Efficacy of Compounds Isolated from the Essential Oil of Artemisia lavandulaefolia in Control of the Cigarette Beetle, Lasioderma serricorne
Abstract
:1. Introduction
2. Results
2.1. Composition Analysis
2.2. Isolated Compounds
2.3. Bioactivity Analysis
2.3.1. Fumigant and Contact Toxicity
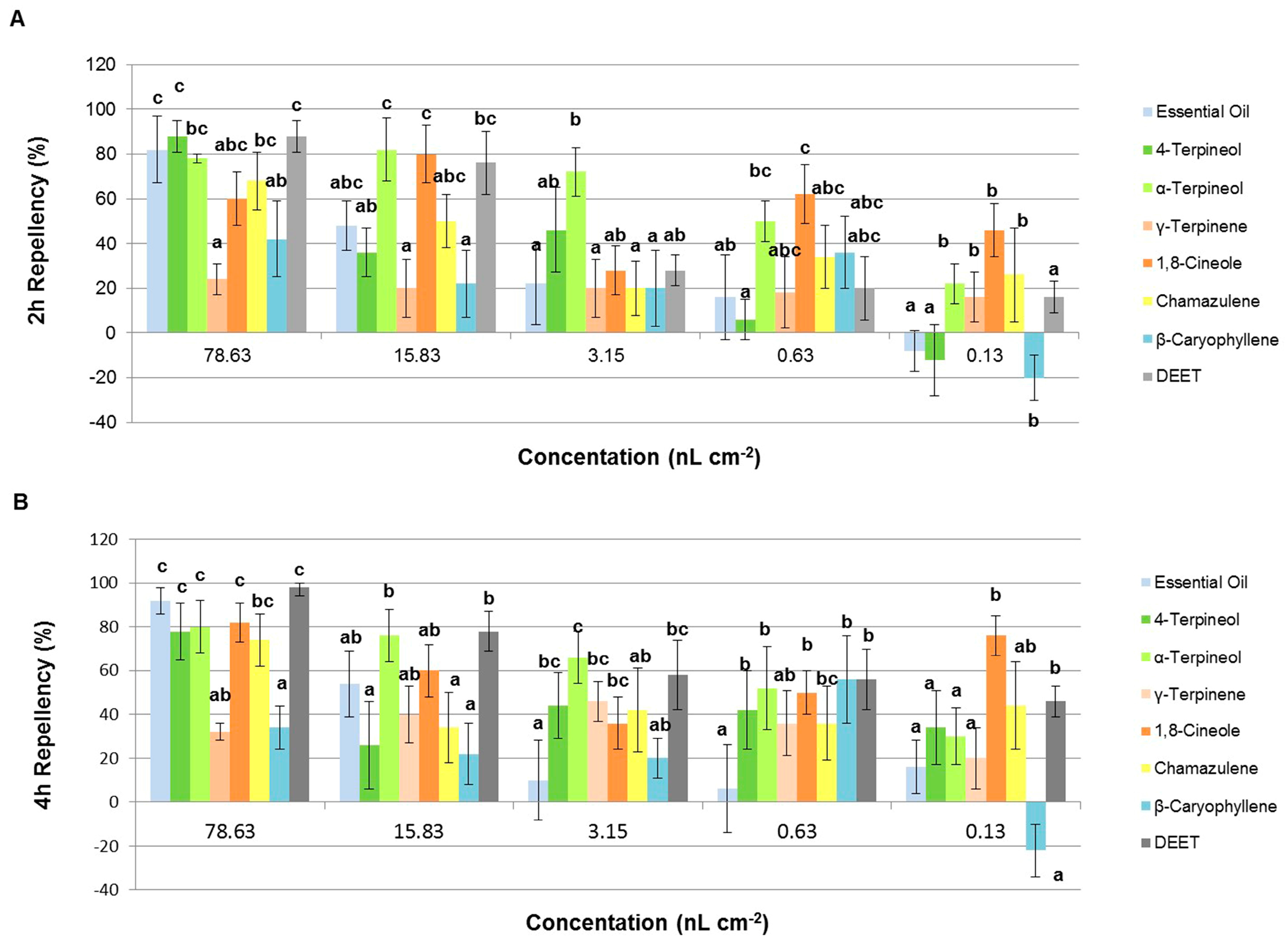
2.3.2. Repellent Activity
3. Discussion
3.1. Composition of the Essential Oil
3.2. Structure-Bioactivity Relationship of the Four Monoterpenoids
3.3. Characteristics of Chamazulene
4. Materials and Methods
4.1. Material
4.1.1. Chemicals
4.1.2. Plants
4.1.3. Insects
4.3. Extraction and Analysis of the Essential Oil
4.4. Purification of Three Compounds
4.5. Bioactivity Assay
4.5.1. Fumigant Toxicity
4.5.2. Contact Toxicity
4.5.3. Repellency Tests
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Times | Treatments | Concentrations (nL/cm2) | ||||
|---|---|---|---|---|---|---|
| 78.63 | 15.83 | 3.15 | 0.63 | 0.13 | ||
| 2 h | A. lavandulaefolia | 82 ± 15 c | 48 ± 11 abc | 22 ± 18 a | 16 ± 19 ab | −8 ± 9 a |
| 4-Terpineol | 88± 7 c | 36 ± 11ab | 46 ± 19 ab | 6 ± 9 a | −12 ± 16 a | |
| α-Terpineol | 78 ± 2 bc | 82 ± 14 c | 72 ± 11 b | 50 ± 9 bc | 22 ± 9 b | |
| γ-Terpinene | 24 ± 7 a | 20 ± 13 a | 20 ± 13 a | 18 ± 16 abc | 16 ± 11 b | |
| 1,8-Cineole | 60 ± 12 abc | 80 ± 13 c | 28 ± 11 ab | 62 ± 13 c | 46 ± 12 b | |
| Chamazulene | 68 ± 13 bc | 50 ± 12 abc | 20 ± 12 a | 34 ± 14 abc | 26 ± 21 b | |
| β-Caryophyllene | 42 ± 17 ab | 22 ± 15 a | 20 ± 17 a | 36 ± 16 abc | −20 ± 10 b | |
| DEET | 88 ± 7 c | 76 ± 14 bc | 28 ± 7 ab | 20 ± 14 abc | 16 ± 7 b | |
| 4 h | A. lavandulaefolia | 92 ± 6 c | 54 ± 15 ab | 10 ± 18 a | 6 ± 20 a | 16 ± 12 a |
| 4-Terpineol | 78 ± 13 c | 26 ± 20 a | 44 ± 15 bc | 42 ± 18 b | 34 ± 17 a | |
| α-Terpineol | 80 ± 12 c | 76 ± 12 b | 66 ± 12 c | 52 ± 19 b | 30 ± 13 a | |
| γ-Terpinene | 32 ± 4 ab | 40 ± 13 ab | 46 ± 9 bc | 36 ± 15 ab | 20 ± 14 a | |
| 1,8-Cineole | 82 ± 9 c | 60 ± 12 ab | 36 ± 12 bc | 50 ± 10 b | 76 ± 9b | |
| Chamazulene | 74 ± 12 bc | 34 ± 16 a | 42 ± 19 ab | 36 ± 17 bc | 44 ± 20 ab | |
| β-Caryophyllene | 34 ± 10 a | 22 ± 14 a | 20 ± 9 ab | 56 ± 20 b | −22 ± 12 a | |
