Review of Current Cell-Penetrating Antibody Developments for HIV-1 Therapy
Abstract
:1. Introduction
2. Targeting HIV-1 Intracellular Proteins as Therapeutic Targets
2.1. Capsid Proteins (CA), p24
2.2. Nef
2.3. Tat
2.4. Rev
2.5. Other Proteins
3. Development of Cell-Penetrating Antiviral Antibodies
3.1. Targeting the Viral Proteins Essential for Replication
3.2. Targeting the Accessory Proteins
4. Challenges and Future Directions
4.1. Technologies in Cell-Penetrating Antibody Development
4.2. Viral Resistance and Escape Mutants
4.3. Future Directions
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ADIN | antibody-dependent intracellular neutralization |
| AIDS | acquired immunodeficiency syndrome |
| bnAb | broad-neutralizing antibody |
| CA | capsid |
| CD | cluster of differentiation |
| CPP | cell-penetrating peptide |
| Fabs | antibody fragment |
| Env | envelope |
| Fc | fragment crystallizable fragment |
| FDA | Food and Drug Administration |
| gp | glycoprotein |
| HAART | highly active antiretroviral therapy |
| HIV-1 | Human immunodeficiency virus type-I |
| IFITM1 | interferon-induced transmembrane |
| IN | Integrase |
| kDa | kilodalton |
| LTR | long terminal repeat |
| MA | Matrix protein |
| mAbs | monoclonal antibodies |
| MHC | major histocompatibility complex |
| MPER | membrane-proximal external region |
| MSW | mutant selection window |
| NC | nucleocapsid |
| NF-κB | Nuclear factor kappa B |
| PBMC | peripheral blood mononuclear cell |
| PR | protease |
| PTD | protein transduction domain |
| RNA | ribonucleic acid |
| RRE | Rev response element |
| RT | reverse transcriptase |
| scFvs/sFvs | Single-chain variable fragments |
| sdAb | single-domain antibody |
| SHPR | SUMO-1 heptapeptide protein transduction domain for binding Rev |
| SH3 | Src homology-3 |
| SP | spironolactone |
| SV40 | Simian virus 40 |
| TAR | transactivation-responsive region |
| TRIM21 | tripartite motif-containing 21 |
| VCP | valosin-containing protein |
References
- Palmisano, L.; Vella, S. A brief history of antiretroviral therapy of HIV infection: Success and challenges. Ann. Ist. Super. Sanita 2011, 47, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Arts, E.J.; Hazuda, D.J. HIV-1 antiretroviral drug therapy. Cold Spring Harb. Perspect. Med. 2012, 2, a007161. [Google Scholar] [CrossRef] [PubMed]
- Rolón, M.J.; Sued, O.; Cahn, P. Simplifying HAART: The role of two-drug therapy. Curr. Treat. Options Infect. Dis. 2017, 9, 250. [Google Scholar] [CrossRef]
- Ebele, I.J.; Daniel, I.M.; Vivian, A.N. Effect of short and long term exposure of HIV patients to highly active antiretroviral therapy (HAART) on lipid profile. BAOJ HIV 2017, 3, 020. [Google Scholar]
- Montessori, V.; Press, N.; Harris, M.; Akagi, L.; Montaner, J.S.G. Adverse effects of antiretroviral therapy for HIV infection. CMAJ 2004, 170, 229–238. [Google Scholar] [PubMed]
- Sedaghat, A.R.; Siliciano, J.D.; Brennan, T.P.; Wilke, C.O.; Siliciano, R.F. Limits on replenishment of the resting CD4+ T cell reservoir for HIV in patients on HAART. PLoS Pathog. 2007, 3, e122. [Google Scholar] [CrossRef] [PubMed]
- Siliciano, R.F.; Greene, W.C. HIV Latency. Cold Spring Harb. Perspect. Med. 2011, 1, a007096. [Google Scholar] [CrossRef] [PubMed]
- Trono, D.; Marzetta, F. Profaning the ultimate sanctuary: HIV latency in hematopoietic stem cells. Cell Host Microbe 2011, 9, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Adler, M.J.; Dimitrov, D.S. Therapeutic Antibodies Against Cancer. Hematol. Oncol. Clin. N. Am. 2012, 26, 447–481. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody therapy of cancer. Nat. Rev. Cancer 2012, 12, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Caskey, M.; Klein, F.; Lorenzi, J.C.C.; Seaman, M.S.; West, A.P.; Buckley, N.; Kremer, G.; Nogueira, L.; Braunschweig, M.; Scheid, J.F.; et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 2016, 535, 580. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H.; Whitney, J.B.; Moldt, B.; Klein, F.; Oliveira, T.Y.; Liu, J.; Stephenson, K.E.; Chang, H.-W.; Shekhar, K.; Gupta, S.; et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 2013, 503, 224–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, K.H.; Seaton, K.E.; Huang, Y.; Grunenberg, N.; Isaacs, A.; Allen, M.; Ledgerwood, J.E.; Frank, I.; Sobieszczyk, M.E.; Baden, L.R.; et al. Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: Results of a phase 1 randomized trial. PLoS Med. 2017, 14, e1002435. [Google Scholar] [CrossRef]
- Muller, S.; Zhao, Y.; Brown, T.L.; Morgan, A.C.; Kohler, H. TransMabs: Cell-penetrating antibodies, the next generation. Vaccines Antib. 2005, 5, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Marschall, A.L.J.J.; Frenzel, A.; Schirrmann, T.; Schüngel, M.; Dubel, S.; Dübel, S. Targeting antibodies to the cytoplasm. MAbs 2011, 3, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.M.; Hope, T.J. HIV-1 capsid: The multifaceted key player in HIV-1 infection. Nat. Rev. Microbiol. 2015, 13, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Le Sage, V.; Mouland, A.J.; Valiente-Echeverría, F. Roles of HIV-1 capsid in viral replication and immune evasion. Virus Res. 2014, 193, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Levesque, K.; Finzi, A.A.; Binette, J.; Cohen, E.A. Role of CD4 receptor down-regulation during HIV-1 infection. Curr. HIV Res. 2004, 2, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, W.; Verhasselt, B. Contributions of HIV-1 Nef to immune dysregulation in HIV-infected patients: A therapeutic target? Expert Opin. Ther. Targets 2013, 17, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, J.; Halambage, U.D.; Jurado, K.A.; Jamin, A.V.; Wang, Y.; Engelman, A.N.; Aiken, C. Inhibition of HIV-1 Maturation via Small-Molecule Targeting of the Amino-Terminal Domain in the Viral Capsid Protein. J. Virol. 2017, 91, e02155-16. [Google Scholar] [CrossRef] [PubMed]
