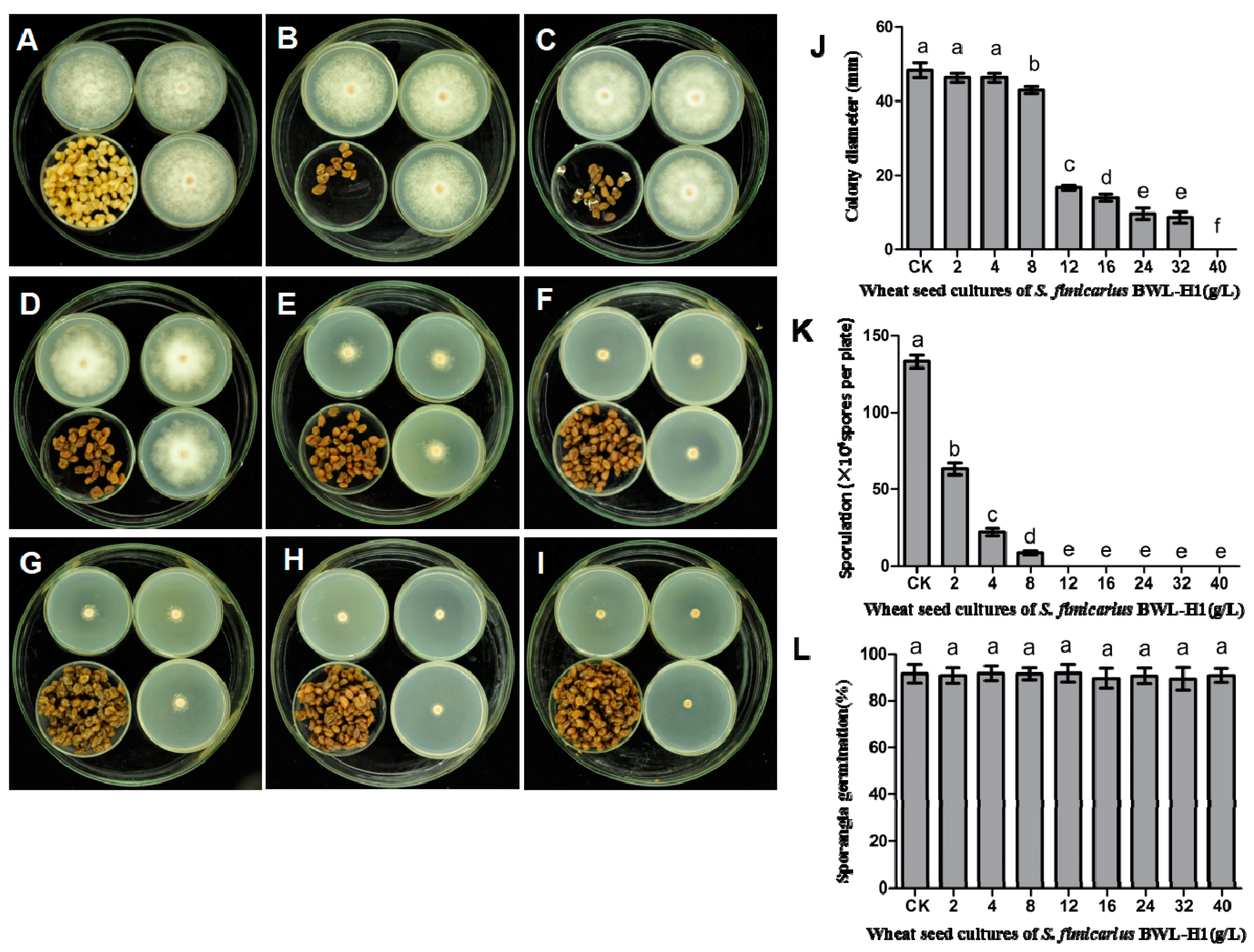
Figure 1.
Suppression of P. litchii mycelial growth, sporangial production and germination by S. fimicarius BWL-H1volatiles. P. litchii was fumigated with 16 g/L of wheat seeds without S. fimicarius BWL-H1 culture (A); or with various amounts (2, 4, 8, 12, 16, 24, 32, 40 g/L) of wheat seed culture of S. fimicarius BWL-H1 (B–I) for 6 d and photographed. Barchart represents mean ± S.E. of colony diameter (J); sporangial production (K); or percentage of sporangia germination (L); grown as in (A–I). CK represents unfumigated control as shown in (A). For each instance, three independent experiments with triplicates were performed and values followed by different letters were significantly different (p < 0.05).
Figure 1.
Suppression of P. litchii mycelial growth, sporangial production and germination by S. fimicarius BWL-H1volatiles. P. litchii was fumigated with 16 g/L of wheat seeds without S. fimicarius BWL-H1 culture (A); or with various amounts (2, 4, 8, 12, 16, 24, 32, 40 g/L) of wheat seed culture of S. fimicarius BWL-H1 (B–I) for 6 d and photographed. Barchart represents mean ± S.E. of colony diameter (J); sporangial production (K); or percentage of sporangia germination (L); grown as in (A–I). CK represents unfumigated control as shown in (A). For each instance, three independent experiments with triplicates were performed and values followed by different letters were significantly different (p < 0.05).
