Synthesis and Magnetic Properties of Stable Radical Derivatives Carrying a Phenylacetylene Unit
Abstract
:1. Introduction
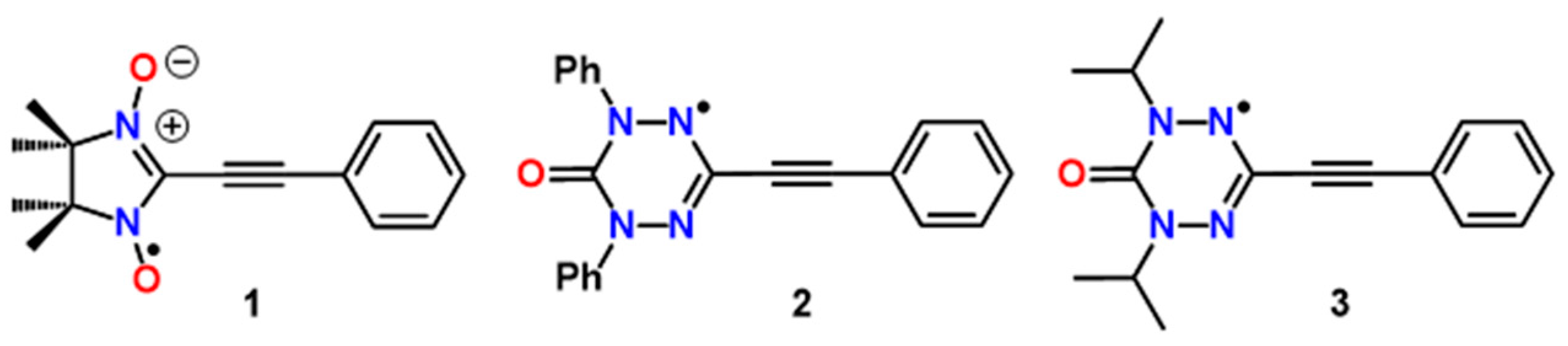
2. Results
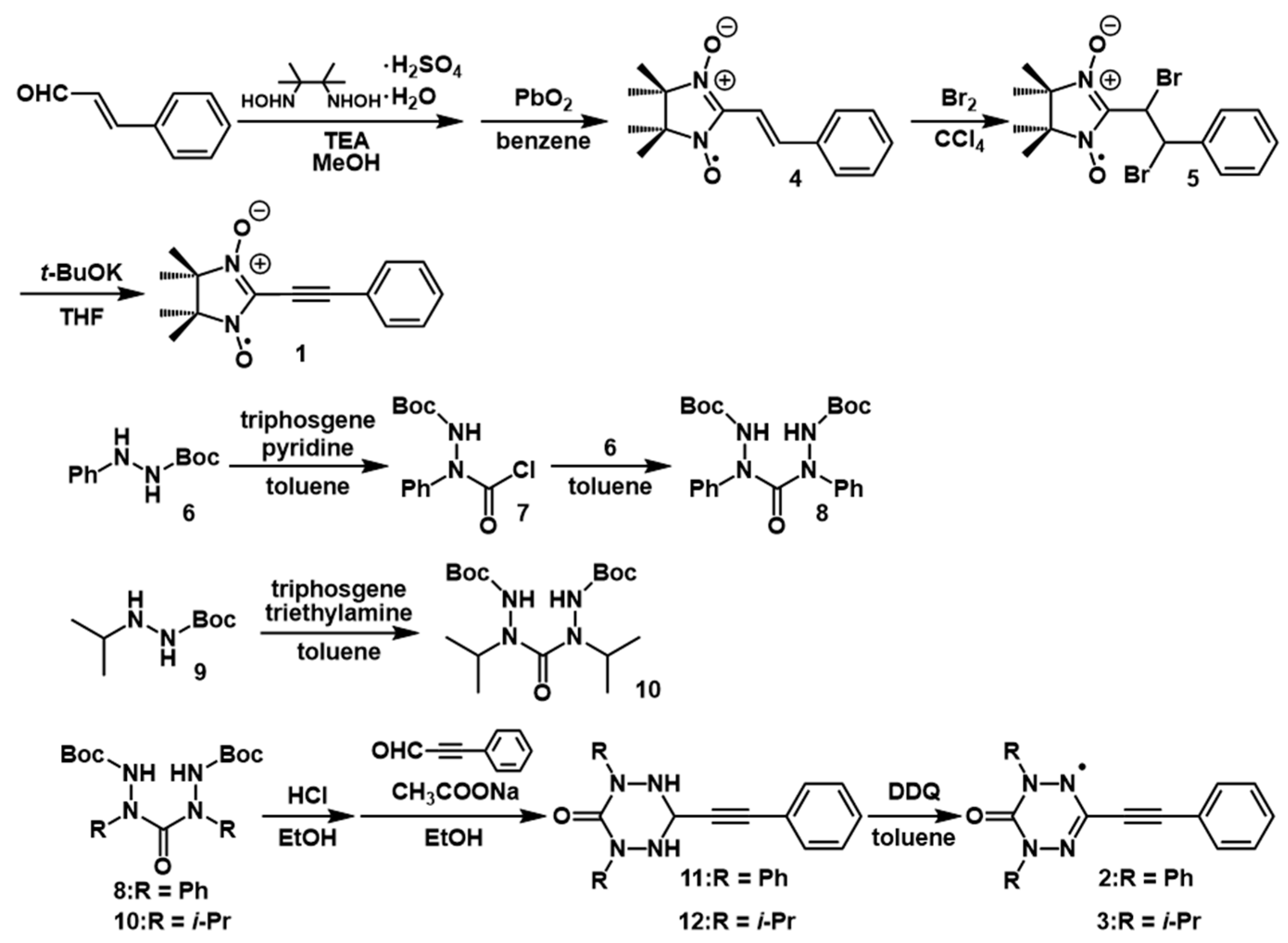
2.1. Synthesis and Electron Paramagnetic Resonance Spectra
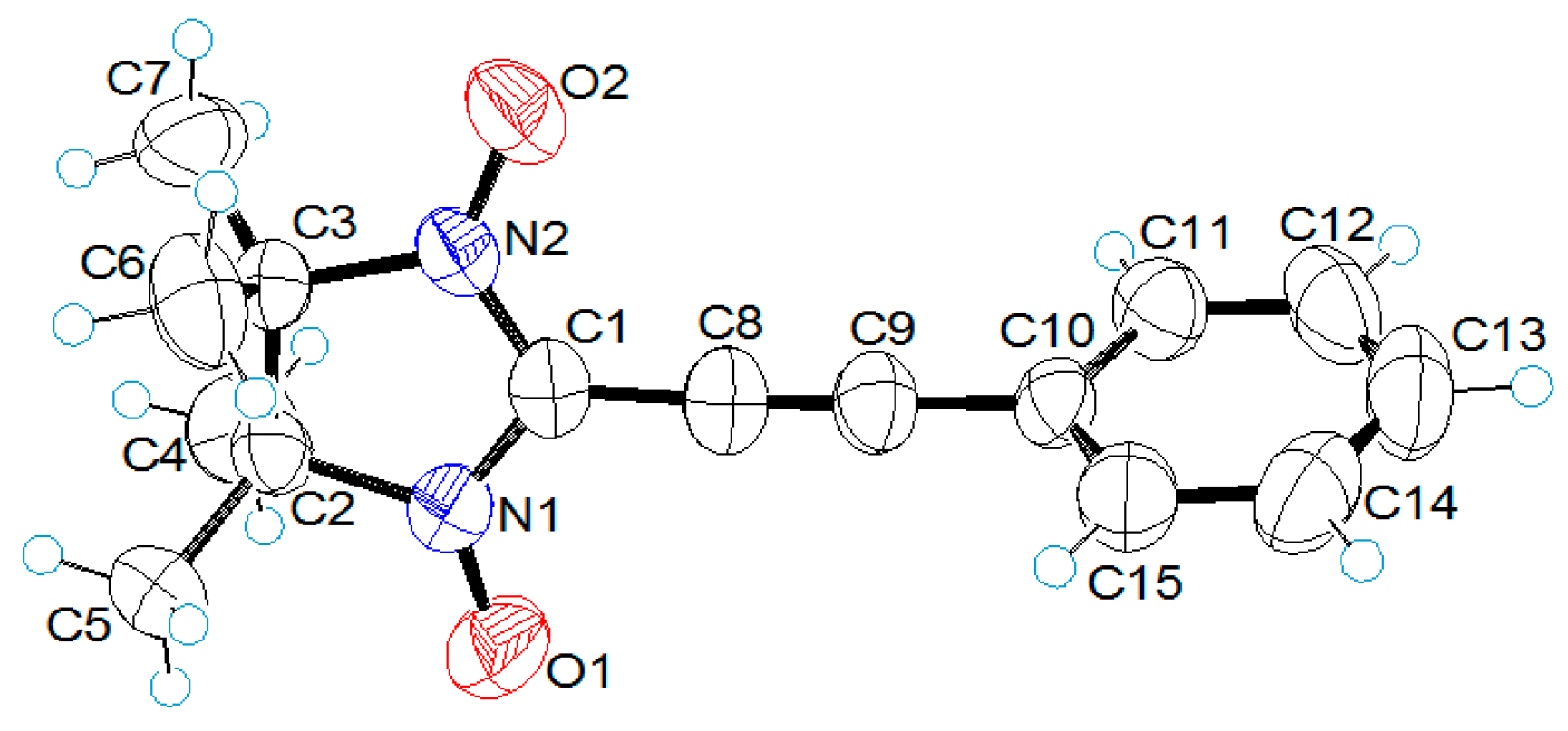
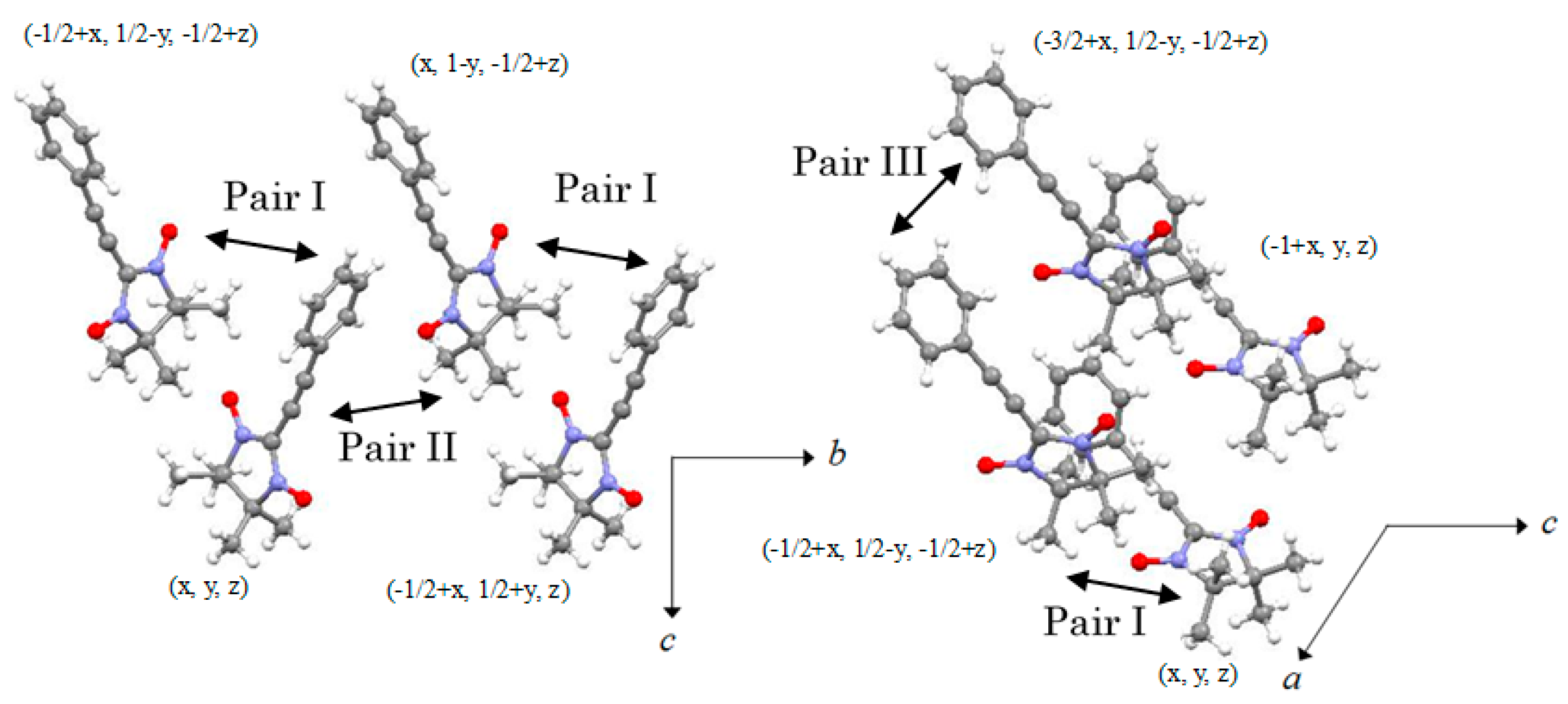
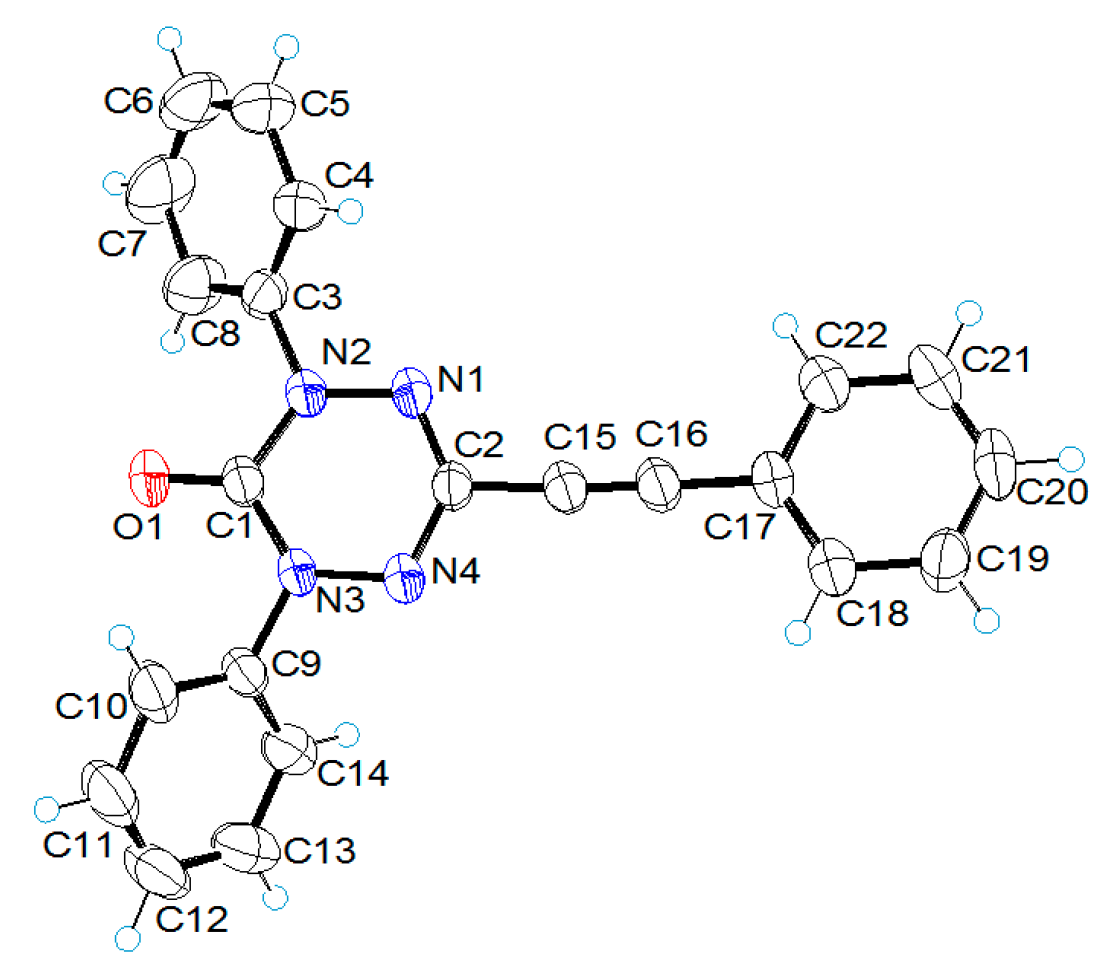
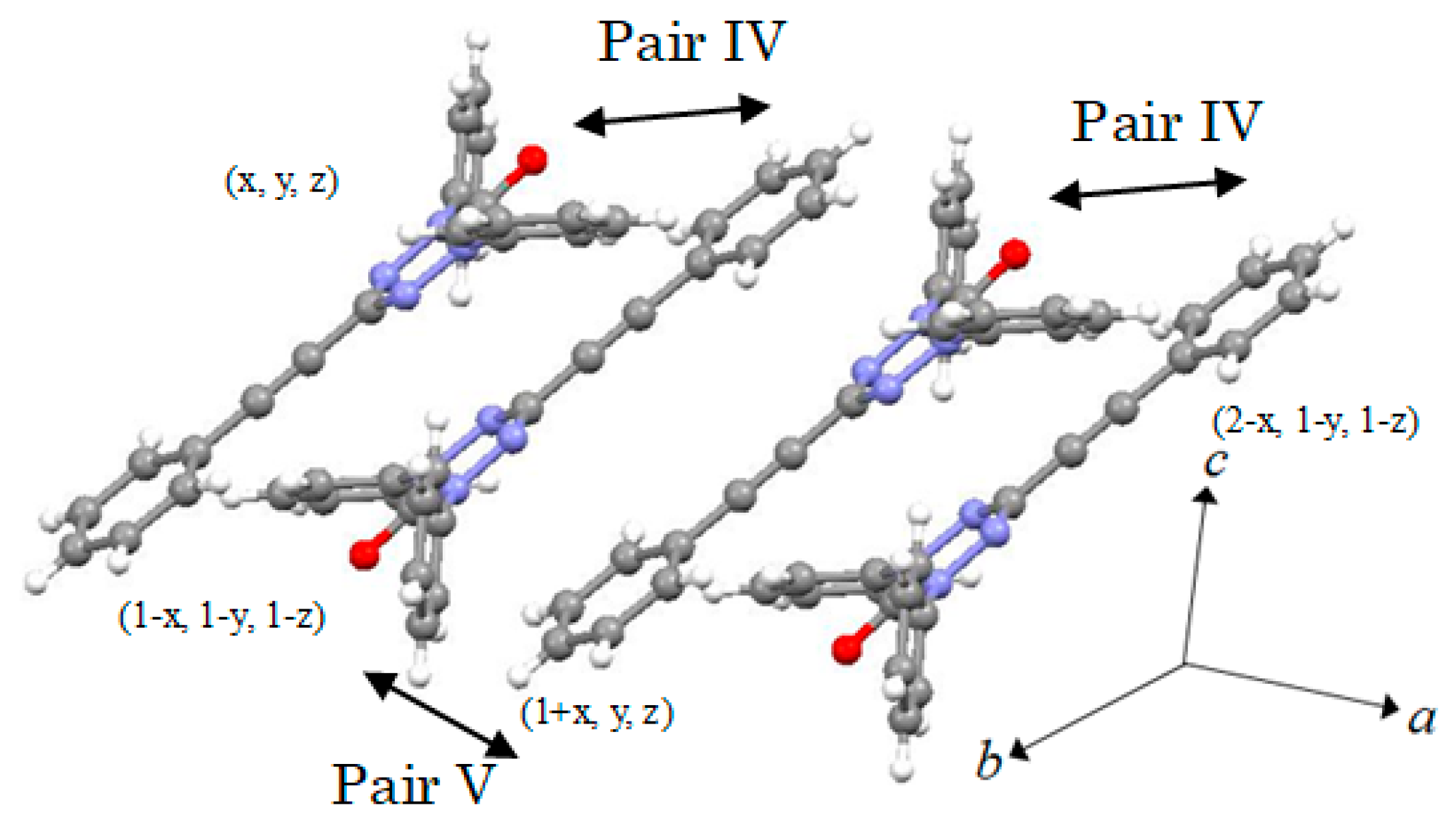
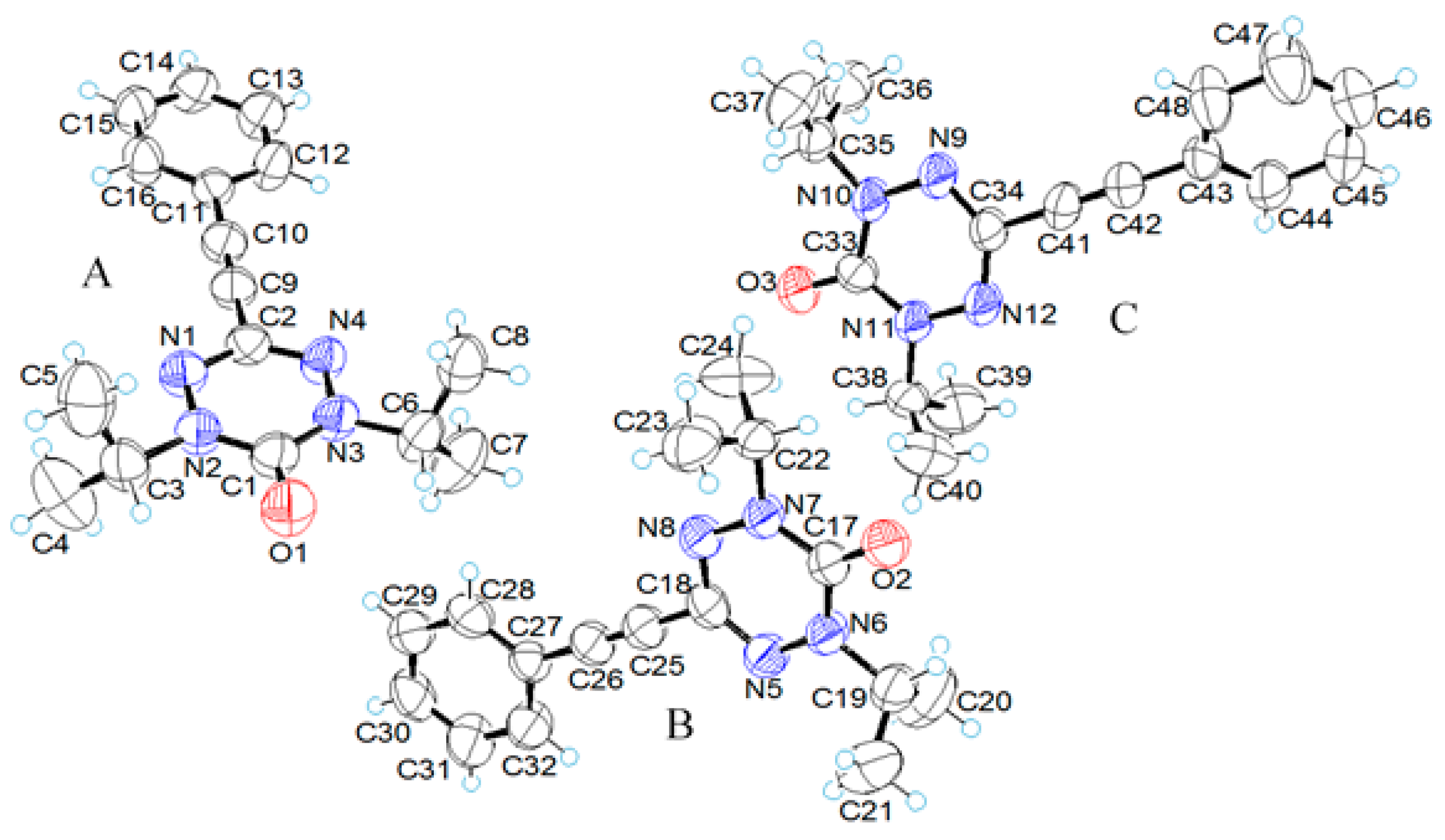
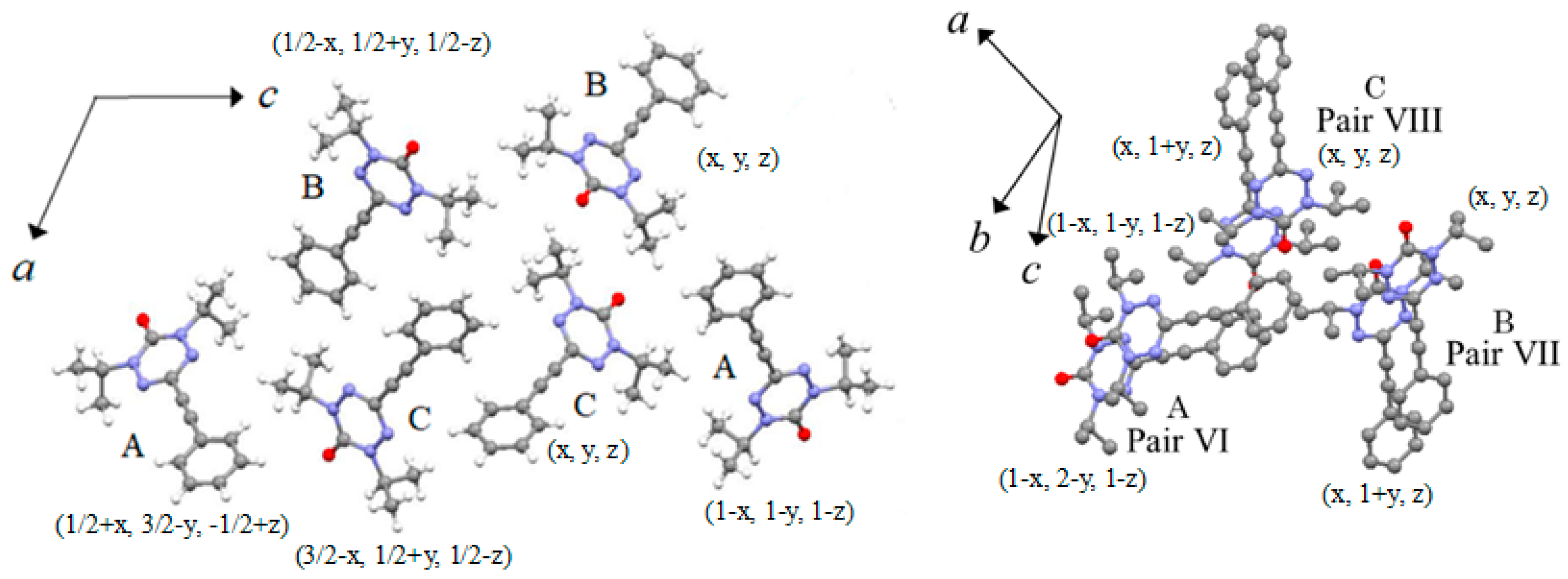
2.2. X-ray Structural Analysis
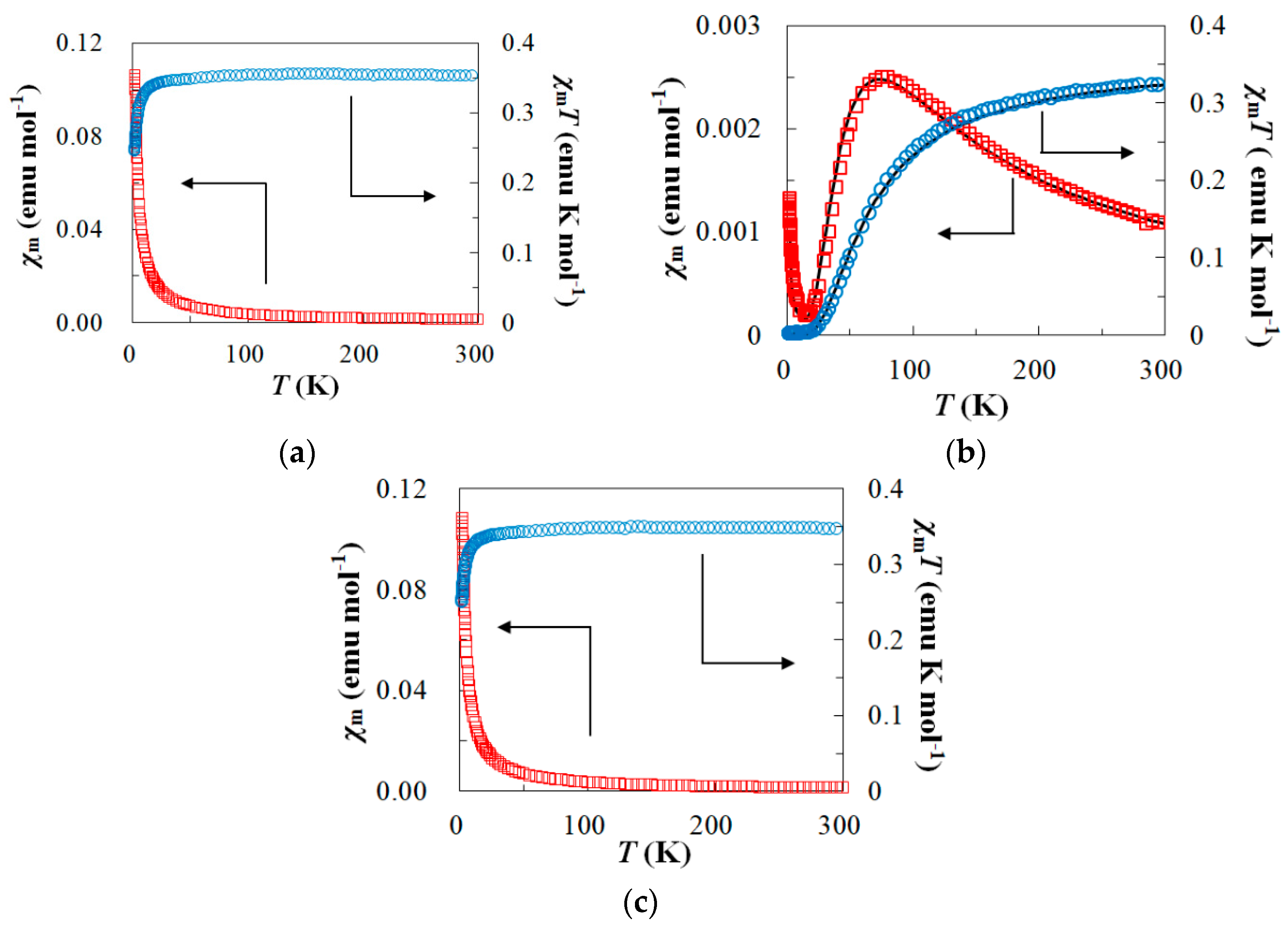
2.3. Magnetic Properties
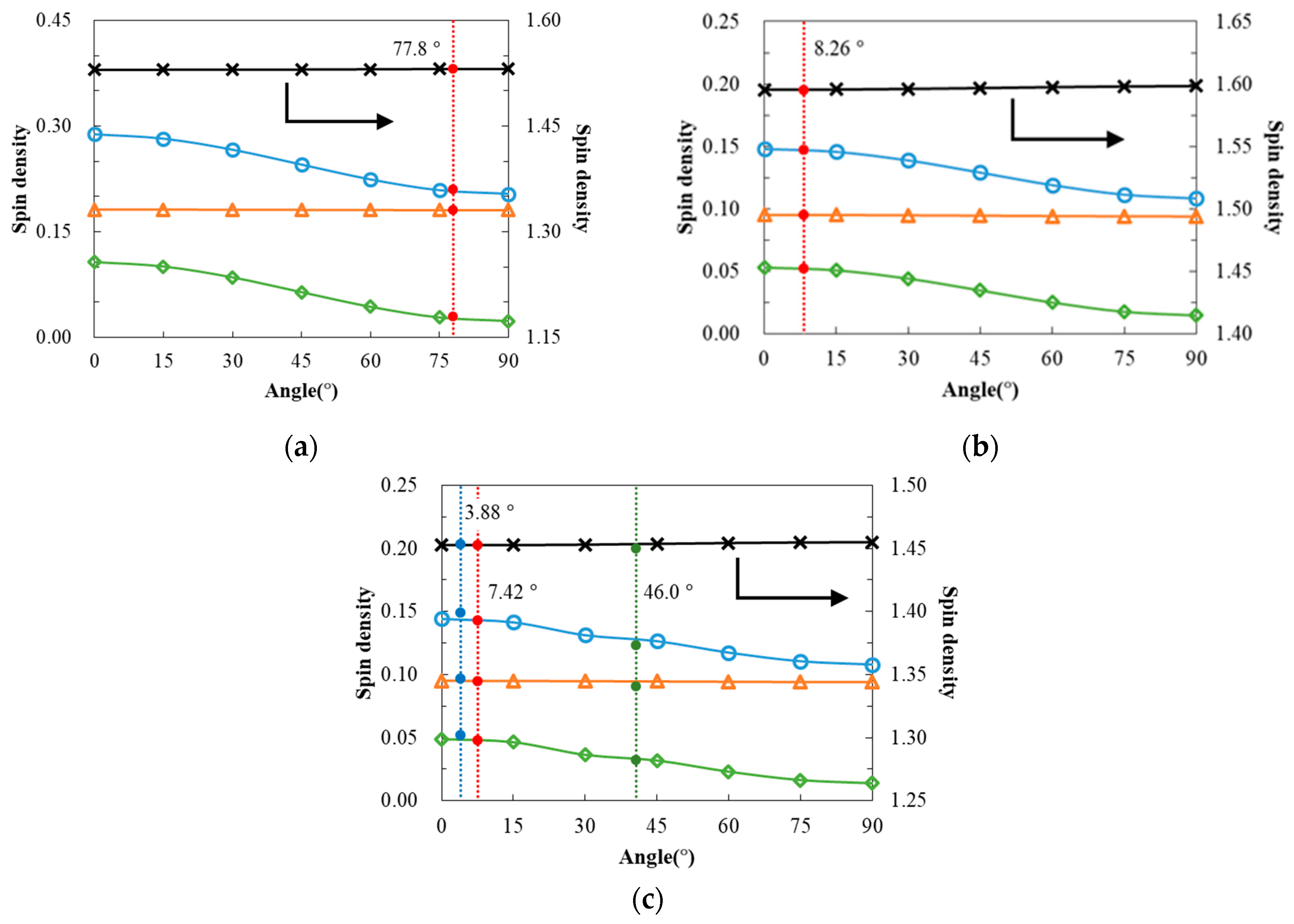
3. Discussion
4. Experimental
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lahti, P.M. (Ed.) Magnetic Properties of Organic Materials; Marcel Dekker: New York, NY, USA, 1999; ISBN 9780824719760. [Google Scholar]
- Miller, J.S.; Drillon, M. (Eds.) Magnetism: Molecules to Materials; Wiley–VCH: Weinheim, Germany, 2005; Volume I–V. [Google Scholar] [CrossRef]
- Hicks, R.G. (Ed.) Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds; Wiley: Chichester, UK, 2010; ISBN 978-0-470-77083-2. [Google Scholar]
- Chatgilialoglu, C.; Studer, A. (Eds.) Encyclopedia of Radicals in Chemistry, Biology and Materials; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Koivisto, B.D.; Hicks, R.G. The magnetochemistry of verdazyl radical-based materials. Coord. Chem. Rev. 2005, 249, 2612–2630. [Google Scholar] [CrossRef]
- Vostrikova, K.E. High-spin molecules based on metal complexes of organic free radicals. Coord. Chem. Rev. 2008, 252, 1409–1419. [Google Scholar] [CrossRef]
- Retera, I.; Veciana, J. Playing with organic radicals as building blocks for functional molecular materials. Chem. Soc. Rev. 2012, 41, 303–349. [Google Scholar] [CrossRef] [PubMed]