- Thenin-Houssier, S.; Valente, S.T. HIV-1 Capsid Inhibitors as Antiretroviral Agents. Curr. HIV Res. 2016, 14, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Teow, S.Y.; Mualif, S.A.; Omar, T.C.; Wei, C.Y.; Yusoff, N.M.; Ali, S.A. Production and purification of polymerization-competent HIV-1 capsid protein p24 (CA) in NiCo21(DE3) Escherichia coli. BMC Biotechnol. 2013, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- Mualif, S.A.; Teow, S.-Y.Y.; Omar, T.C.; Chew, Y.W.; Yusoff, N.M.; Ali, S.A. Engineering and validation of a vector for concomitant expression of rare transfer RNA (tRNA) and HIV-1 nef genes in Escherichia coli. PLoS ONE 2015, 10, e0130446. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Teow, S.-Y.Y.; Omar, T.C.; Khoo, A.S.-B.B.; Choon, T.S.; Yusoff, N.M. A cell internalizing antibody targeting capsid protein (p24) inhibits the replication of HIV-1 in T cells lines and PBMCS: A proof of concept study. PLoS ONE 2016, 11, e0145986. [Google Scholar] [CrossRef] [PubMed]
- Teow, S.Y. Cell-Penetrating Antibodies for Targeting HIV-1 P24 Capsid Protein. Ph.D. Thesis, Universiti Sains Malaysia, Penang, Malaysia, 2015. Chapter 4. pp. 281–329. [Google Scholar]
- Mhashilkar, A.M.; Biswas, D.K.; LaVecchio, J.; Pardee, A.B.; Marasco, W.A. Inhibition of human immunodeficiency virus type 1 replication in vitro by a novel combination of anti-Tat single-chain intrabodies and NF-kappa B antagonists. J. Virol. 1997, 71, 6486–6494. [Google Scholar] [PubMed]
- Mhashilkar, A.M.; Lavecchio, J.; Eberhardt, B.; Porter-Brooks, J.; Boisot, S.; Dove, J.H.; Pumphrey, C.; Li, X.; Weissmahr, R.N.; Ring, D.B.; et al. Inhibition of Human Immunodeficiency Virus Type 1 Replication in Vitro in Acutely and Persistently Infected Human CD4+ Mononuclear Cells Expressing Murine and Humanized Anti-Human Immunodeficiency Virus Type 1 Tat Single-Chain Variable Fragment Intrabodie. Hum. Gene Ther. 1999, 10, 1453–1467. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Stahl, S.J.; Watts, N.R.; DiMattia, M.A.; Steven, A.C.; Wingfield, P.T. A cell-penetrating antibody fragment against HIV-1 Rev has high antiviral activity: Characterization of the paratope. J. Biol. Chem. 2014, 289, 20222–20233. [Google Scholar] [CrossRef] [PubMed]
- Boons, E.; Li, G.; Vanstreels, E.; Vercruysse, T.; Pannecouque, C.; Vandamme, A.M.; Daelemans, D. A stably expressed llama single-domain intrabody targeting Rev displays broad-spectrum anti-HIV activity. Antivir. Res. 2014, 112, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Fassati, A. Multiple roles of the capsid protein in the early steps of HIV-1 infection. Virus Res. 2012, 170, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Bharat, T.A.M.; Davey, N.E.; Ulbrich, P.; Riches, J.D.; de Marco, A.; Rumlova, M.; Sachse, C.; Ruml, T.; Briggs, J.A.G. Structure of the immature retroviral capsid at 8 Å resolution by cryo-electron microscopy. Nature 2012, 487, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J.A.G.; Simon, M.N.; Gross, I.; Kräusslich, H.-G.; Fuller, S.D.; Vogt, V.M.; Johnson, M.C. The stoichiometry of Gag protein in HIV-1. Nat. Struct. Mol. Biol. 2004, 11, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Aiken, C. Viral and cellular factors that regulate HIV-1 uncoating. Curr. Opin. HIV AIDS 2006, 1, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Ganser-Pornillos, B.K.; von Schwedler, U.K.; Stray, K.M.; Aiken, C.; Sundquist, W.I. Assembly Properties of the Human Immunodeficiency Virus Type 1 CA Protein. J. Virol. 2004, 78, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Neira, J.L. The capsid protein of human immunodeficiency virus: Designing inhibitors of capsid assembly. FEBS J. 2009, 276, 6110–6117. [Google Scholar] [CrossRef] [PubMed]
- Thenin-Houssier, S.; De Vera, I.M.S.; Pedro-Rosa, L.; Brady, A.; Richard, A.; Konnick, B.; Opp, S.; Buffone, C.; Fuhrmann, J.; Kota, S.; et al. Ebselen, a small-Molecule capsid inhibitor of HIV-1 replication. Antimicrob. Agents Chemother. 2017, 60, 2195–2208. [Google Scholar] [CrossRef] [PubMed]
- Lemke, C.T.; Titolo, S.; von Schwedler, U.; Goudreau, N.; Mercier, J.-F.J.-F.; Wardrop, E.; Faucher, A.-M.A.-M.; Coulombe, R.; Banik, S.S.R.; Fader, L.; et al. Distinct Effects of Two HIV-1 Capsid Assembly Inhibitor Families That Bind the Same Site within the N-Terminal Domain of the Viral CA Protein. J. Virol. 2012, 86, 6643–6655. [Google Scholar] [CrossRef] [PubMed]