| DEET | 98 ± 4 c | 78 ± 9 b | 58 ± 16 bc | 56 ± 14 b | 46 ± 7 ab | |
References
- Kim, M.; Yang, J.; Lee, H. Phototactic behavior: Repellent effects of cigarette beetle, Lasioderma serricorne (Coleoptera: Anobiidae), to light-emitting diodes. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 331–333. [Google Scholar] [CrossRef]
- Hill, D.S. Pests of Stored Products and Their Control; Belhaven Press: London, UK, 1990; p. 274. [Google Scholar]
- Hori, M. Repellency of essential oils against the cigarette beetle, Lasioderma serricorne (Fabricius) (Coleoptera: Anobiidae). Appl. Entomol. Zool. 2003, 38, 467–473. [Google Scholar] [CrossRef]
- Baur, F.J. Chemical methods to control insect pests of processed foods. In Ecology and Management of Food Industry Pests; Gorham, J.R., Ed.; FDA Technical Bulletin; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1991; Volume 4, pp. 1–2. [Google Scholar]
- Reed, W.D.; Vinzant, J.P. Control of insects attacking stored tobacco and tobacco products. United States department of agriculture, division of truck crop and garden insect investigations. Bureau of entomology and plant quarantine. Circular 1942, 635, 5–6. [Google Scholar]
- Phillips, T.W.; Throne, J.E. Bioragional approaches to managing stored-product insects. Annu. Rev. Entomol. 2010, 55, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.; Sriranjini, V. Plant products as fumigants for stored-product insect control. J. Stored Prod. Res. 2008, 44, 126–135. [Google Scholar] [CrossRef]
- Ahmadi, M.; Abd-alla, A.M.M.; Moharramipour, S. Combination of gamma radiation and essential oils from medicinal plants in managing Tribolium castaneum contamination of stored products. Appl. Radiat. Isot. 2013, 78, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Becnel, J.J.; Tsikolia, M.; Bernier, U.R.; Tabanca, N.; Baser, K.H.; Wedge, D.E.; Agramonte, N.M.; Sakhanokho, H.F.; Sampson, B.J.; Demirci, B. Chemical composition, antifungal and insecticidal activities of Hedychium essential Oils. Molecules 2013, 18, 4308–4327. [Google Scholar]