- Lipunova, G.N.; Fedorchenko, T.G.; Chupakhin, O.N. Verdazyls: Synthesis, properties, application. Russ. Chem. Rev. 2013, 82, 701–734. [Google Scholar] [CrossRef]
- Mukai, K. Anomalous magnetic properties of stable crystalline phenoxyl radicals. Bull. Chem. Soc. Jpn. 1969, 42, 40–46. [Google Scholar] [CrossRef]
- Chouteau, G.; Veyret-Jeandey, C. Metamagnetism in tanol suberate. J. Phys. Paris 1981, 42, 1441–1444. [Google Scholar] [CrossRef]
- Sugawara, T.; Murata, S.; Kimura, K.; Iwamura, H.; Sugawara, Y.; Iwasaki, H. Design of molecular assembly of diphenylcarbenes having ferromagnetic intermolecular interactions. J. Am. Chem. Soc. 1985, 107, 5293–5294. [Google Scholar] [CrossRef]
- Awaga, K.; Sugano, T.; Kinoshita, M. Ferromagnetic intermolecular interactions in a series of organic mixed crystals of galvinoxyl radical and its precursory closed shell compound. J. Chem. Phys. 1986, 85, 2211–2218. [Google Scholar] [CrossRef]
- Tamura, M.; Nakazawa, Y.; Shiomi, D.; Nozawa, K.; Hosokoshi, Y.; Ishikawa, M.; Takahashi, M.; Kinoshita, M. Bulk ferromagnetism in the β-phase crystal of the p-nitrophenyl nitronyl nitroxide radical. Chem. Phys. Lett. 1991, 186, 401–404. [Google Scholar] [CrossRef]
- Chiarelli, R.; Novak, M.A.; Rassat, A.; Tholence, J.L. A ferromagnetic transition at 1.48 K in an organic nitroxide. Nature 1993, 363, 147–149. [Google Scholar] [CrossRef]
- Lanfranc de Panthou, F.; Luneau, D.; Laugier, J.; Rey, P. Crystal structures and magnetic properties of a nitronyl nitroxide and of its imino analog. Crystal packing and spin distribution dependence of ferromagnetic intermolecular interactions. J. Am. Chem. Soc. 1993, 115, 9095–9100. [Google Scholar] [CrossRef]
- Pei, Y.; Kahn, O.; Aebersold, M.A.; Ouahab, L.; Le Berre, F.; Pardi, L.; Jean Louis, T. Synthesis, Crystal Structure, Magnetic Properties, and Spin Densities of a Triazole-Nitronyl Nitroxide Radical. Adv. Mater. 1994, 6, 681–683. [Google Scholar] [CrossRef]