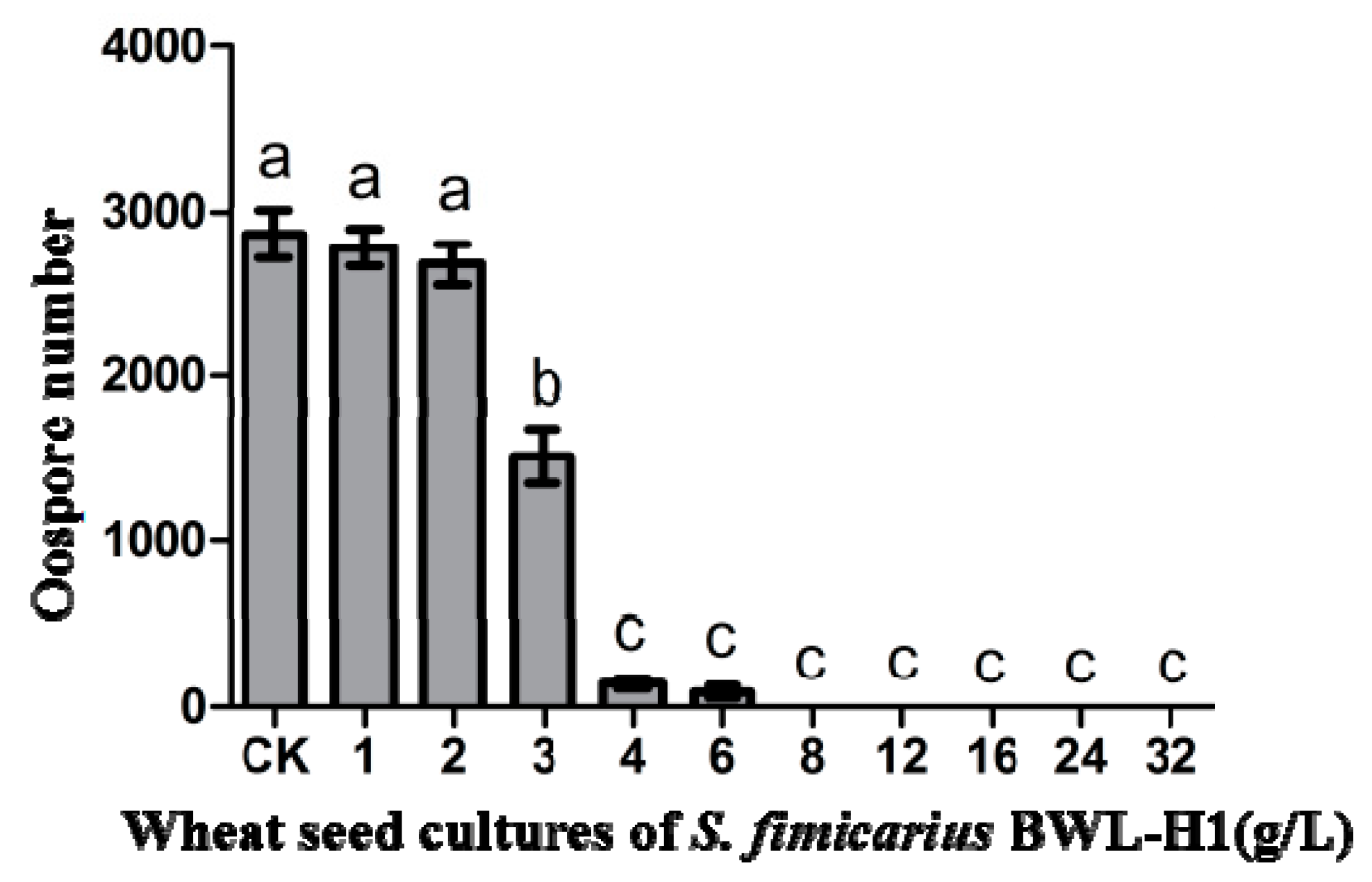
Figure 2.
Suppression of P. litchii oospore production by S. fimicarius BWL-H1 volatiles. Barchart represents mean ± S.E. of oospores per cm2 area from 10 mm around the inoculation site. Data represent three independent experiments with triplicates. Values followed by different letters were significantly different (p < 0.05).
Figure 2.
Suppression of P. litchii oospore production by S. fimicarius BWL-H1 volatiles. Barchart represents mean ± S.E. of oospores per cm2 area from 10 mm around the inoculation site. Data represent three independent experiments with triplicates. Values followed by different letters were significantly different (p < 0.05).
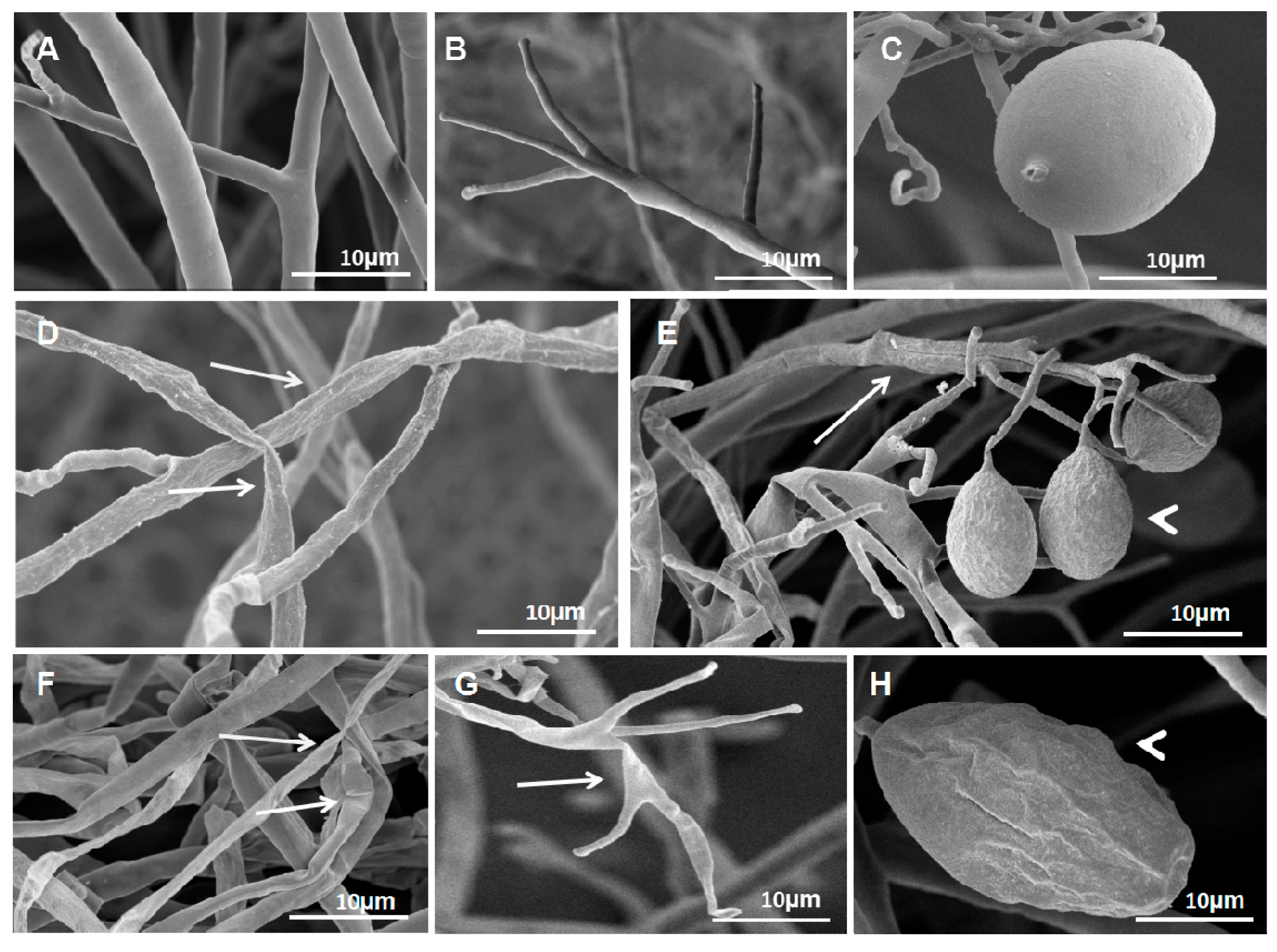
Figure 3.