- Kortagere, S.; Madani, N.; Mankowski, M.K.; Schön, A.; Zentner, I.; Swaminathan, G.; Princiotto, A.; Anthony, K.; Oza, A.; Sierra, L.-J.; et al. Inhibiting Early-Stage Events in HIV-1 Replication by Small-Molecule Targeting of the HIV-1 Capsid. J. Virol. 2012, 86, 8472–8481. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhou, J.; Shah, V.B.; Aiken, C.; Whitby, K. Small-Molecule Inhibition of Human Immunodeficiency Virus Type 1 Infection by Virus Capsid Destabilization. J. Virol. 2011, 85, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Feasley, C.L.; Jackson, K.W.; Nitz, T.J.; Salzwedel, K.; Air, G.M.; Sakalian, M. The prototype HIV-1 maturation inhibitor, bevirimat, binds to the CA-SP1 cleavage site in immature Gag particles. Retrovirology 2011, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Keller, P.W.; Adamson, C.S.; Heymann, J.B.; Freed, E.O.; Steven, A.C. HIV-1 maturation inhibitor bevirimat stabilizes the immature Gag lattice. J. Virol. 2011, 85, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Loeliger, E.; Kinde, I.; Kyere, S.; Mayo, K.; Barklis, E.; Sun, Y.; Huang, M.; Summers, M.F. Antiviral inhibition of the HIV-1 capsid protein. J. Mol. Biol. 2003, 327, 1013–1020. [Google Scholar] [CrossRef]
- Ternois, F.; Sticht, J.; Duquerroy, S.; Kräusslich, H.G.; Rey, F.A. The HIV-1 capsid protein C-terminal domain in complex with a virus assembly inhibitor. Nat. Struct. Mol. Biol. 2005, 12, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Q.; Bhattacharya, S.; Waheed, A.A.; Tong, X.; Hong, A.; Heck, S.; Curreli, F.; Goger, M.; Cowburn, D.; et al. A cell penetrating helical peptide as a potential HIV-1 inhibitor. J. Mol. Biol. 2008, 378, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Lamorte, L.; Titolo, S.; Lemke, C.T.; Goudreau, N.; Mercier, J.F.; Wardrop, E.; Shah, V.B.; von Schwedler, U.K.; Langelier, C.; Banik, S.S.; et al. Discovery of novel small-molecule HIV-1 replication inhibitors that stabilize capsid complexes. Antimicrob. Agents Chemother. 2013, 57, 4622–4631. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Alam, S.L.; Fricke, T.; Zadrozny, K.; Sedzicki, J.; Taylor, A.B.; Demeler, B.; Pornillos, O.; Ganser-Pornillos, B.K.; Diaz-Griffero, F.; et al. Structural basis of HIV-1 capsid recognition by PF74 and CPSF6. Proc. Natl. Acad. Sci. USA 2014, 111, 18625–18630. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.K.; Shrivastava, G.; Tiwari, S.; Nair, M.P.N. HIV-1 Nef: Hacker of the host cell. Future Virol. 2012, 7, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.K.; Shrivastava, G.; Tiwari, S.; Swamy, M.L.A.; Nair, M.P.N. Modulation of HIV pathogenesis and T-cell signaling by HIV-1 Nef. Future Virol. 2012, 7, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Alves, C. Viral pathogenesis: HIV-1 Nef targets restriction factors. Nat. Rev. Microbiol. 2015, 13, 660–661. [Google Scholar] [CrossRef] [PubMed]
- Lamers, S.L.; Fogel, G.B.; Singer, E.J.; Salemi, M.; Nolan, D.J.; Huysentruyt, L.C.; McGrath, M.S. HIV-1 Nef in macrophage-mediated disease pathogenesis. Int. Rev. Immunol. 2012, 31, 432–450. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.; Kuo, L.S.; Krisko, J.F.; Tomchick, D.R.; Garcia, J.V.; Foster, J.L. Dynamic Evolution of the Human Immunodeficiency Virus Type 1 Pathogenic Factor, Nef. J. Virol. 2006, 80, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Huang, M.-B.; Campbell, P.E.; Roth, W.W.; Campbell, T.; Khan, M.; Newman, G.; Villinger, F.; Powell, M.D.; Bond, V.C. Genetic Characterization of HIV Type 1 Nef-Induced Vesicle Secretion. AIDS Res. Hum. Retrovir. 2010, 26, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Teow, S.-Y.; Nordin, A.C.; Ali, S.A.; Khoo, A.S. Exosomes in Human Immunodeficiency Virus Type I Pathogenesis: Threat or Opportunity? Adv. Virol. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aiken, C. Pseudotyping Human Immunodeficiency Virus Type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 1997, 71, 5871–5877. [Google Scholar] [PubMed]
- Schaeffer, E.; Geleziunas, R.; Greene, W.C. Human Immunodeficiency Virus Type 1 Nef Functions at the Level of Virus Entry by Enhancing Cytoplasmic Delivery of Virions. J. Virol. 2001, 75, 2993–3000. [Google Scholar] [CrossRef] [PubMed]
- Lülf, S.; Matz, J.; Rouyez, M.-C.; Järviluoma, A.; Saksela, K.; Benichou, S.; Geyer, M. Structural basis for the inhibition of HIV-1 Nef by a high-affinity binding single-domain antibody. Retrovirology 2014, 11, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchet, J.; Basmaciogullari, S.E.; Chrobak, P.; Stolp, B.; Bouchard, N.; Fackler, O.T.; Chames, P.; Jolicoeur, P.; Benichou, S.; Baty, D. Inhibition of the Nef regulatory protein of HIV-1 by a single-domain antibody. Blood 2011, 117, 3559–3568. [Google Scholar] [CrossRef] [PubMed]
- Emert-Sedlak, L.A.; Narute, P.; Shu, S.T.; Poe, J.A.; Shi, H.; Yanamala, N.; Alvarado, J.J.; Lazo, J.S.; Yeh, J.I.; Johnston, P.A.; et al. Effector kinase coupling enables high-throughput screens for direct HIV-1 Nef antagonists with antiretroviral activity. Chem. Biol. 2013, 20, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, A.; Sato, K.; Aron, Z.D.; Cohen, F.; Harris, A.; McDougall, B.R.; Robinson, W.E.; Overman, L.E.; Weiss, G.A. Guanidine alkaloid analogs as inhibitors of HIV-1 Nef interactions with p53, actin, and p56lck. Proc. Natl. Acad. Sci. USA 2004, 101, 14079–14084. [Google Scholar] [CrossRef] [PubMed]