- Cirujeda, J.; Mas, M.; Molins, E.; Lanfranc de Panthou, F.; Laugier, J.; Park, J.G.; Paulsen, C.; Rey, P.; Rovira, C.; Veciana, J. Control of the structural dimensionality in hydrogen-bonded self-assemblies of open-shell molecules. Extension of intermolecular ferromagnetic interactions in α-phenyl nitronyl nitroxide radicals into three dimensions. J. Chem. Soc. Chem. Commun. 1995, 709–710. [Google Scholar] [CrossRef]
- Lang, A.; Pei, Y.; Ouahab, L.; Kahn, O. Synthesis, crystal structure, and magnetic properties of 5-methyl-1,2,4-triazole-nitronyl nitroxide: A one-dimensional compound with unusually large ferromagnetic intermolecular interactions. Adv. Mater. 1996, 8, 60–62. [Google Scholar] [CrossRef]
- Matsushita, M.M.; Izuoka, A.; Sugawara, T.; Kobayashi, T.; Wada, N.; Takeda, N.; Ishikawa, M. Hydrogen-Bonded Organic Ferromagnet. J. Am. Chem. Soc. 1997, 119, 4369–4379. [Google Scholar] [CrossRef]
- Yoshioka, N.; Irisawa, M.; Mochizuki, Y.; Kato, T.; Inoue, H.; Ohba, S. Unusually Large Magnetic Interactions Observed in Hydrogen-Bonded Nitronyl Nitroxides. Chem. Lett. 1997, 26, 251–257. [Google Scholar] [CrossRef]
- Nagashima, H.; Hashimoto, N.; Inoue, H.; Yoshioka, N. Coexistence of an antiferromagnetically coupled dimer and isolated paramagnetic spin in 4-azaindol-2-yl nitronyl nitroxide crystal. New J. Chem. 2003, 27, 805–810. [Google Scholar] [CrossRef]
- Nagashima, H.; Inoue, H.; Yoshioka, N. An ideal one-dimensional antiferromagnetic spin system observed in hydrogen-bonded naphth[2,3-d]imidazol-2-yl nitronyl nitroxide crystal: The role of the hydrogen bond. J. Phys. Chem. B 2004, 108, 6144–6151. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, H.; Fujita, S.Y.; Inoue, H.; Yoshioka, N. Metamagnetic Behavior Observed in Purely Organic 5-Azaindol-2-yl Nitronyl Nitroxide Brick-Wall Architecture. Cryst. Growth Des. 2004, 4, 19–21. [Google Scholar] [CrossRef]