S. fimicarius BWL-H1 volatiles caused morphological changes in P. litchii hyphae, sporangiophores and sporangia. (A–C) untreated control; (D–E) culture fumigated with 40 g/L wheat seed culture of S. fimicarius BWL-H1; (F–H) culture fumigated with 100 g/L wheat seed culture of S. fimicarius BWL-H1. Arrows denote shrinking or distorted mycelia and sporangiophores, and arrowhead for deformed sporangia.
Figure 3.
S. fimicarius BWL-H1 volatiles caused morphological changes in P. litchii hyphae, sporangiophores and sporangia. (A–C) untreated control; (D–E) culture fumigated with 40 g/L wheat seed culture of S. fimicarius BWL-H1; (F–H) culture fumigated with 100 g/L wheat seed culture of S. fimicarius BWL-H1. Arrows denote shrinking or distorted mycelia and sporangiophores, and arrowhead for deformed sporangia.
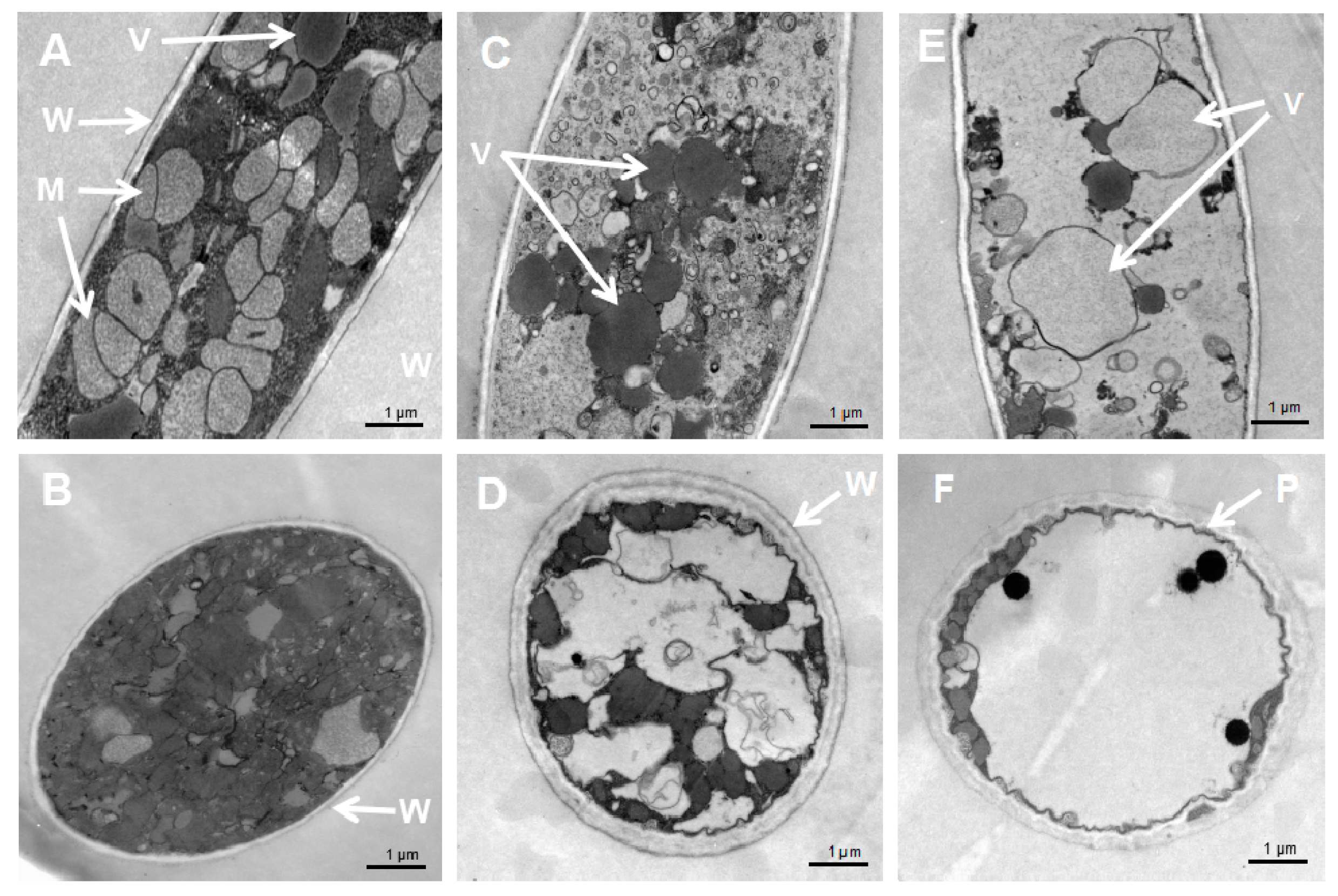
Figure 4.