- Trible, R.P.; Narute, P.; Emert-Sedlak, L.A.; Alvarado, J.J.; Atkins, K.; Thomas, L.; Kodama, T.; Yanamala, N.; Korotchenko, V.; Day, B.W.; et al. Discovery of a diaminoquinoxaline benzenesulfonamide antagonist of HIV-1 Nef function using a yeast-based phenotypic screen. Retrovirology 2013, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- BouHamdan, M.; Duan, L.-X.; Pomerantz, R.J.; Strayer, D.S. Inhibition of HIV-1 by an anti-integrase single-chain variable fragment (SFv): Delivery by SV40 provides durable protection against HIV-1 and does not require selection. Gene Ther. 1999, 6, 660–666. [Google Scholar] [CrossRef] [PubMed]
- BouHamdan, M.; Kulkosky, J.; Duan, L.X.; Pomerantz, R.J. Inhibition of HIV-1 replication and infectivity by expression of a fusion protein, VPR-anti-integrase single-chain variable fragment (SFv): Intravirion molecular therapies. J. Hum. Virol. 2000, 3, 6–15. [Google Scholar] [PubMed]
- Franke, L.; Grunow, R.; Meissner, K.; Porstmann, T.; von Baehr, R. Inhibition of HIV-1 infection in vitro by murine monoclonal anti-p24 antibodies. J. Med. Virol. 1992, 37, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Grunow, R.; Franke, L.; Hinkula, J.; Wahren, B.; Fenyö, E.M.; Jondal, M.; von Baehr, R. Monoclonal antibodies to p24-core protein of HIV-1 mediate ADCC and inhibit virus spread in vitro. Clin. Diagn. Virol. 1995, 3, 221–231. [Google Scholar] [CrossRef]
- Bouchet, J.; Herate, C.; Guenzel, C.A.; Verollet, C.; Jarviluoma, A.; Mazzolini, J.; Rafie, S.; Chames, P.; Baty, D.; Saksela, K.; et al. Single-Domain Antibody-SH3 Fusions for Efficient Neutralization of HIV-1 Nef Functions. J. Virol. 2012, 86, 4856–4867. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Zhang, H.; Oakes, J.W.; Bagasra, O.; Pomerantz, R.J. Molecular and virological effects of intracellular anti-Rev single-chain variable fragments on the expression of various human immunodeficiency virus-1 strains. Hum. Gene Ther. 1994, 5, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Zhu, M.; Bagasra, O.; Pomerantz, R.J. Intracellular immunization against HIV-1 infection of human T lymphocytes: Utility of anti-rev single-chain variable fragments. Hum. Gene Ther. 1995, 6, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Duan, L.; Zhu, M.; Hu, B.; Kubota, S.; Bagasra, O.; Pomerantz, R.J. Binding of intracellular anti-Rev single chain variable fragments to different epitopes of human immunodeficiency virus type 1 rev: Variations in viral inhibition [published erratum appears in J Virol 1998 Apr;72(4):3505-6]. J. Virol. 1996, 70, 3290–3297. [Google Scholar] [PubMed]
- Ho, W.-Z.; Lai, J.-P.; Bouhamdan, M.; Duan, L.; Pomerantz, R.J.; Starr, S.E. Inhibition of HIV Type 1 Replication in Chronically Infected Monocytes and Lymphocytes by Retrovirus-Mediated Gene Transfer of Anti-Rev Single-Chain Variable Fragments. AIDS Res. Hum. Retrovir. 1998, 14, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, T.; Pardon, E.; Vanstreels, E.; Steyaert, J.; Daelemans, D. An intrabody based on a llama single-domain antibody targeting the N-terminal α-helical multimerization domain of HIV-1 Rev prevents viral production. J. Biol. Chem. 2010, 285, 21768–21780. [Google Scholar] [CrossRef] [PubMed]
- Mhashilkar, A.M.; Bagley, J.; Chen, S.Y.; Szilvay, A.M.; Helland, D.G.; Marasco, W.A. Inhibition of HIV-1 Tat-mediated LTR transactivation and HIV-1 infection by anti-Tat single chain intrabodies. EMBO J. 1995, 14, 1542–1551. [Google Scholar] [PubMed]
- Cruikshank, W.W.; Doctrow, S.R.; Falvo, M.S.; Huffman, K.; Maciaszek, J.; Viglianti, G.; Raina, J.; Kornfeld, H.; Malfroy, B. A lipidated anti-Tat antibody enters living cells and blocks HIV-1 viral replication. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1997, 14, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, J.; Silva, F.; Freitas-Vieira, A.; Santa-Marta, M.; Malhó, R.; Yang, X.; Gabuzda, D.; Barbas, C.; Malhó, R.; Yang, X.; et al. Functional neutralization of HIV-1 Vif protein by intracellular immunization inhibits reverse transcription and viral replication. J. Biol. Chem. 2002, 277, 32036–32045. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, A.; Jans, D. The HIV-1 Tat Transactivator Protein: A Therapeutic Target? IUBMB Life (Int. Union Biochem. Mol. Biol. Life) 2003, 55, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Das, A.T.; Harwig, A.; Berkhout, B. The HIV-1 Tat Protein Has a Versatile Role in Activating Viral Transcription. J. Virol. 2011, 85, 9506–9516. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.-C.; Wang, S.-W.; Cheng, L.; Tarn, W.-Y.; Tsai, S.-J.; Sun, H.S. Human DDX3 Interacts with the HIV-1 Tat Protein to Facilitate Viral mRNA Translation. PLoS ONE 2013, 8, e68665. [Google Scholar] [CrossRef] [PubMed]
- Mousseau, G.; Valente, S. Strategies to Block HIV Transcription: Focus on Small Molecule Tat Inhibitors. Biology (Basel) 2012, 1, 668–697. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Palu, G. Inhibitors of HIV-1 Tat-Mediated Transactivation. Curr. Med. Chem. 2006, 13, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Hamy, F.; Gelus, N.; Zeller, M.; Lazdins, J.L.; Bailly, C.; Klimkait, T. Blocking HIV replication by targeting Tat protein. Chem. Biol. 2000, 7, 669–676. [Google Scholar] [CrossRef]