- Yao, M.; Asakura, S.; Abe, M.; Inoue, H.; Yoshioka, N. Formation of a One-Dimensional Stacking Structure of π-Conjugated Nitroxyl Radical Bearing a 1,2,5-Thiadiazole Ring and Its Magnetic Property. Cryst. Growth Des. 2005, 5, 413–417. [Google Scholar] [CrossRef]
- Murata, H.; Mague, J.T.; Aboaku, S.; Yoshioka, N.; Lahti, P.M. An Organic Radical Solid Solution with Strong Ferromagnetic Exchange. Chem. Mater. 2007, 19, 4111–4113. [Google Scholar] [CrossRef]
- Murata, H.; Miyazaki, Y.; Inaba, A.; Paduan-Filho, A.; Bindilatti, V.; Oliveira, N.F., Jr.; Delen, Z.; Lahti, P.M. 2-(4,5,6,7-Tetrafluorobenzimidazol-2-yl)-4,4,5,5-tetramethyl-4,5-dihydro-1-H-imidazole-3-oxide-1-oxyl, A Hydrogen-Bonded Organic Quasi-1D Ferromagnet. J. Am. Chem. Soc. 2008, 130, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Miura, Y.; Yoshioka, N. Synthesis and properties of the 3-tert-butyl-7-trifluoromethyl-1,4-dihydro-1-phenyl-1,2,4-benzotriazin-4-yl radical. New J. Chem. 2015, 39, 4783–4789. [Google Scholar] [CrossRef]
- Azuma, N.; Yamauchi, J.; Mukai, K.; Ohya, H.N.; Deguchi, Y. The Magnetic Properties of Verdazyl Free Radicals. III. The Anomalous Magnetic Behavior of Symmetrical Triphenylverdazyl. Bull. Chem. Soc. Jpn. 1973, 46, 2728–2734. [Google Scholar] [CrossRef]
- Allemand, P.M.; Srdanov, G.; Fred, F. Molecular engineering in the design of short-range ferromagnetic exchange in organic solids: The 1,3,5-triphenylverdazyl system. J. Am. Chem. Soc. 1990, 112, 9391–9392. [Google Scholar] [CrossRef]
- Mito, M.; Takeda, K.; Mukai, K.; Azuma, N.; Gleiter, M.R.; Krieger, C.; Neugebauer, F.A. Magnetic Properties and Crystal Structures of 1,5-Diphenylverdazyls with Electron Acceptor Groups in the 3-Position. J. Phys. Chem. B 1997, 101, 9517–9524. [Google Scholar] [CrossRef]