Cellular damages caused by S. fimicarius BWL-H1 volatiles. (A,B) untreated control; (C,D) mycelial samples fumigated with 40 g/L wheat seed culture of S. fimicarius BWL-H1; (E,F) mycelial samples fumigated with 100 g/L wheat seed culture of S. fimicarius BWL-H1. (A), (C) and (E) were longitudinal section through P. litchii hyphae, while (B,D,F) were tangential section. M, mitochondria; P, plasma membrane; V, vacuoles; and W, cell wall.
Figure 4.
Cellular damages caused by S. fimicarius BWL-H1 volatiles. (A,B) untreated control; (C,D) mycelial samples fumigated with 40 g/L wheat seed culture of S. fimicarius BWL-H1; (E,F) mycelial samples fumigated with 100 g/L wheat seed culture of S. fimicarius BWL-H1. (A), (C) and (E) were longitudinal section through P. litchii hyphae, while (B,D,F) were tangential section. M, mitochondria; P, plasma membrane; V, vacuoles; and W, cell wall.
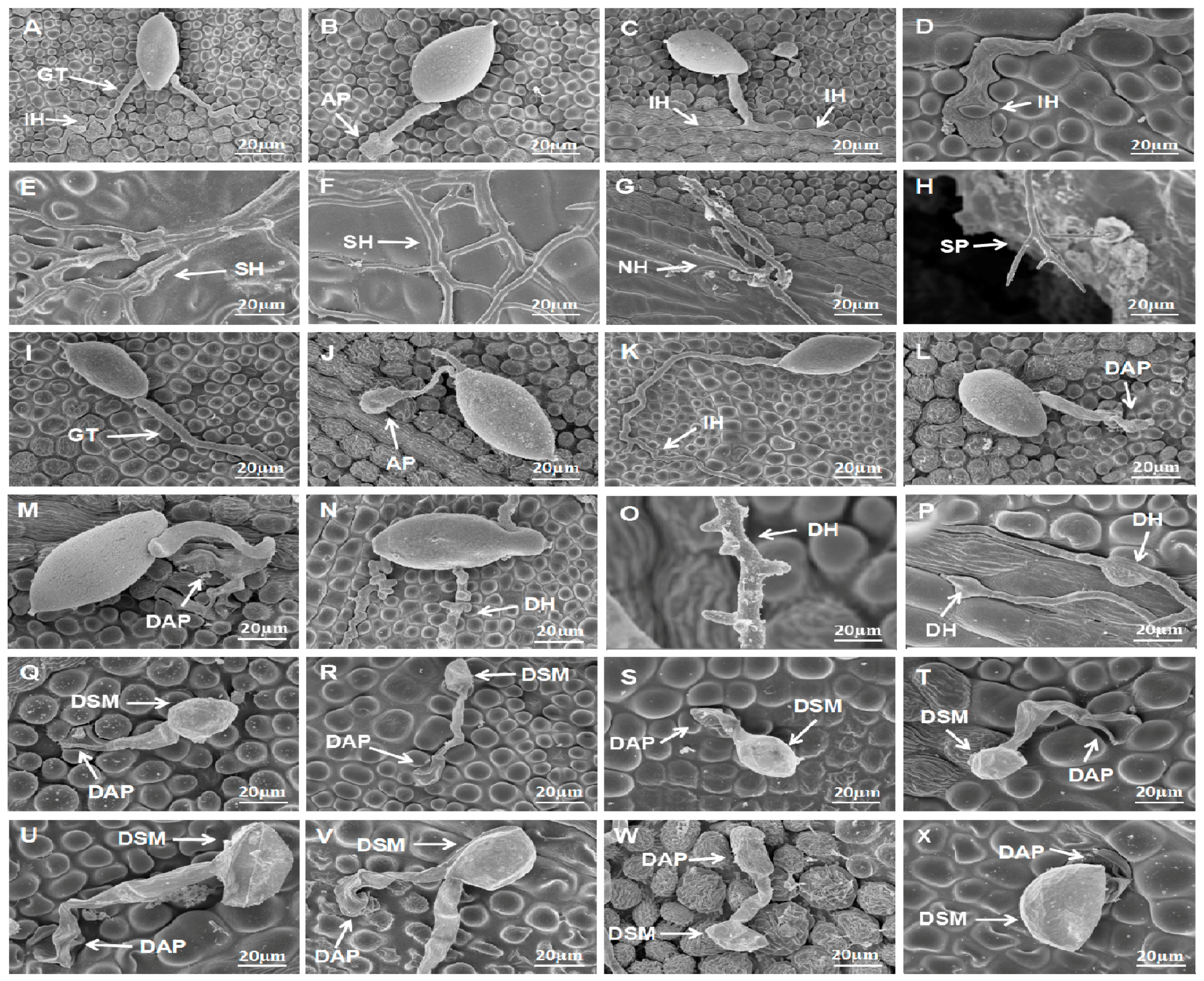
Figure 5.
Effect of S. fimicarius BWL-H1 volatiles in P. litchii infection process. Inoculation of P. litchii on litchi leaves, without volatiles fumigation, were sampled at 3 h (A,B); 6 h (C); 9 h (D); 12 h (E); 24 h (F); and 48 h (G,H), and imaged. Fumigated with 12 g/L wheat seed culture of S. fimicarius BWL-H1, the inoculated leaves were sampled and imaged at 3 h (I,J); 6 h (K); 9 h (L); 12 h (M); 24 h (N); and 48 h (O,P). Inoculation of P. litchii on litchi leaves fumigating with 16 g/L wheat seed culture of S. fimicarius BWL-H1 were imaged at 3 h (Q,R); 6 h (S); 9 h (T); 12 h (U); 24 h (V); and 48 h (W,X). SEM images for each instance were representative of three independent biological replications. GT, germ tube; IH, infection hyphae; SH, secondary hyphae; NH, new hyphae; AP, appressorium; DAP, deformed appressorium; SP, sporangiophore; DSM, deformed sporangia; DH: deformed hyphae.