- Ali, A.; Banerjea, A.C. Curcumin inhibits HIV-1 by promoting Tat protein degradation. Sci. Rep. 2016, 6, 27539. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Chen, X. Triptolide inhibits Human Immunodeficiency Virus type 1 replication by promoting proteasomal degradation of Tat protein. Retrovirology 2014, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, B.; Morel, M.; Margottin-Goguet, F.; Ramirez, B.C. Specific Inhibition of HIV Infection by the Action of Spironolactone in T Cells. J. Virol. 2016, 90, 10972–10980. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, M.S.; Lobritz, M.A.; Ratcliff, A.; Chamanian, M.; Athanassiou, Z.; Tyagi, M.; Wong, J.; Robinson, J.A.; Karn, J.; Varani, G.; et al. Inhibition of both HIV-1 reverse transcription and gene expression by a cyclic peptide that binds the Tat-Transactivating response element (TAR) RNA. PLoS Pathog. 2011, 7, e1002038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.Y.; Dinh, A.; Cronin, J.; Li, S.C.; Reiser, J. Cellular uptake and lysosomal delivery of galactocerebrosidase tagged with the HIV Tat protein transduction domain. J. Neurochem. 2008, 104, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.S.; Dunsmore, K.E.; Wong, H.R. Intracellular delivery of HSP70 using HIV-1 Tat protein transduction domain. Biochem. Biophys. Res. Commun. 2003, 301, 54–59. [Google Scholar] [CrossRef]
- Secchiero, P.; Zella, D.; Capitani, S.; Gallo, R.C.; Zauli, G. Extracellular HIV-1 tat protein up-regulates the expression of surface CXC-chemokine receptor 4 in resting CD4+ T cells. J. Immunol. 1999, 162, 2427–2431. [Google Scholar] [PubMed]
- Ferrari, A.; Pellegrini, V.; Arcangeli, C.; Fittipaldi, A.; Giacca, M.; Beltram, F. Caveolae-mediated internalization of extracellular HIV-1 tat fusion proteins visualized in real time. Mol. Ther. 2003, 8, 284–294. [Google Scholar] [CrossRef]
- Nielsen, M.; Pedersen, F.; Kjems, J. Molecular strategies to inhibit HIV-1 replication. Retrovirology 2005, 2, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grewe, B.; Überla, K. The Human Immunodeficiency Virus type 1 Rev protein: Ménage à trois during the early phase of the lentiviral replication cycle. J. Gen. Virol. 2010, 91, 1893–1897. [Google Scholar] [CrossRef] [PubMed]
- Blissenbach, M.; Grewe, B.; Hoffmann, B.; Brandt, S.; Uberla, K. Nuclear RNA Export and Packaging Functions of HIV-1 Rev Revisited. J. Virol. 2010, 84, 6598–6604. [Google Scholar] [CrossRef] [PubMed]
- Daelemans, D.; Afonina, E.; Nilsson, J.; Werner, G.; Kjems, J.; De Clercq, E.; Pavlakis, G.N.; Vandamme, A.-M. A synthetic HIV-1 Rev inhibitor interfering with the CRM1-mediated nuclear export. Proc. Natl. Acad. Sci. USA 2002, 99, 14440–14445. [Google Scholar] [CrossRef] [PubMed]
- Roisin, A.; Robin, J.-P.P.; Dereuddre-Bosquet, N.; Vitte, A.-L.L.; Dormont, D.; Clayette, P.; Jalinot, P. Inhibition of HIV-1 Replication by Cell-penetrating Peptides Binding Rev. J. Biol. Chem. 2004, 279, 9208–9214. [Google Scholar] [CrossRef] [PubMed]
- Shuck-Lee, D.; Chen, F.F.; Willard, R.; Raman, S.; Ptak, R.; Hammarskjold, M.-L.M.-L.; Rekosh, D. Heterocyclic Compounds That Inhibit Rev-RRE Function and Human Immunodeficiency Virus Type 1 Replication. Antimicrob. Agents Chemother. 2008, 52, 3169–3179. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.W.; Balachandran, A.; Haaland, M.; Stoilov, P.; Cochrane, A. Characterization of novel inhibitors of HIV-1 replication that function via alteration of viral RNA processing and rev function. Nucleic Acids Res. 2013, 41, 9471–9483. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, A.; Wong, R.; Stoilov, P.; Pan, S.; Blencowe, B.; Cheung, P.; Harrigan, P.R.; Cochrane, A. Identification of small molecule modulators of HIV-1 Tat and Rev protein accumulation. Retrovirology 2017, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.A.; Freed, E.O. HIV type 1 Gag as a target for antiviral therapy. AIDS Res. Hum. Retrovir. 2012, 28, 54–75. [Google Scholar] [CrossRef] [PubMed]
- González, M.E.; Eugenia, M. The HIV-1 vpr protein: A multifaceted target for therapeutic intervention. Int. J. Mol. Sci. 2017, 18, 126. [Google Scholar] [CrossRef] [PubMed]
- González, M.E.; Eugenia, M. Vpu protein: The viroporin encoded by HIV-1. Viruses 2015, 7, 4352–4368. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Liu, D.; Lv, W.; Wang, X.; Wang, J.; Lv, M.; Huang, W.; Wu, J.; Zhang, H.; Jin, H.; et al. Small-Molecule Inhibition of Human Immunodeficiency Virus Type 1 Replication by Targeting the Interaction between Vif and ElonginC. J. Virol. 2012, 86, 5497–5507. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.-L.L.; Varghese, V.; Rhee, S.-Y.Y.; Gatell, J.M.; Shafer, R.W. HIV-1 integrase inhibitor resistance and its clinical implications. J. Infect. Dis. 2011, 203, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Weber, I.T.; Kneller, D.W.; Wong-Sam, A. Highly resistant HIV-1 proteases and strategies for their inhibition. Future Med. Chem. 2015, 7, 1023–1038. [Google Scholar] [CrossRef] [PubMed]