- Hicks, R.G.; Lemaire, M.T.; Oehrstroem, L.; Richardson, J.F.; Thompson, L.K.; Xu, Z. Strong Supramolecular-Based Magnetic Exchange in π-Stacked Radicals. Structure and Magnetism of a Hydrogen-Bonded Verdazyl Radical: Hydroquinone Molecular Solid. J. Am. Chem. Soc. 2001, 123, 7154–7159. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Matsubara, M.; Hisatou, H.; Hosokoshi, Y.; Inoue, K.; Azuma, N. Anomalous Magnetic Behavior in Three Kinds of 3-(Aryl-substituted)-1,5-diphenylverdazyl Radical Crystals (p-FPDV, p-PyDV and m-PyDV) Induced by Frustrated Spin Interaction. J. Phys. Chem. B 2002, 106, 8632–8638. [Google Scholar] [CrossRef]
- Merhi, A.; Roisnel, T.; Rigaut, S.; Train, C.; Norel, L. Ferromagnetic intermolecular exchange interaction in ethynyl-verdazyl radical crystals. Cryst. Eng. Comm. 2014, 16, 9783–9787. [Google Scholar] [CrossRef]
- Eusterwiemann, S.; Dresselhaus, T.; Doerenkamp, C.; Janka, O.; Niehaus, O.; Massolle, A.; Daniliuc, C.G.; Eckert, H.; Poettgen, R.; Neugebauer, J.; et al. Cooperative Magnetism in Crystalline N-Aryl-Substituted Verdazyl Radicals: First-Principles Predictions and Experimental Results. Chem. Eur. J. 2017, 23, 6069–6082. [Google Scholar] [CrossRef] [PubMed]
- Eusterwiemann, S.; Doerenkamp, C.; Dresselhaus, T.; Janka, O.; de Oliveira, M., Jr.; Daniliuc, C.G.; Eckert, H.; Neugebauer, J.; Poettgen, R.; Studer, A. Strong intermolecular antiferromagnetic verdazyl-verdazyl coupling in the solid state. Phys. Chem. Chem. Phys. 2017, 19, 15681–15685. [Google Scholar] [CrossRef] [PubMed]
- Ducharme, Y.; Wuest, J.D. Use of Hydrogen Bonds to Control Molecular Aggregation. Extensive, Self-complementary Arrays of Donors and Acceptors. J. Org. Chem. 1988, 53, 5787–5789. [Google Scholar] [CrossRef]
- Simard, M.; Su, D.; Wuest, J.D. Use of Hydrogen Bonds to Control Molecular Aggregation. Self-Assembly of Three-Dimensional Networks with Large Chambers. J. Am. Chem. Soc. 1991, 113, 4696–4698. [Google Scholar] [CrossRef]