Figure 5.
Effect of S. fimicarius BWL-H1 volatiles in P. litchii infection process. Inoculation of P. litchii on litchi leaves, without volatiles fumigation, were sampled at 3 h (A,B); 6 h (C); 9 h (D); 12 h (E); 24 h (F); and 48 h (G,H), and imaged. Fumigated with 12 g/L wheat seed culture of S. fimicarius BWL-H1, the inoculated leaves were sampled and imaged at 3 h (I,J); 6 h (K); 9 h (L); 12 h (M); 24 h (N); and 48 h (O,P). Inoculation of P. litchii on litchi leaves fumigating with 16 g/L wheat seed culture of S. fimicarius BWL-H1 were imaged at 3 h (Q,R); 6 h (S); 9 h (T); 12 h (U); 24 h (V); and 48 h (W,X). SEM images for each instance were representative of three independent biological replications. GT, germ tube; IH, infection hyphae; SH, secondary hyphae; NH, new hyphae; AP, appressorium; DAP, deformed appressorium; SP, sporangiophore; DSM, deformed sporangia; DH: deformed hyphae.
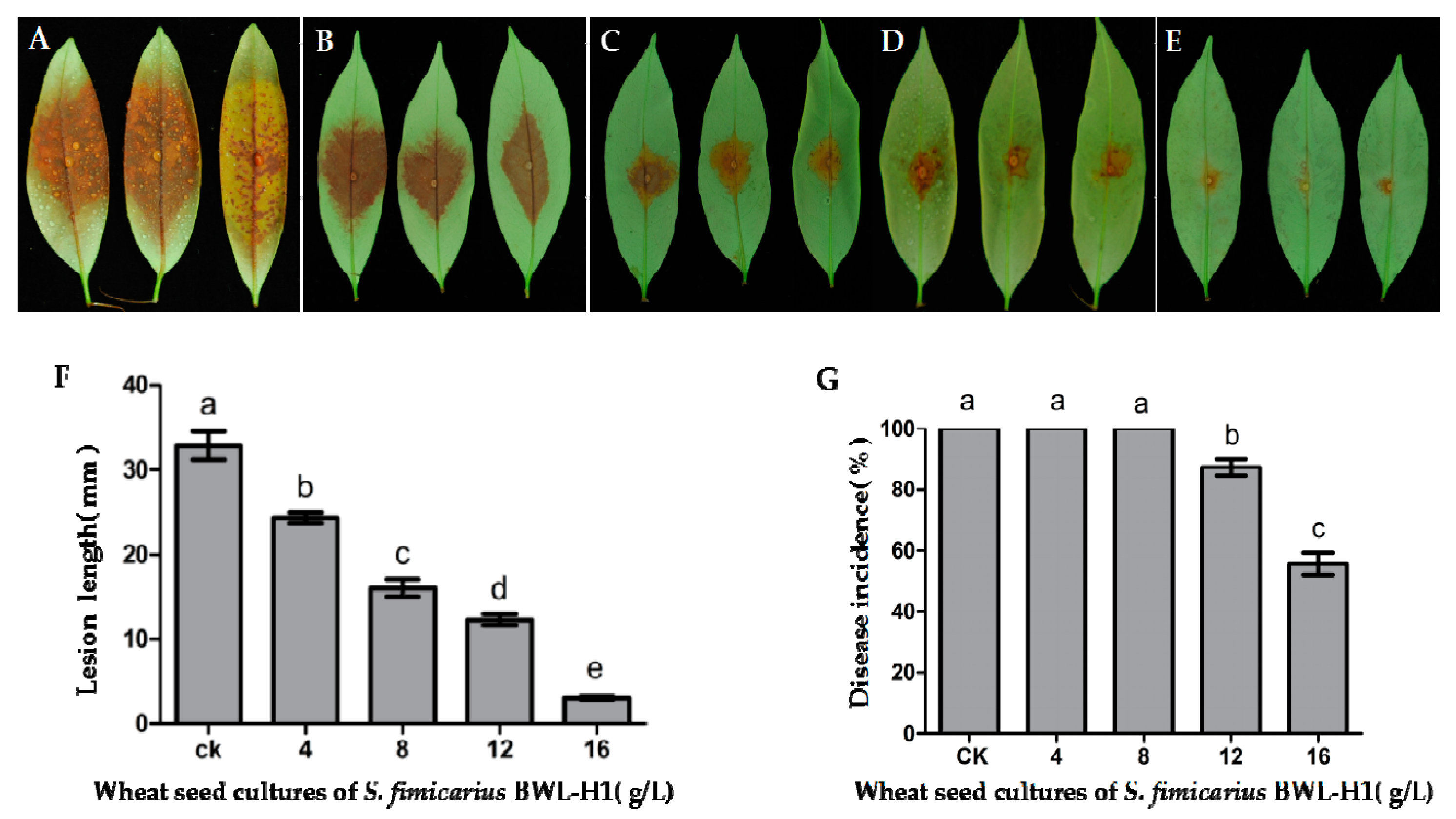
Figure 6.
Antifungal activity of VOCs against P. litchii on detached leaves. (A) unfumigated control; (B–E) P. litchii inoculated leaves were fumigated with various amounts (4, 8, 12, 16 g/L) of wheat seed culture of S. fimicarius BWL-H1 volatiles; (F,G) Lesion length and Disease incidence were measured at 48 h post inoculation. Mean ± S.E. was based on 3 replicates. Values followed by different letters were significantly different (p < 0.05).
Figure 6.
Antifungal activity of VOCs against P. litchii on detached leaves. (A) unfumigated control; (B–E) P. litchii inoculated leaves were fumigated with various amounts (4, 8, 12, 16 g/L) of wheat seed culture of S. fimicarius BWL-H1 volatiles; (F,G) Lesion length and Disease incidence were measured at 48 h post inoculation. Mean ± S.E. was based on 3 replicates. Values followed by different letters were significantly different (p < 0.05).