- Fun, A.; van Maarseveen, N.M.; Pokorná, J.; Maas, R.E.M.; Schipper, P.J.; Konvalinka, J.; Nijhuis, M. HIV-1 protease inhibitor mutations affect the development of HIV-1 resistance to the maturation inhibitor bevirimat. Retrovirology 2011, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Price, A.J.; Halambage, U.D.; James, L.C.; Aiken, C. HIV-1 Resistance to the Capsid-Targeting Inhibitor PF74 Results in Altered Dependence on Host Factors Required for Virus Nuclear Entry. J. Virol. 2015, 89, 9068–9079. [Google Scholar] [CrossRef] [PubMed]
- Shuck-Lee, D.; Chang, H.; Sloan, E.A.; Hammarskjold, M.-L.M.-L.; Rekosh, D. Single-Nucleotide Changes in the HIV Rev-Response Element Mediate Resistance to Compounds That Inhibit Rev Function. J. Virol. 2011, 85, 3940–3949. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Pan, Q.; Liu, S.L.; Liang, C. HIV-1 mutates to evade IFITM1 restriction. Virology 2014, 454–455, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Chande, A.; Ziglio, S.; De Sanctis, V.; Bertorelli, R.; Goh, S.L.; McCauley, S.M.; Nowosielska, A.; Antonarakis, S.E.; Luban, J.; et al. HIV-1 Nef promotes infection by excluding SERINC5 from virion incorporation. Nature 2015, 526, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Usami, Y.; Wu, Y.; Göttlinger, H.G. SERINC3 and SERINC5 restrict HIV-1 infectivity and are counteracted by Nef. Nature 2015, 526, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Archin, N.M.; Sung, J.M.; Garrido, C.; Soriano-Sarabia, N.; Margolis, D.M. Eradicating HIV-1 infection: Seeking to clear a persistent pathogen. Nat. Rev. Microbiol. 2014, 12, 750–764. [Google Scholar] [CrossRef] [PubMed]
- Cihlar, T.; Fordyce, M. Current status and prospects of HIV treatment. Curr. Opin. Virol. 2016, 18, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Delagrèverie, H.M.; Delaugerre, C.; Lewin, S.R.; Deeks, S.G.; Li, J.Z. Ongoing clinical trials of Human Immunodeficiency Virus latency-reversing and immunomodulatory agents. Open Forum Infect. Dis. 2016, 3, ofw189. [Google Scholar] [CrossRef] [PubMed]
- Tse, W.C.; Link, J.O.; Mulato, A.; Niedziela-Majka, A.; Rowe, W.; Somoza, J.R.; Villasenor, A.G.; Yant, S.R.; Zhang, J.R.; Zheng, J. Discovery of Novel Potent HIV Capsid Inhibitors with Long-Acting Potential. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Seattle, WA, USA, 13–16 February 2017. [Google Scholar]
- Dejnirattisai, W.; Wongwiwat, W.; Supasa, S.; Zhang, X.; Dai, X.; Rouvinsky, A.; Jumnainsong, A.; Edwards, C.; Quyen, N.T.H.; Duangchinda, T.; et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat. Immunol. 2014, 16, 170–177. [Google Scholar] [CrossRef] [PubMed]
- De Jong, Y.P.; Dorner, M.; Mommersteeg, M.C.; Xiao, J.W.; Balazs, A.B.; Robbins, J.B.; Winer, B.Y.; Gerges, S.; Vega, K.; Labitt, R.N.; et al. Broadly neutralizing antibodies abrogate established hepatitis C virus infection. Sci. Transl. Med. 2014, 6, 254ra129. [Google Scholar] [CrossRef] [PubMed]
- Caskey, M.; Klein, F.; Nussenzweig, M.C. Broadly Neutralizing Antibodies for HIV-1 Prevention or Immunotherapy. N. Engl. J. Med. 2016, 375, 2019–2021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, S.; Gu, Y.; Xia, N. Antiviral therapy by HIV-1 broadly neutralizing and inhibitory antibodies. Int. J. Mol. Sci. 2016, 17, 1901. [Google Scholar] [CrossRef] [PubMed]
- Margolis, D.M.; Koup, R.A.; Ferrari, G. HIV antibodies for treatment of HIV infection. Immunol. Rev. 2017, 275, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Klein, F.; Mouquet, H.; Dosenovic, P.; Scheid, J.F.; Scharf, L.; Nussenzweig, M.C. Antibodies in HIV-1 vaccine development and therapy. Science 2013, 341, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Weissleder, R. Intracellular Cargo Delivery Using Tat Peptide and Derivatives. Med. Res. Rev. 2004, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Toro, A.; Grunebaum, E. TAT-mediated intracellular delivery of purine nucleoside phosphorylase corrects its deficiency in mice. J. Clin. Investig. 2006, 116, 2717–2726. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Liu, E.; Yu, Z.; Pei, X.; Chen, S.; Zhang, P.; Shin, M.-C.C.; Gong, J.; He, H.; Yang, V.C. CPP-assisted intracellular drug delivery, what is next? Int. J. Mol. Sci. 2016, 17, 1892. [Google Scholar] [CrossRef] [PubMed]
- Fales, A.M.; Yuan, H.; Vo-Dinh, T. Cell-penetrating peptide enhanced intracellular Raman imaging and photodynamic therapy. Mol. Pharm. 2013, 10, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Bullok, K.E.; Gammon, S.T.; Violini, S.; Prantner, A.M.; Villalobos, V.M.; Sharma, V.; Piwnica-Worms, D. Permeation peptide conjugates for in vivo molecular imaging applications. Mol. Imaging 2006, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Harrich, D.; Apolloni, A.; Warrilow, D. HIV-1 Tat implicated as a key factor in viral spread. Futur. HIV Ther. 2008, 2, 323–326. [Google Scholar] [CrossRef]
- Marchiò, S.; Alfano, M.; Primo, L.; Gramaglia, D.; Butini, L.; Gennero, L.; De Vivo, E.; Arap, W.; Giacca, M.; Pasqualini, R.; et al. Cell surface-associated Tat modulates HIV-1 infection and spreading through a specific interaction with gp120 viral envelope protein. Blood 2005, 105, 2802–2811. [Google Scholar] [CrossRef] [PubMed]