- Chu, S.S.; Liu, S.L.; Liu, Q.Z.; Jiang, G.H.; Liu, Z.L. Chemical composition and insecticidal activities of the essential oil of the flowering aerial parts of Aster ageratoides. J. Serbian Chem. Soc. 2013, 78, 209–216. [Google Scholar] [CrossRef]
- Fang, R.; Jiang, C.H.; Wang, X.Y.; Zhang, H.M.; Liu, Z.M.; Zhou, L.; Du, S.S.; Deng, Z.W. Insecticidal activity of essential oil of Carum carvi fruits from China and its main components against two grain storage insects. Molecules 2010, 15, 9391–9402. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, J.L.; Xu, S.; Zhao, N.N.; Zhou, L.; Cheng, J.; Liu, Z.L. Evaluation of repellency of some Chinese medicinal herbs essential oils against Liposcelis bostrychophila (Psocoptera: Liposcelidae) and Tribolium castaneum (Coleoptera: Tenebrionidae). J. Econ. Entomol. 2013, 106, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Du, S.S. Fumigant components from the essential oil of Evodia rutaecarpa Hort unripe fruits. Eur. J. Chem. 2011, 8, 1937–1943. [Google Scholar]
- Liu, Z.L.; Zhao, N.N.; Liu, C.M.; Zhou, L.; Du, S.S. Identification of insecticidal constituents of the essential oil of Curcuma wenyujin rhizomes active against Liposcelis bostrychophila Badonnel. Molecules 2012, 17, 12049–12060. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Liu, S.L.; Yang, K.; Chu, S.S.; Liu, Q.Z.; Du, S.S. Chemical composition and toxicity of essential oil of Boenninghausenia sessilicarpa (Rutaceae) against two grain storage insects. J. Med. Plants Res. 2014, 6, 2920–2924. [Google Scholar]
- Lu, J.; Su, X.; Zhong, J. Fumigant activity of Elsholtzia stauntonii extract against Lasioderma serricorne: Research letter. S. Afr. J. Sci. 2012, 108, 77–79. [Google Scholar] [CrossRef]
- Zhang, J.S.; Zhao, N.N.; Liu, Q.Z.; Liu, Z.L.; Du, S.S.; Zhou, L.; Deng, Z.W. Repellent constituents of essential oil of Cymbopogon distans aerial parts against two stored-product insects. J. Agric. Food Chem. 2011, 59, 9910–9915. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q. General Biology; Central China Normal University Press: Wuhan, China, 2006. [Google Scholar]