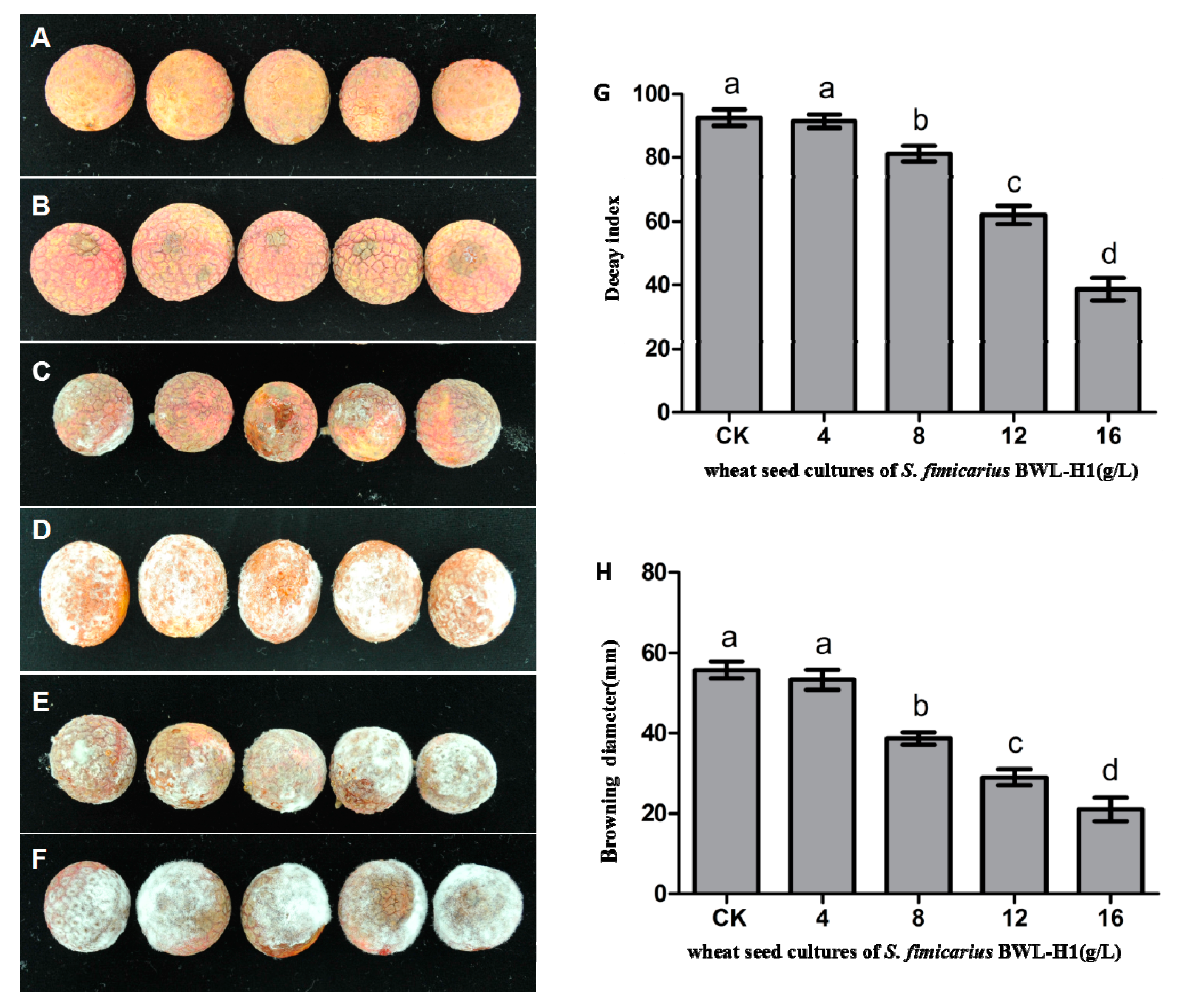
Figure 7.
Antifungal activity of VOCs against P. litchii on fruits. (A) Negative control (without P. litchii inoculation); (B–E) P. litchii inoculated fruits fumigated with 16, 12, 8, 4 g/L of wheat seed culture of S. fimicarius BWL-H1 volatiles; (F) unfumigated control, with autoclaved wheat seeds. Each treatment consisted of three replicates with 30 fruits in each replicate; (G,H) Decay index and browning diameter of harvested litchi fruits inoculated with P. litchii after 5 d storage at 25 °C. Data are the mean ± S.E. from three replicates per treatment.
Figure 7.
Antifungal activity of VOCs against P. litchii on fruits. (A) Negative control (without P. litchii inoculation); (B–E) P. litchii inoculated fruits fumigated with 16, 12, 8, 4 g/L of wheat seed culture of S. fimicarius BWL-H1 volatiles; (F) unfumigated control, with autoclaved wheat seeds. Each treatment consisted of three replicates with 30 fruits in each replicate; (G,H) Decay index and browning diameter of harvested litchi fruits inoculated with P. litchii after 5 d storage at 25 °C. Data are the mean ± S.E. from three replicates per treatment.
Table 1.
Quantification of deformed structures of P. litchii caused by treatment of wheat seed culture of S. fimicarius BWL-H1, during infection process.
Table 1.
Quantification of deformed structures of P. litchii caused by treatment of wheat seed culture of S. fimicarius BWL-H1, during infection process.
| Condition | Inoculation on Untreated Leaves | P. litchii Inoculation on Leaves Treated with 12 g/L Wheat Seed Culture of S. fimicarius BWL-H1 |
|---|
| IH% (6 hpi) | 78.74% ± 0.79% (n = 66) | 9.84 ± 1.33% * (n = 43) |
| DAP% (9–12 hpi) | --- | 77.11 ± 5.58% (n = 48) |
| DH% (24–48 hpi) | --- | 79.70 ± 6.56% (n = 54) |
| | --- | P. litchii inoculation on leaves treated with 16 g/L wheat seed culture of S. fimicarius BWL-H1 |
| DSM% (3–48 hpi) | --- | 83.33% ± 3.33% (n = 25) |
Table 2.