- Papkalla, A.; Münch, J.; Otto, C.; Kirchhoff, F.; Munch, J.; Otto, C.; Kirchhoff, F. Nef Enhances Human Immunodeficiency Virus Type 1 Infectivity and Replication Independently of Viral Coreceptor Tropism. J. Virol. 2002, 76, 8455–8459. [Google Scholar] [CrossRef] [PubMed]
- Jere, A.; Fujita, M.; Adachi, A.; Nomaguchi, M. Role of HIV-1 Nef protein for virus replication in vitro. Microbes Infect. 2010, 12, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, T.; Boons, E.; Venken, T.; Vanstreels, E.; Voet, A.; Steyaert, J.; De Maeyer, M.; Daelemans, D. Mapping the Binding Interface between an HIV-1 Inhibiting Intrabody and the Viral Protein Rev. PLoS ONE 2013, 8, e60259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.M.Y.; Iorno, N.; Sierro, F.; Christ, D. Selection of human antibody fragments by phage display. Nat. Protoc. 2007, 2, 3001–3008. [Google Scholar] [CrossRef] [PubMed]
- Chames, P.; Van Regenmortel, M.; Weiss, E.; Baty, D. Therapeutic antibodies: Successes, limitations and hopes for the future. Br. J. Pharmacol. 2009, 157, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Kontermann, R.E. Strategies to extend plasma half-lives of recombinant antibodies. BioDrugs 2009, 23, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Kontermann, R.E. Half-life extended biotherapeutics. Expert Opin. Biol. Ther. 2016, 16, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Hutt, M.; Färber-Schwarz, A.; Unverdorben, F.; Richter, F.; Kontermann, R.E. Plasma half-life extension of small recombinant antibodies by fusion to immunoglobulin-binding domains. J. Biol. Chem. 2012, 287, 4462–4469. [Google Scholar] [CrossRef] [PubMed]
- Trüssel, S.; Dumelin, C.; Frey, K.; Villa, A.; Buller, F.; Neri, D.; Trüssel, S.; Dumelin, C.; Frey, K.; Villa, A.; et al. New strategy for the extension of the serum half-life of antibody fragments. Bioconjug. Chem. 2009, 20, 2286–2292. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.L.; Hearn, B.R.; Pfaff, S.J.; Fontaine, S.D.; Reid, R.; Ashley, G.W.; Grabulovski, S.; Strassberger, V.; Vogt, L.; Jung, T.; et al. Approach for Half-Life Extension of Small Antibody Fragments That Does Not Affect Tissue Uptake. Bioconjug. Chem. 2016, 27, 2534–2539. [Google Scholar] [CrossRef] [PubMed]
- Descotes, J. Immunotoxicity of monoclonal antibodies. MAbs 2009, 1, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J.T. The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 2010, 9, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Harding, F.A.; Stickler, M.M.; Razo, J.; DuBridge, R.B. The immunogenicity of humanized and fully human antibodies: Residual immunogenicity resides in the CDR regions. MAbs 2010, 2, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Shafer, R.W.; Schapiro, J.M. HIV-1 drug resistance mutations: An updated framework for the second decade of HAART. AIDS Rev. 2008, 10, 67–84. [Google Scholar] [PubMed]
- Klein, F.; Nogueira, L.; Nishimura, Y.; Phad, G.; West, A.P.; Halper-Stromberg, A.; Horwitz, J.A.; Gazumyan, A.; Liu, C.; Eisenreich, T.R.; et al. Enhanced HIV-1 immunotherapy by commonly arising antibodies that target virus escape variants. J. Exp. Med. 2014, 211, 2361–2372. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Kuwata, T.; Shimura, K.; Yokoyama, M.; Ramirez Valdez, K.P.; Tanaka, K.; Maruta, Y.; Oishi, S.; Fujii, N.; Sato, H.; et al. Enhanced antibody-mediated neutralization of HIV-1 variants that are resistant to fusion inhibitors. Retrovirology 2016, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Wibmer, C.K.; Bhiman, J.N.; Gray, E.S.; Tumba, N.; Abdool Karim, S.S.; Williamson, C.; Morris, L.; Moore, P.L. Viral Escape from HIV-1 Neutralizing Antibodies Drives Increased Plasma Neutralization Breadth through Sequential Recognition of Multiple Epitopes and Immunotypes. PLoS Pathog. 2013, 9, e1003738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.; Li, Y.; Zheng, H.; Shao, Y. Recent progress toward engineering HIV-1-specific neutralizing monoclonal antibodies. Front. Immunol. 2016, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Manrique, A.; Rusert, P.; Joos, B.; Fischer, M.; Kuster, H.; Leemann, C.; Niederöst, B.; Weber, R.; Stiegler, G.; Katinger, H.; et al. In Vivo and In Vitro Escape from Neutralizing Antibodies 2G12, 2F5, and 4E10. J. Virol. 2007, 81, 8793–8808. [Google Scholar] [CrossRef] [PubMed]
- Magnus, C.; Reh, L.; Trkola, A. HIV-1 resistance to neutralizing antibodies: Determination of antibody concentrations leading to escape mutant evolution. Virus Res. 2015, 218, 57–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, F.; Halper-Stromberg, A.; Horwitz, J.A.; Gruell, H.; Scheid, J.F.; Bournazos, S.; Mouquet, H.; Spatz, L.A.; Diskin, R.; Abadir, A.; et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 2012, 492, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, J.A.; Halper-Stromberg, A.; Mouquet, H.; Gitlin, A.D.; Tretiakova, A.; Eisenreich, T.R.; Malbec, M.; Gravemann, S.; Billerbeck, E.; Dorner, M.; et al. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc. Natl. Acad. Sci. USA 2013, 110, 16538–16543. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.A.; Isenberg, D.A. TRIM21 and the function of antibodies inside cells. Trends Immunol. 2017, 38, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Mallery, D.L.; McEwan, W.A.; Bidgood, S.R.; Towers, G.J.; Johnson, C.M.; James, L.C. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21). Proc. Natl. Acad. Sci. USA 2010, 107, 19985–19990. [Google Scholar] [CrossRef] [PubMed]