- Wang, Y.; Li, X.; Jiang, Q.; Sun, H.; Jiang, J.; Chen, S.; Guan, Z.; Fang, W.; Chen, F. GC-MS Analysis of the volatile constituents in the leaves of 14 Compositae plants. Molecules 2018, 23, E166. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. genus: A review of bioactive essential oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef] [PubMed]
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Borjigidai, A.; Zhuang, L.; Li, Y.W.; Xi, Y.; Pang, Z.R.; Liu, T.X.; Cui, J. Investigation and application of Artemisia plant resources for Chinese medicine and Mongolian medicine in inner Mongolia. Liaoning Tradit. Chin. Med. J. 2013, 40, 770–774. [Google Scholar]
- Liu, Z.L.; Liu, Q.R.; Chu, S.S.; Jiang, G.H. Insecticidal activity and chemical composition of the essential oils of Artemisia lavandulaefolia and Artemisia sieversiana from China. Chem. Biodivers. 2010, 7, 2040–2045. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Chu, S.S.; Liu, Q.R. Chemical composition and insecticidal activity against Sitophilus zeamais of the essential oils of Artemisia capillaris and Artemisia mongolica. Molecules 2010, 15, 2600–2608. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.S.; Liu, Z.L.; Du, S.S.; Deng, Z.W. Chemical composition and insecticidal activity against Sitophilus zeamais of the essential oils derived from Artemisia giraldii and Artemisia subdigitata. Nat. Prod. Commun. 2012, 17, 7255–7265. [Google Scholar] [CrossRef] [PubMed]
- You, C.X.; Guo, S.S.; Zhang, W.J.; Yang, K.; Geng, Z.F.; Du, S.S.; Wang, C.F.; Deng, Z.W. Identification of repellent and insecticidal constituents from Artemisia mongolica essential oil against Lasioderma serricorne. J. Chem. 2015, 2015, 1–7. [Google Scholar]
- Liang, J.Y.; Wang, W.T.; Zheng, Y.F.; Zhang, D.; Wang, J.L.; Guo, S.S.; Zhang, W.J.; Du, S.S.; Zhang, J. Bioactivities and chemical constituents of essential oil extracted from Artemisia anethoides against two stored product insects. J. Oleo Sci. 2017, 66, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Prajapati, V.; Aggarwal, K.K.; Khanuja, S.P.S.; Kumar, S. Repellency and toxicity of oil from Artemisia annua to certain stored-product beetles. J. Econ. Entomol. 