Volatile compounds identified from 14 d culture of S. fimicarius BWL-H1.
Table 2.
Volatile compounds identified from 14 d culture of S. fimicarius BWL-H1.
| Tentatively Identified Compounds a (CAS Number) | RT b (min) | Area (%) |
|---|
| o-Methoxyaniline (90-04-0) | 18.8833 | 4.1906 |
| Methyl anthranilate (134-20-3) | 26.1807 | 0.4783 |
| α-Copaene (3856-25-5) | 27.6194 | 0.5516 |
| Geosmin (19700-21-1) | 28.6784 | 10.8232 |
| cis-Caryophyllene (13877-93-5) | 29.4235 | 5.202 |
| (+)-Calarene (1733-45-5) | 29.9298 | 7.3629 |
| Germacrene D (37839-63-7) | 31.9711 | 5.9553 |
| γ-Cadinene (39029-41-9) | 32.6238 | 0.9447 |
| α-Muurolene (10208-80-7) | 32.8013 | 4.222 |
| trans-Calamenene (6617-49-8) | 33.6999 | 3.8527 |
| 1,6-Dimethyl-4-(1-methylethyl)-(1,2,3,4,4a,7)hexahydronaphthalene (16728-99-7) | 34.0353 | 5.709 |
| Caryophyllenyl alcohol (472-97-9) | 35.4697 | 2.2033 |
Table 3.
Volatile compounds identified from 21 d culture of S. fimicarius BWL-H1.
Table 3.
Volatile compounds identified from 21 d culture of S. fimicarius BWL-H1.
| Tentatively Identified Compounds a (CAS Number) | RT b (min) | Area (%) |
|---|
| Phenylethyl Alcohol (60-12-8) | 15.9498 | 23.013 |
| o-Methoxyaniline (90-04-0) | 18.8742 | 4.6636 |
| Methyl salicylate (9041-28-5) | 19.6214 | 3.5798 |
| 2,6-Dimethoxyphenol (91-10-1) | 22.0447 | 0.7572 |
| 2-Methoxy-6-methylaniline (50868-73-0) | 22.4178 | 0.77 |
| Ethyl phenylacetate (101-97-3) | 22.475 | 3.4846 |
| Methyl anthranilate (134-20-3) | 26.1925 | 4.4317 |
| α-Copaene (3856-25-5) | 27.6234 | 6.1812 |
| trans-1,10-Dimethyl-trans-9-decalol (19700-21-1) | 28.6737 | 4.3395 |
| (+)-Calarene (1733-45-5) | 29.0428 | 1.2989 |
| Caryophyllene (87-44-5) | 29.4244 | 3.1322 |
| 4-Ethylphenol (123-07-9) | 29.5647 | 3.9247 |
| (+)-Calarene (1733-45-5) | 29.9329 | 9.3446 |
| Humulene (6753-98-6) | 30.807 | 3.2429 |
| 1,5,9,9-Tetramethyl-1,4,7-cycloundecatriene (N.A. c) | 30.8328 | 1.4562 |
| Germacrene D (37839-63-7) | 31.9703 | 1.6972 |
| α-Muurolene (10208-80-7) | 32.7906 | 3.2963 |
| cis-Calamenene (6617-49-8) | 33.7001 | 2.6585 |
| 1,6-Dimethyl-4-(1-methylethyl)-(1,2,3,4,4a,7)hexahydronaphthalene (16728-99-7) | 34.0368 | 7.2795 |
| Caryophyllenyl alcohol (472-97-9) | 35.4702 | 0.6922 |
Table 4.
Volatile compounds used for individual test against P. litchii in vitro.
Table 4.
Volatile compounds used for individual test against P. litchii in vitro.
| Compound | Source | Purity |
|---|
| Phenylethyl alcohol | Aldrich | ≥99.0% (GC) |
| Methyl salicylate | Sigma–Aldrich | ≥99.0% (GC) |
| Ethyl phenylacetate | Sigma–Aldrich | ≥98.5% (GC) |
| Methyl anthranilate | Aldrich | ≥98%, FCC, FG |
| α-Copaene | Sigma–Aldrich | ≥99% (GC) |
| Caryophyllene | Sigma | ≥98.5% (sum of enantiomers, GC) |
| 4-Ethylphenol | Sigma–Aldrich | ≥99% (GC) |
| Humulene | Aldrich | ≥96.0% (GC) |
Table 5.
Effect of volatile compounds on P. litchii mycelial growth.
Table 5.
Effect of volatile compounds on P. litchii mycelial growth.
| Volatile Compound | Mycelial Growth (mm) at Different Concentrations of Volatiles |
|---|
| 0 µL/L | 2 µL/L | 20 µL/L | 200 µL/L | 500 µL/L | 1000 µL/L |
|---|
| Phenylethyl alcohol | 51.0a | 46.1 ± 0.2b | 45.7 ± 0.1b | 39.1 ± 0.2c | 38.7 ± 0.2c | 38.1 ± 0.2c |
| Methyl salicylate | 51.0a | 47.8 ± 0.2a | 21.8 ± 0.2b | 0.0c | 0.0c | 0.0c |
| Ethyl phenylacetate | 51.0a | 47.5 ± 0.1a | 11.8 ± 0.3b | 9.2 ± 0.1c | 0.0d | 0.0d |
| Methyl anthranilate | 51.0a | 46.5 ± 0.2b | 23.8 ± 0.2c | 18.2 ± 0.3d | 15.8 ± 0.4d | 12.3 ± 0.2e |
| α-Copaene | 51.0a | 49.6 ± 0.3a | 49.2 ± 0.1a | 12.4 ± 0.2b | 0.0c | 0.0c |
| Caryophyllene | 51.0a | 49.3 ± 0.2a | 43.6 ± 0.4b | 22.6 ± 0.2c | 20.4 ± 0.1c | 18.4 ± 0.3c |
| 4-Ethylphenol | 51.0a | 4.1 ± 0.2b | 3.8 ± 0.1b | 2.2 ± 0.1b | 1.7 ± 0.2b | 0.0b |
| Humulene | 51.0a | 51.2 ± 0.2a | 50.8 ± 0.3a | 50.1 ± 0.2a | 49.7 ± 0.3a | 49.5 ± 0.2a |
Table 6.