- Ullman, E.F.; Osiecki, J.H.; Boocock, D.G.B.; Darcy, R. Stable free radicals. X. Nitronyl nitroxide monoradicals and biradicals as possible small molecule spin labels. J. Am. Chem. Soc. 1972, 94, 7049–7059. [Google Scholar] [CrossRef]
- Dulog, L.; Kim, J.S. Stable free radical paramagnetic monomers containing aminoxylamine oxide moieties. An intermediate step toward organic ferromagnetic polyradicals. Makromol. Chem. 1989, 190, 2604–2614. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Romanenko, G.V.; Podoplelov, A.; Ovcharenko, V. Synthesis of alkynyl-substituted nitronyl nitroxides through an organosilicon derivative. Eur. J. Org. Chem. 2006, 2695–2702. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Romanenko, G.V.; Ovcharenko, V.I. Alkynyl-substituted nitronyl nitroxide. Russ. Chem. Bull. 2006, 55, 591–592. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Tolstikov, S.E.; Romanenko, G.V.; Bogomyakov, A.S.; Stass, D.V.; Kadirov, M.K.; Holin, K.V.; Sinyashin, O.G.; Ovcharenko, V.I. Synthesis, structure, and magnetic properties of 2,2′-(buta-1,3-diyne-1,4-diyl)bis(4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazole 3-oxide 1-oxyl). Polyhedron 2011, 30, 3232–3237. [Google Scholar] [CrossRef]
- Ko, K.C.; Cho, D.; Lee, J.Y. Systematic Approach to Design Organic Magnetic Molecules: Strongly Coupled Diradicals with Ethylene Coupler. J. Phys. Chem. A 2012, 116, 6837–6844. [Google Scholar] [CrossRef] [PubMed]
- Sarbadhikary, P.; Shil, S.; Panda, A.; Misra, A. A Perspective on Designing Chiral Organic Magnetic Molecules with Unusual Behavior in Magnetic Exchange Coupling. J. Org. Chem. 2016, 81, 5623–5630. [Google Scholar] [CrossRef] [PubMed]