2000, 93, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Yang, K.; You, C.X.; Wang, Y.; Wang, C.F.; Wu, Y.; Geng, Z.F.; Su, Y.; Du, S.S.; Deng, Z.W. Bioactivity of essential oil from Artemisia stolonifera (Maxim.) Komar. and its main compounds against two stored-product insects. J. Oleo Sci. 2015, 64, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; You, C.X.; Yang, K.; Chen, R.; Wang, Y.; Wu, Y.; Geng, Z.F.; Chen, H.P.; Jiang, H.Y.; Su, Y.; et al. Bioactivity of essential oil of Artemisia argyi Lévl. et Van. and its main compounds against Lasioderma serricorne. J. Oleo Sci. 2014, 63, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.B.; Liu, Y.; Ding, Y.J.; Wang, J.; Li, Y.S. Effects of essential oil from Artemisia lavandulaefolia DC. on fumigation activity and several enzymes activities of Monolepta hieroglyphica (Motschulsky) Adults. J. Jilin Agric. Univ. 2014, 36, 30–35. [Google Scholar]
- Han, X.B.; Xie, X.K.; Qiao, R.X. Toxicity test for Lymantria dispar from five Artenisia species. Forest. By-Prod. Spec. China 2011, 12, 14–17. [Google Scholar]
- Yuan, H.B.; Shang, L.N.; Wei, C.Y.; Ren, B.Z. Comparison of constituents and insecticidal activities of essential oil from Artemisia lavandulaefolia by steam distillation and supercritical-CO2 Fluid Extraction. Chem. Res. Chin. Univ. 2010, 26, 888–892. [Google Scholar]
- Wang, X.S. Study on the Fumigation Activity of Three Plant Essential Oils of Artemisia and Ethyl Formate against Two Stored Grain Insects. Master’s Thesis, Northeast Normal University, Changchun, China, 2012. [Google Scholar]
- Adam, K.P.; Zapp, J. Biosynthesis of the isoprene units of chamomile sesquiterpenes. Phytochem. 1998, 48, 953–959. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Espineli, D.L.; Agoo, E.; Del Fierro, R.S. Chemical constituents of Cinnamomum cebuense. Chin. J. Nat. Med. 2013, 11, 264–268. [Google Scholar] [CrossRef]
- Ashnagar, A.; Naseri, N.G.; Bayemani, A. Isolation and determination of the major chemical compounds present in essential oil of the leaves of Myrtus plant grown in Khuzestan Province of Iran. Asian J. Chem. 2009, 21, 4969–4975. [Google Scholar]