Effect of volatile compounds on P. litchii sporulation.
Table 6.
Effect of volatile compounds on P. litchii sporulation.
| Volatile Compound | Sporulation (×104 Spores Per Plate) at Different Concentrations of Volatiles |
|---|
| 0 µL/L | 2 µL/L | 20 µL/L | 200 µL/L | 500 µL/L | 1000 µL/L |
|---|
| Phenylethyl alcohol | 132.8 ± 22a | 134.2 ± 1a | 133.4 ± 13a | 63.6 ± 2b | 61.3 ± 2b | 58.5 ± 4b |
| Methyl salicylate | 132.8 ± 22a | 137.4 ± 7a | 52.8 ± 3b | 0.0c | 0.0c | 0.0c |
| Ethyl phenylacetate | 132.8 ± 22a | 127.5 ± 3b | 00c | 0.0c | 0.0c | 0.0c |
| Methyl anthranilate | 132.8 ± 22a | 136.3 ± 4a | 20.1 ± 2b | 18.4 ± 2b | 17.5 ± 1b | 7.6 ± 1c |
| α-Copaene | 132.8 ± 22a | 134.6 ± 9a | 132.3 ± 6a | 17.7 ± 2b | 0.0c | 0.0c |
| Caryophyllene | 132.8 ± 22a | 138.3 ± 5a | 95.4 ± 12b | 6.1 ± 13c | 2.9 ± 4c | 0.0c |
| 4-Ethylphenol | 132.8 ± 22a | 0.0b | 0.0b | 0.0b | 0.0b | 0.0b |
| Humulene | 132.8 ± 22a | 133.8 ± 14a | 134.6 ± 17a | 132.5 ± 20a | 131.8 ± 23a | 131.4 ± 21a |
Table 7.
Effect of volatile compounds on P. litchii sporangia germination.
Table 7.
Effect of volatile compounds on P. litchii sporangia germination.
| Volatile Compound | Sporangia Germination (%) at Different Concentrations of Volatiles |
|---|
| 0 µL/L | 2 µL/L | 20 µL/L | 200 µL/L | 500 µL/L | 1000 µL/L |
|---|
| Phenylethyl alcohol | 94.7 ± 0.9a | 93.4 ± 0.5a | 92.2 ± 0.3ab | 88.9 ± 0.7b | 63.8 ± 0.4c | 39.8 ± 0.3d |
| Methyl salicylate | 95.4 ± 1.1a | 92.6 ± 0.8b | 89.7 ± 1.3c | 0.0c | 00.0c | 0.0c |
| Ethyl phenylacetate | 94.9 ± 0.7a | 94 ± 1.0a | 90.8 ± 0.9ab | 87.7 ± 1.2b | 68.4 ± 0.3c | 41.3 ± 0.2d |
| Methyl anthranilate | 95.1 ± 1.1a | 92.8 ± 1.4a | 91.5 ± 0.8ab | 88.2 ± 0.7b | 38.8 ± 0.2c | 0.0d |
| α-Copaene | 94.7 ± 0.9a | 91.3 ± 0.7a | 78.6 ± 0.9b | 0.0c | 0.0c | 0.0c |
| Caryophyllene | 95.4 ± 1.1a | 92.7 ± 1.2ab | 92.4 ± 0.8ab | 88.2 ± 0.3b | 52.6 ± 0.2c | 27.6 ± 0.2c |
| 4-Ethylphenol | 95.1a ± 1.1a | 23.9b ± 0.1b | 0.0c | 0.0c | 0.0c | 0.0c |
| Humulene | 94.9a ± 0.7a | 93.7 ± 1.5ab | 92.6 ± 2.0ab | 91.5 ± 1.8ab | 91.4 ± 1.5ab | 91.2 ± 1.2b |
Table 8.
Effect of volatile compounds on P. litchii germ tube growth.
Table 8.
Effect of volatile compounds on P. litchii germ tube growth.
| Volatile Compound | The Length of Germ Tube (µm) at Different Concentrations of Volatiles |
|---|
| 0 µL/L | 2 µL/L | 20 µL/L | 200 µL/L | 500 µL/L | 1000 µL/L |
|---|
| Phenylethyl alcohol | 251.3 ± 35.4a | 151.0 ± 2.5b | 75.6 ± 3.1c | 68.4 ± 4.1c | 19.2 ± 2.5d | 18.2 ± 1.9d |
| Methyl salicylate | 244.6 ± 8.8a | 142.7 ± 2.0b | 123.9 ± 3.4c | 0.0d | 0.0d | 0.0d |
| Ethyl phenylacetate | 254.6 ± 44.0a | 202.8 ± 4.7b | 198.3 ± 2.7b | 87.7 ± 2.3c | 73.6 ± 2.0c | 65.2 ± 5.6c |
| Methyl anthranilate | 241.3 ± 8.8a | 137.7 ± 3.4b | 119.4 ± 2.4c | 62.8 ± 3.5d | 23.4 ± 3.6e | 0.0f |
| α-Copaene | 251.3 ± 35.4a | 49.7 ± 2.4b | 26.8 ± 2.3bc | 0.0c | 0.0c | 0.0c |
| Caryophyllene | 244.6 ± 8.8a | 195.1 ± 5.0b | 151.4 ± 4.2c | 99.3 ± 4.5d | 30.2 ± 3.8e | 18.4 ± 3.9f |
| 4-Ethylphenol | 241.3 ± 8.8a | 36 ± 3.1b | 0.0c | 0.0c | 0.0c | 0.0c |
| Humulene | 254.6 ± 44.0a | 256.9 ± 5.7a | 234.3 ± 3.3ab | 230.5 ± 0.7ab | 229.4 ± 2.1ab | 220.5 ± 2.7b |