- Steenbock, T.; Shultz, D.A.; Kirk, M.L.; Herrmann, C. Influence of Radical Bridges on Electron Spin Coupling. J. Phys. Chem. A 2017, 121, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, F.A.; Fischer, H.; Krieger, C. Verdazyls. Part 33. EPR and ENDOR studies of 6-oxo- and 6-thioxoverdazyls. X-Ray molecular structure of 1,3,5-triphenyl-6-oxoverdazyl and 3-tert-butyl-1,5-diphenyl-6-thioxoverdazyl. J. Chem. Soc. Perkin Trans. 1993, 2, 535–544. [Google Scholar] [CrossRef]
- Milcent, R.; Barbier, G.; Capelle, S.; Catteau, J.P. New general synthesis of tetrahydro-1,2,4,5-tetrazin-3(2H)-one derivatives and stable 3,4-dihydro-3-oxo-1,2,4,5-tetrazin-1(2H)-yl radical derivatives. J. Heterocycl. Chem. 1994, 31, 319–324. [Google Scholar] [CrossRef]
- Paré, E.C.; Brook, D.J.R.; Brieger, A.; Badik, M.; Schinke, M. Synthesis of 1,5-diisopropyl substituted 6-oxoverdazyls. Org. Biomol. Chem. 2005, 3, 4258–4261. [Google Scholar] [CrossRef] [PubMed]
- Tayama, E.; Kobayashi, Y.; Toma, Y. Diaza [1,4] Wittig-type rearrangement of N-allylicN-Boc-hydrazines into γ-amino-N-Boc-enamines. Chem. Commun. 2016, 52, 10570–10573. [Google Scholar] [CrossRef] [PubMed]
- Melendez, R.E.; Lubell, W.D. Aza-Amino Acid Scan for Rapid Identification of Secondary Structure Based on the Application of N-Boc-Aza1-Dipeptides in Peptide Synthesis. J. Am. Chem. Soc. 2004, 126, 6759–6764. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, H.; Hanaki, E.; Izawa, H.; Kano, M.; Itahashi, H. A convenient method for the preparation of α-vinylfurans by phosphine-initiated reactions of various substituted enynes bearing a carbonyl group with aldehydes. Tetrahedron 2004, 60, 1913–1920. [Google Scholar] [CrossRef]
- Masuda, Y.; Kuratsu, M.; Suzuki, S.; Kozaki, M.; Shiomi, D.; Sato, K.; Takui, T.; Okada, K. Preparation and magnetic properties of verdazyl-substituted dihydrophenazine radical cation tetrachloroferrate salts. Polyhedron 2009, 28, 1950–1954. [Google Scholar] [CrossRef]