- Wang, Y.; You, C.X.; Yang, K.; Wu, Y.; Chen, R.; Zhang, W.J.; Liu, Z.L.; Du, S.S.; Deng, Z.W.; Geng, Z.F.; et al. Bioactivity of essential oil of Zingiber purpureum rhizomes and its main compounds against two stored product insects. J. Econ. Entomol. 2015, 108, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, C.F.; You, C.X.; Geng, Z.F.; Sun, R.Q.; Guo, S.S.; Du, S.S.; Liu, Z.L.; Deng, Z.W. Bioactivity of essential oil of Litsea cubeba from China and its main compounds against two stored product insects. J. Asia-Pac. Entomol. 2014, 17, 459–466. [Google Scholar] [CrossRef]
- Cha, J.D.; Jeong, M.R.; Choi, H.J.; Jeong, S.I.; Moon, S.E.; Yun, S.I.; Kim, Y.H.; Kil, B.S.; Song, Y.H. Chemical composition and antimicrobial activity of the essential oil of Artemisia lavandulaefolia. Planta Medica 2005, 71, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Suleimenov, E.M.; Ozek, T.; Demirci, B.; Baser, K.H.C.; Adekenov, S.M. Component composition of essential oils of Artemisia lercheana and A. sieversiana of the flora of Kazakhstan. Antimicrobial activity of A. sieversiana essential oil. Chem. Nat. Compd. 2009, 45, 120–123. [Google Scholar] [CrossRef]
- Carnat, A.P.; Madesclaire, M.; Chavignon, O.; Lamaison, J.L. cis-Chrysanthenol, a main component in essential oil of Artemisia absinthium L. growing in Auvergne (Massif Central), France. J. Essent. Oil Res. 1992, 4, 487–490. [Google Scholar] [CrossRef]
- Raal, A.; Orav, A.; Arak, E. Essential oil content and composition in commercial Achillea millefolium L. herbs from different countries. J. Essent. Oil Bear. Plants. 2012, 15, 22–31. [Google Scholar] [CrossRef]
- Zhang, W.J.; You, C.X.; Yang, K.; Wang, Y.; Su, Y.; Geng, Z.F.; Du, S.S.; Wang, C.F.; Deng, Z.W.; Wang, Y.Y. Bioactivity and chemical constituents of the essential oil from Dendranthema indicum (L.) Des moul. against two stored insects. J. Oleo Sci. 2015, 64, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Chizzola, R. Regular Monoterpenes and Sesquiterpenes (Essential Oils); Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Capuzzo, A.; Occhipinti, A.; Maffei, M.E. Antioxidant and radical scavenging activities of chamazulene. Nat. Prod. Res. 2014, 28, 2321–2323. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.B. Biology Activity, Mechanism and Security of Essential Oil from Artemisia lavandulaefolia DC. Ph.D. Thesis, Northeast Normal University, Changchun, China, 2010. [Google Scholar]
