Kodama, H.; Chen, G. Anti-inflammatory effect of three iridoids in human neutrophils. Nat. Prod. Res. 2013, 27, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Pietrzak, W.; Kawa-Rygielska, J. Simultaneous saccharification and ethanol fermentation of waste wheat–rye bread at very high solids loading: Effect of enzymatic liquefaction conditions. Fuel 2015, 147, 236–242. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.-C.; Chen, H.-Y. Antioxidant activity of varous tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Variety of Cornelian Cherry Fruit | Method of Fermentation | Extract (°Bx) | pH | Glycerol (g/L) | Alcohol (g/L) | Acetic Acid (g/L) |
|---|---|---|---|---|---|---|
| Y 1 | I 2 | 10.00 a ± 0.28 | 2.81 b ± 0.01 | 0.99 f ± 0.01 | 1.79 d ± 0.01 | 41.65 f ± 0.23 |
| II | 8.00 a ± 0.45 | 2.76 a ± 0.01 | 1.20 e ± 0.04 | 3.77 b ± 0.01 | 47.06 a ± 0.44 | |
| C | I | 12.80 a ± 0.28 | 2.90 d ± 0.01 | 1.56 d ± 0.03 | 7.46 a ± 0.01 | 42.30 d ± 0.24 |
| II | 11.50 a ± 0.71 | 2.86 c ± 0.01 | 1.72 c ± 0.03 | 0.00 | 42.13 e ± 0.19 | |
| R | I | 11.40 a ± 0.57 | 2.85 c ± 0.00 | 1.92 b ± 0.03 | 2.3 c ± 0.01 | 42.64 c ± 0.76 |
| II | 9.30 a ± 0.42 | 2.76 a ± 0.01 | 2.12 a ± 0.03 | 0.0 | 45.38 b ± 0.04 |
| Variety of Cornelian Cherry Fruit | Method of Fermentation | L* | a* | b* |
|---|---|---|---|---|
| Y 1 | I 2 | 70.73 ± 0.00 a,3 | 10.98 ± 0.01 f | 48.22 ± 0.04 d |
| II | 64.46 ± 0.01 b | 15.07 ± 0.01 e | 52.64 ± 0.00 c | |
| C | I | 49.85 ± 0.00 c | 36.05 ± 0.00 c | 59.97 ± 0.02 b |
| II | 41.06 ± 0.01 d | 45.79 ± 0.01 b | 61.01 ± 0.13 a | |
| R | I | 16.92 ± 0.01 e | 46.67 ± 0.00 a | 9.39 ± 0.10 e |
| II | 5.72 ± 0.26 f | 32.17 ± 0.34 d | 28.59 ± 0.40 f |
| Compound | RI | RII | CI | CII | YI | YII |
|---|---|---|---|---|---|---|
| Iridoids | ||||||
| LA | 140.71 ± 0.27 e,1 | 178.46 ± 0.73 c | 175.54 ± 1.01 c | 190.75 ± 1.19 a | 158.38 ± 0.52 d | 185.07 ± 4.18 b |
| S+Lo | 15.24 ± 0.42 e | 34.72 ± 0.80 a | 19.83 ± 0.23 d | 27.38 ± 0.97 c | 15.63 ± 0.12 f | 29.41 ± 0.03 b |
| Co. | 17.20 ± 0.02 e | 30.30 ± 0.19 c | 22.49 ± 0.04 d | 35.04 ± 0.39 a | 10.42 ± 0.08 f | 32.92 ± 0.10 b |
| Phenolic acids | ||||||
| EA | 0.87 ± 0.02 e | 1.99 ± 0.02 a | 1.13 ± 0.02 d | 1.69 ± 0.01 b | 0.62 ± 0.00 f | 1.52 ± 0.01 c |
| Total CQA d | 10.47 ± 0.00 e | 19.66 ± 0.24 b | 15.16 ± 0.15 d | 24.18 ± 0.27 a | 9.64 ± 0.05 f | 17.10 ± 0.07 b |
| Total p-CA d | 3.50 ± 0.00 f | 6.70 ± 0.43 d | 7.39 ± 0.01 c | 10.85 ± 0.02 a | 4.89 ± 0.06 e | 9.21 ± 0.01 b |
| Anthocyanins | ||||||
| Cy 3-gal | 0.29 ± 0.02 b | 0.75 ± 0.03 a | nd | nd | nd | nd |
| Cy 3-rob | 0.19 ± 0.02 b | 0.41 ± 0.00 a | nd | nd | nd | nd |
| Pg 3-gal | 0.65 ± 0.04 b | 1.69 ± 0.02 a | nd | nd | nd | nd |
| Pg 3-rob | 0.20 ± 0.01 b | 0.30 ± 0.20 a | nd | nd | nd | nd |
| Flavonols | ||||||
| A 7-glu | 1.80 ± 0.02 b | 3.35 ± 0.06 a | nd | nd | nd | nd |
| Q 3-gal | 0.32 ± 0.02 c | 0.58 ± 0.01 a | 0.39 ± 0.05 b | 0.59 ± 0.01 a | 0.19 ± 0.00 d | 0.34 ± 0.00 c |
| Q 3-gluc | 2.63 ± 0.02 e | 4.60 ± 0.05 b | 4.07 ± 0.05 c | 6.00 ± 0.05 a | 2.80 ± 0.01 d | 4.58 ± 0.00 b |
| Kp 3-gal | 1.38 ± 0.00 b | 2.53 ± 0.06 a | 0.10 ± 0.00 c | 0.17 ± 0.01 c | nd | nd |
| Symbol | Description | |
|---|---|---|
| I | Single-stage (spontaneous) alcoholic-acetic fermentation under controlled aerobic conditions. | Vinegar production method |
| II | Two-stage fermentation including first alcoholic fermentation with the use of Saccharomyces bayanus—Safspirit fruit yeast, followed by spontaneous acetic fermentation. | |
| Y | Yellow (‘Yantarnyi’ variety with yellow fruits) | Cornelian cherry variety/fruit color |
| C | Coral (‘Koralovyi’ variety with coral fruits) | |
| R | Red (‘Podolski’ variety with red fruits) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Piórecki, N. Bioactive Compounds in Cornelian Cherry Vinegars. Molecules 2018, 23, 379. https://doi.org/10.3390/molecules23020379
Kawa-Rygielska J, Adamenko K, Kucharska AZ, Piórecki N. Bioactive Compounds in Cornelian Cherry Vinegars. Molecules. 2018; 23(2):379. https://doi.org/10.3390/molecules23020379
Chicago/Turabian StyleKawa-Rygielska, Joanna, Kinga Adamenko, Alicja Z. Kucharska, and Narcyz Piórecki. 2018. "Bioactive Compounds in Cornelian Cherry Vinegars" Molecules 23, no. 2: 379. https://doi.org/10.3390/molecules23020379