- CCDC 1587567, 1587568 and 1587569 for 1, 2, and 3, Respectively, Contain the Supplementary Crystallographic Data for This Paper. Available online: http://www.ccdc.cam.ac.uk/conts/retrieving.html (11 November 2017).
- Yamaguchi, K.; Fukui, H.; Fueno, T. Molecular orbital (MO) theory for magnetically interacting organic compounds ab-initio MO calculations of the effective exchange integrals for cyclophane-type carbene dimers. Chem. Lett. 1986, 625–628. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D.A.; Duling, D.R.; Fann, Y.C. Winsim v.0.96, EPR Spectral Simulation for MS-Windows; Public EPR Software Tools; NIEHS: Bethesda, MD, USA.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 (Revision D.01); Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
Sample Availability: Samples of the compounds 1, 2 and 3 are available from the authors. |
| αN(G) | g | |
|---|---|---|
| 1 | 7.32, 7.35 | 2.0062 |
| 2 | 4.62, 4.64, 6.39, 6.40 | 2.0033 |
| 3 | 5.29, 5.61, 6.52, 6.56 | 2.0031 |
| 1 | 2 | 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Pair I | Pair II | Pair III | Pair IV | Pair V | Pair VI | Pair VII | Pair VIII | |
| 2J/kB (K) | 0.190 | 0.190 | −0.0632 | −79.4 | −1.58 | −1.26 | −2.91 | −0.948 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyashiro, S.; Ishii, T.; Miura, Y.; Yoshioka, N. Synthesis and Magnetic Properties of Stable Radical Derivatives Carrying a Phenylacetylene Unit. Molecules 2018, 23, 371. https://doi.org/10.3390/molecules23020371
Miyashiro S, Ishii T, Miura Y, Yoshioka N. Synthesis and Magnetic Properties of Stable Radical Derivatives Carrying a Phenylacetylene Unit. Molecules. 2018; 23(2):371. https://doi.org/10.3390/molecules23020371
Chicago/Turabian StyleMiyashiro, Shogo, Tomoaki Ishii, Youhei Miura, and Naoki Yoshioka. 2018. "Synthesis and Magnetic Properties of Stable Radical Derivatives Carrying a Phenylacetylene Unit" Molecules 23, no. 2: 371. https://doi.org/10.3390/molecules23020371