- Adam, R.P. Identification of essential oil components by gas chromatography/quadrupole mass spectrometry. J. Am. Soc. Mass Spectrom. 2005, 16, 1902–1903. [Google Scholar]
- Liu, Z.L.; Ho, S.H. Bioactivity of the essential oil extracted from Evodia rutaecarpa Hook f. et Thomas against the grain storage insects, Sitophilus zeamais Motsch and Tribolium castaneum (Herbst). J. Stored Prod. Res. 1999, 35, 317–328. [Google Scholar] [CrossRef]
- Sakuma, M. Probit analysis of preference data. Appl. Entomol. Zool. 1998, 33, 339–348. [Google Scholar] [CrossRef]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
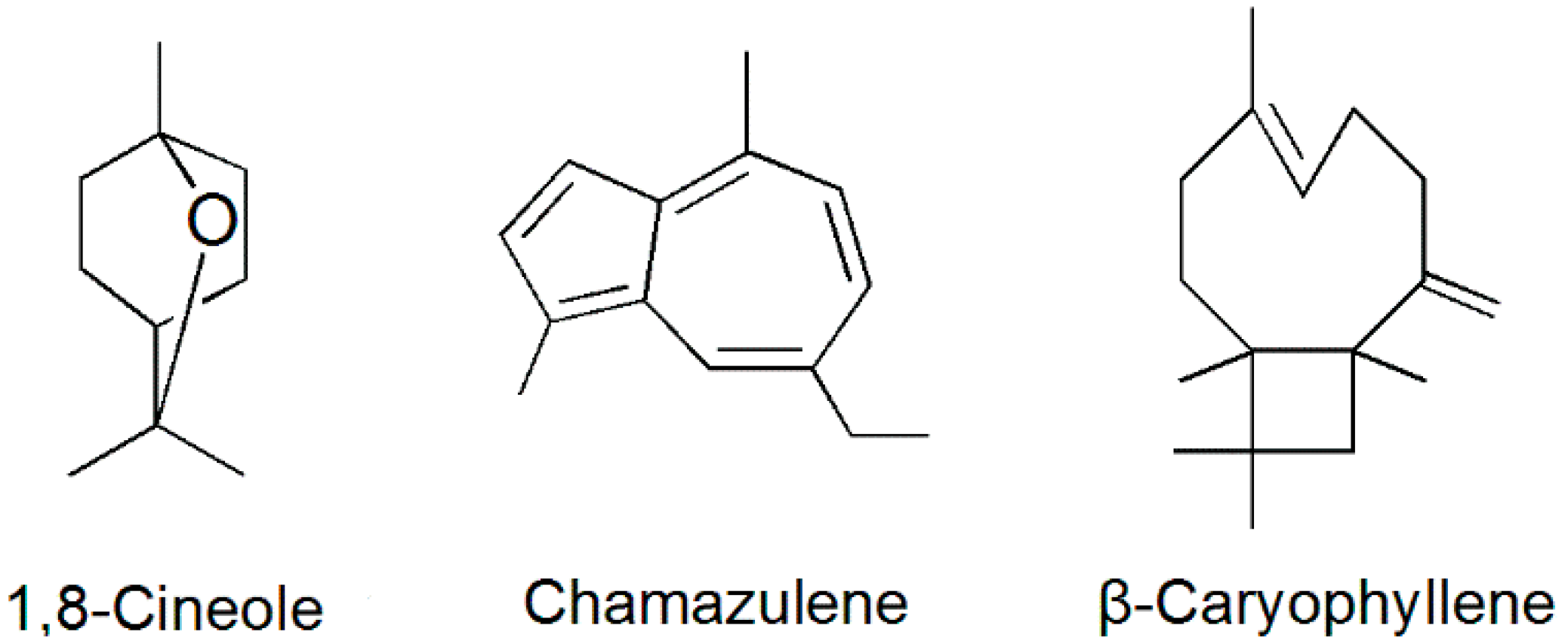
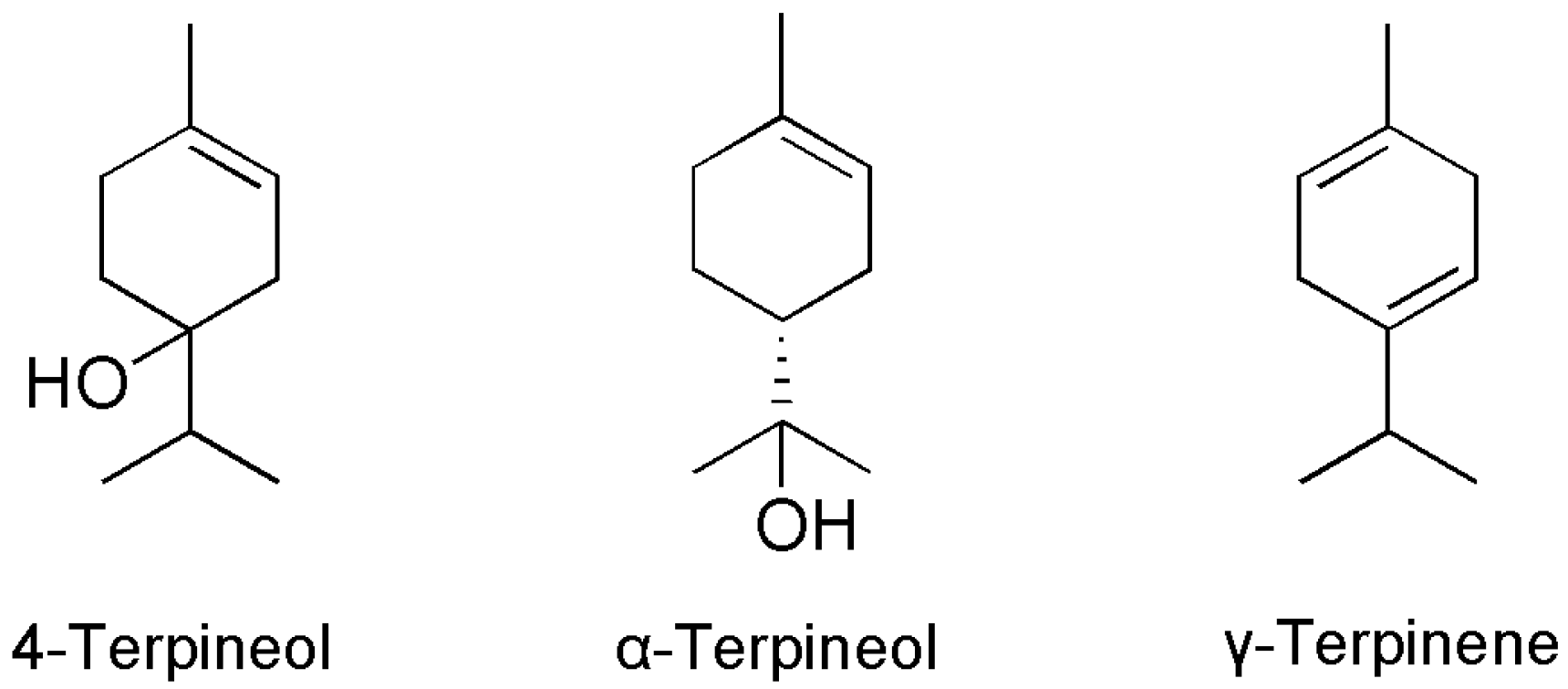
| Peak No. | Components | RI a | RA b (%) | Identification Methods c |
|---|---|---|---|---|
| 1 | p-Xylene | 860 | 0.6 | MS, RI |
| 2 | Santolina triene | 900 | 1.9 | MS, RI |
| 3 | β-Pinene | 974 | 1.5 | MS, RI |
| 4 | o-Cymene | 1014 | 0.1 | MS, RI |
| 5 | 1,8-Cineole | 1031 | 16.0 | MS, RI, NMR |
| 6 | γ-Terpinene | 1056 | 0.6 | MS, RI, Co |
| 7 | Sabinenehydrate | 1071 | 1.1 | MS, RI |
| 8 | Isoterpinolene | 1085 | 0.1 | MS, RI |
| 9 | Camphor | 1143 | 0.3 | MS, RI |
| 10 | 4-Terpineol | 1175 | 4.0 | MS, RI, Co |
| 11 | α-Terpineol | 1188 | 2.0 | MS, RI, Co |
| 12 | γ-Elemene | 1397 | 0.8 | MS, RI |
| 13 | β-Caryophyllene | 1451 | 11.5 | MS, RI, NMR |
| 14 | α-Caryophyllene | 1454 | 0.9 | MS, RI, Co |
| 15 | β-Farnesene | 1457 | 5.3 | MS, RI |
| 16 | Germacrene D | 1479 | 2.7 | MS, RI |
| 17 | Valencene | 1504 | 1.3 | MS, RI |
| 18 | Spathulenol | 1523 | 1.1 | MS, RI |
| 19 | Caryophylladienol II | 1644 | 0.4 | MS, RI |
| 20 | Chamazulene | 1735 | 40.4 | MS, RI, NMR |
| Total | 92.6 |
| Toxicities | Treatments | Concentrations (%) | LC50 e (μg/mL Air); LD50 f (μg/adult) | 95% FL g (μg/mL Air); (μg/Adult) | χ2 | p-Value |
|---|---|---|---|---|---|---|
| Fumigant | A. lavandulaefolia | 3.95–20.00 | 31.81 | 28.17–35.92 | 6.46 | 0.985 |
| 4-Terpineol a | 1.98–10.00 | 6.90 | 6.04–7.84 | 24.84 | 0.963 | |
| α-Terpineol a | 1.98–10.00 | 3.27 | 3.17–3.38 | 8.39 | 0.998 | |
| γ-Terpinene b | 1.98–10.00 | 11.93 | 10.54–13.51 | 8.34 | 0.998 | |
| 1,8-Cineole c | 0.99–5.00 | 5.18 | 4.63–5.70 | 16.79 | 0.951 | |
| Chamazulene | 0–50.00 | – | – | – | – | |
| β-Caryophyllene c | 0–50.00 | – | – | – | – | |
| Phosphine d | 7.20 × 10−3–11.12 × 10−3 | 9.23 × 10−3 | 7.13 × 10−3–11.37 × 10−3 | 11.96 | 0.971 | |
| Contact | A. lavandulaefolia | 1.98–10.00 | 13.51 | 11.51–15.40 | 5.78 | 0.982 |
| 4-Terpineol a | 0.99–5.00 | 8.62 | 7.38–9.85 | 12.65 | 0.976 | |
| α-Terpineol a | 0.59–3.00 | 11.99 | 10.42–13.42 | 18.96 | 0.703 | |
| γ-Terpinene b | 1.98–10.00 | 14.13 | 11.82–16.39 | 13.36 | 0.944 | |
| 1,8-Cineole c | 1.98–10.00 | 15.58 | 12.88–18.02 | 15.18 | 0.935 | |
| Chamazulene | 1.98–10.00 | 15.18 | 13.31–17.02 | 6.39 | 0.961 | |
| β-Caryophyllene c | 3.95–20.00 | 35.52 | 31.89–39.54 | 15.41 | 0.927 | |
| Pyrethrins d | 0.01–0.40 | 0.24 | 0.16–0.35 | 17.36 | 0.791 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Zou, K.; Zhang, W.; Guo, S.; Liu, H.; Sun, J.; Li, J.; Huang, D.; Wu, Y.; Du, S.; et al. Efficacy of Compounds Isolated from the Essential Oil of Artemisia lavandulaefolia in Control of the Cigarette Beetle, Lasioderma serricorne. Molecules 2018, 23, 343. https://doi.org/10.3390/molecules23020343
Zhou J, Zou K, Zhang W, Guo S, Liu H, Sun J, Li J, Huang D, Wu Y, Du S, et al. Efficacy of Compounds Isolated from the Essential Oil of Artemisia lavandulaefolia in Control of the Cigarette Beetle, Lasioderma serricorne. Molecules. 2018; 23(2):343. https://doi.org/10.3390/molecules23020343
Chicago/Turabian StyleZhou, Jun, Kexing Zou, Wenjuan Zhang, Shanshan Guo, Hong Liu, Jiansheng Sun, Jigang Li, Dongye Huang, Yan Wu, Shushan Du, and et al. 2018. "Efficacy of Compounds Isolated from the Essential Oil of Artemisia lavandulaefolia in Control of the Cigarette Beetle, Lasioderma serricorne" Molecules 23, no. 2: 343. https://doi.org/10.3390/molecules23020343