- McEwan, W.A.; Tam, J.C.H.; Watkinson, R.E.; Bidgood, S.R.; Mallery, D.L.; James, L.C. Intracellular antibody-bound pathogens stimulate immune signaling via the Fc receptor TRIM21. Nat. Immunol. 2013, 14, 327–336. [Google Scholar] [CrossRef] [PubMed]
- French, M.A.; Tjiam, M.C.; Abudulai, L.N.; Fernandez, S. Antiviral functions of human immunodeficiency virus type 1 (HIV-1)-specific IgG antibodies: Effects of antiretroviral therapy and implications for therapeutic HIV-1 vaccine design. Front. Immunol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yu, J.; Lanzi, A.; Yao, X.; Andrews, C.D.; Tsai, L.; Gajjar, M.R.; Sun, M.; Seaman, M.S.; Padte, N.N.; et al. Engineered bispecific antibodies with exquisite HIV-1-neutralizing activity. Cell 2016, 165, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Gazumyan, A.; Seaman, M.S.; Nussenzweig, M.C.; Ravetch, J.V. Bispecific Anti-HIV-1 Antibodies with Enhanced Breadth and Potency. Cell 2016, 165, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Bhiman, J.N.; Lynch, R.M. Broadly neutralizing antibodies as treatment: Effects on virus and immune system. Curr. HIV/AIDS Rep. 2017, 14, 54–62. [Google Scholar] [CrossRef] [PubMed]
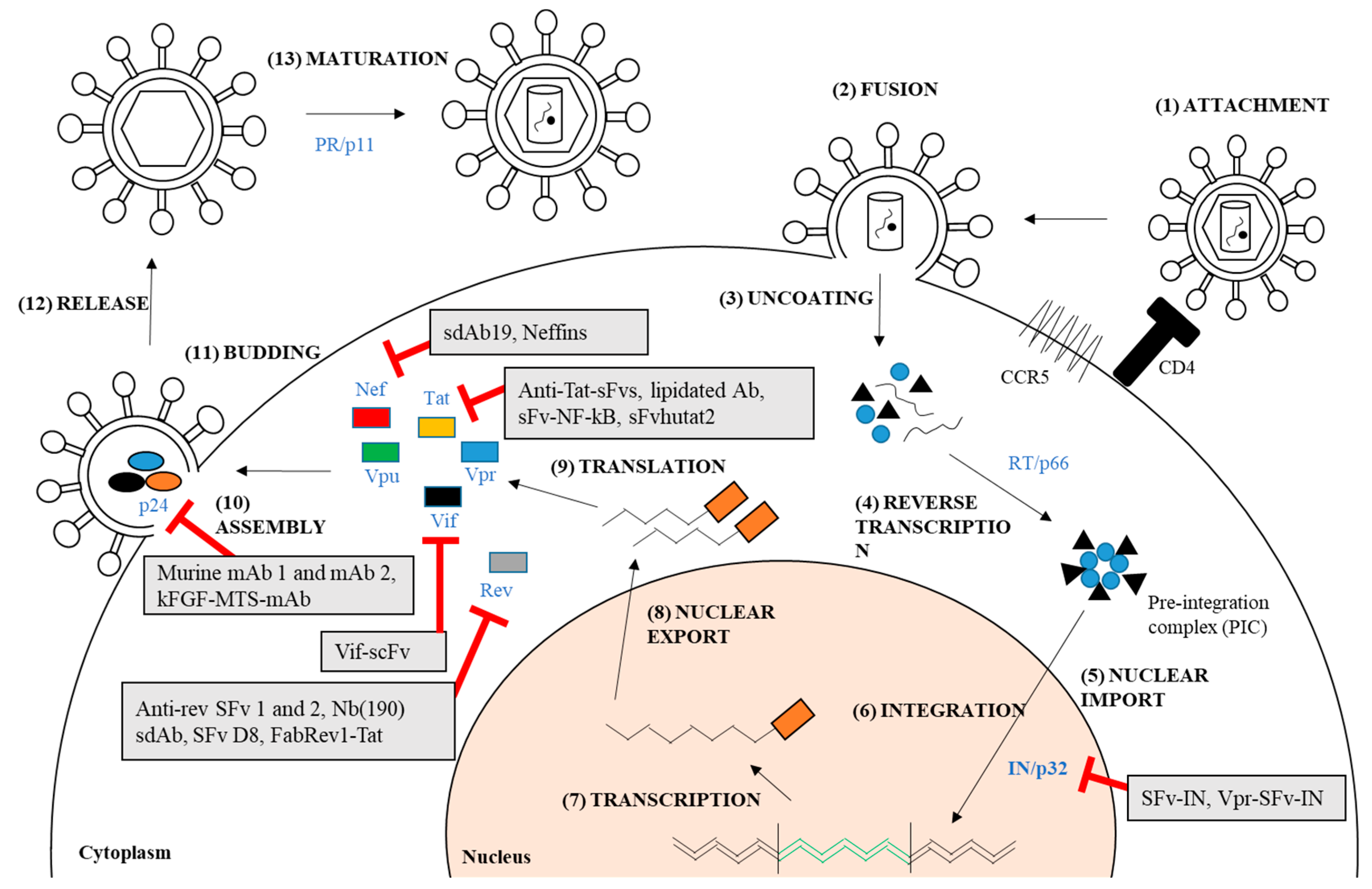
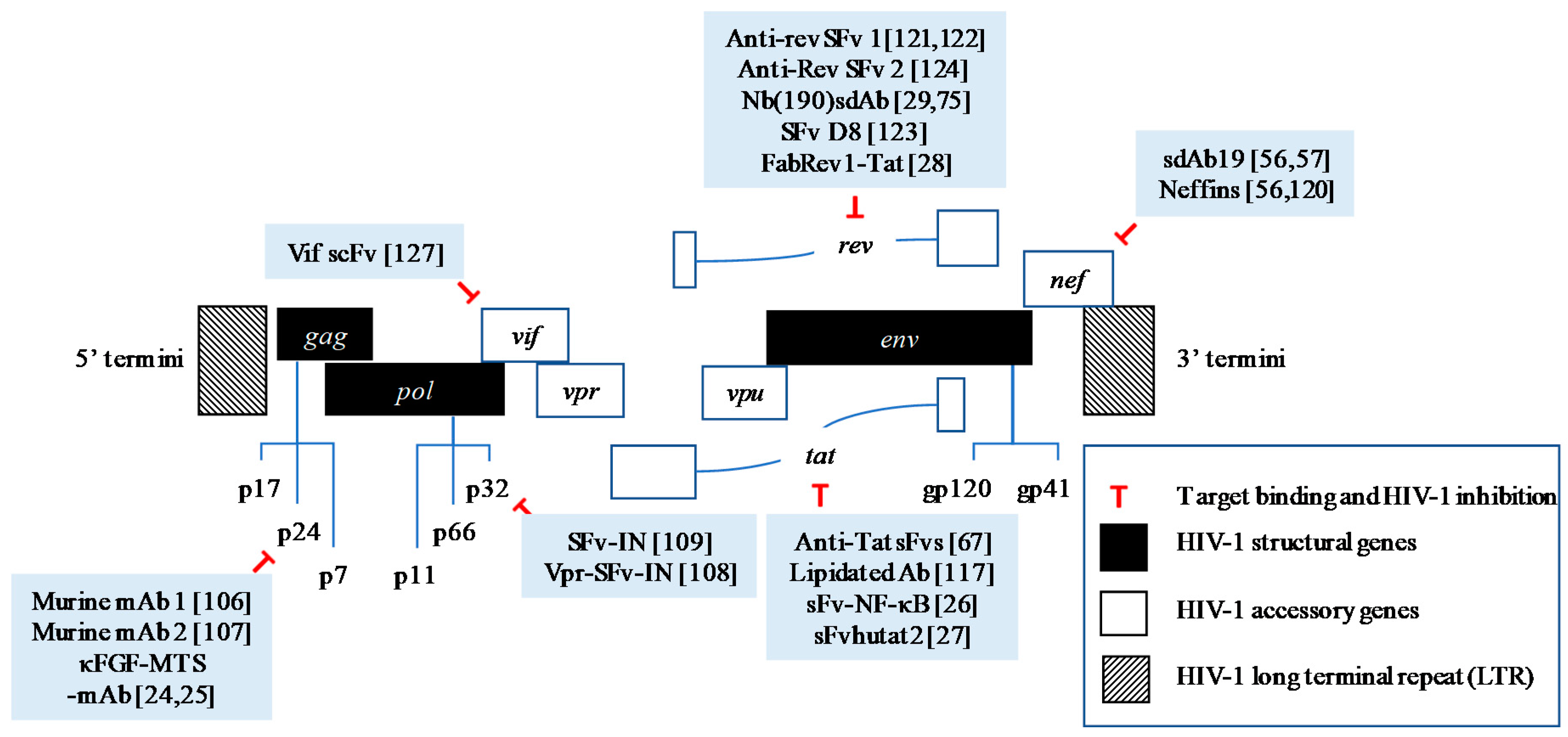
| Target | Antibody | Conjugation/Engineering | Antiviral Effect |
|---|---|---|---|
| Integrase (IN)/p32 | SFv-IN | SV40 as delivery system | Inhibition of HIV-1 replication and syncytium formation in human T-lymphoid cell, SupT1 [61] |
| Vpr-SFv-IN | Phage-display and fusion to Vpr protein | Inhibition of HIV-1 replication in human T-lymphoid cell, SupT1 and reduction of virion infectivity [62] | |
| Capsid (CA)/ p24 | Murine anti-p24 Mabs | Native | Inhibition of active virus particles in up to 60% in HIV-1 infected cell lines or IL-2 stimulated T-cells [63] |
| Murine anti-p24 Mabs | Native | Delay of HIV-1 spread for 6 days in in vitro cell culture [64] | |
| κFGF-MTS-anti-p24-mAbs | κFGF-MTS peptide chemical conjugation | Inhibition of HIV-1 replication up to 73% and 49% in T-cells and PBMCs respectively [24,25] | |
| Nef | sdAb19 | Phage-display | Inhibition of Nef-mediated CD4 down-regulation [56,57] Inhibition of p21-activated kinase 2 interaction and actin remodeling [57] Inhibition of viral infectivity and replication in PBMCs [57] Prevention of Nef-mediated thymic CD4 T-cell maturation and peripheral CD4 T-cell activation in vivo [57] |
| Neffins | Phage-display and fusion to modified SH3 domains | Inhibition of CD4 and MHC-1 cell surface downregulation [56,65] Inhibition of all functions of Nef in both T-cells and macrophages [65] | |
| Rev | Anti-Rev SFv | Phage-display | Inhibition of replication of various laboratory and primary clinical HIV-1 strains in long-term human T-cell lines for several months [66,67] |
| SFv D8 | Phage-display | Inhibition of HIV-1 production in human T-cell lines and PBMCs [68] | |
| Anti-Rev SFv | Phage-display | Inhibition of HIV-1 replication in chronically infected U1 promonocytic cell line, ACH-2 T-cell, and primary monocyte cultures [69] | |
| Nb(190) sdAb | Phage-display | Inhibition of replication of wide range of different HIV-1 subtypes [29,70] | |
| FabRev1-Tat | Phage-display and Tat peptide conjugation | Inhibition of viral replication of CCR5-tropic HIV-1 isolates in PBMCs [28] | |
| Tat | Anti-Tat sFvs | Phage-display | Resistance of antibody-expressing lymphocytes to HIV-1 infection [71] |
| Lipidated anti-Tat antibody | Lipidation chemical modification | Inhibition of HIV-1 replication of several HIV-1 isolates by 85% [72] | |
| Anti-Tat sFv with NF-κB antagonists | Phage-display | Longer inhibition of HIV-1 replication up to 45 days [26] | |
| sFvhutat2 | Phage-display | Inhibition of HIV-1 replication in HxB2- and two syncytium-inducing (SI) primary isolates- challenged PBMCs [27] | |
| Vif | Vif scFv | Phage-display | Inhibition of HIV-1 replication in HIV-1 infected primary cells and cell lines [73] Inhibition of completed reverse transcripts formation [73] |
| Method | Details | Therapeutic Antibody |
|---|---|---|
| Genetic | Phage display | sFv [27] |
| Cloning of SFv into SV40 expression vector | SV(Aw) [61] | |
| Phage display and fusion to Vpr by cloning | Vpr-SFv-IN [62] | |
| Ilama immunization and phage display | sdAb [57] | |
| Phage display and fusion to SH3 by cloning | sdAb-SH3 (Neffins) [65] | |
| Chemical | Lipidation chemical modification | Lipidated antibody [72] |
| Conjugation to κFGF-MTS cell-penetrating peptide | κFGF-MTS-mAbs [24,25] | |
| Genetic & chemical | Phage display and conjugation to Tat cell-penetrating peptide | Fab-Tat [28] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che Nordin, M.A.; Teow, S.-Y. Review of Current Cell-Penetrating Antibody Developments for HIV-1 Therapy. Molecules 2018, 23, 335. https://doi.org/10.3390/molecules23020335
Che Nordin MA, Teow S-Y. Review of Current Cell-Penetrating Antibody Developments for HIV-1 Therapy. Molecules. 2018; 23(2):335. https://doi.org/10.3390/molecules23020335
Chicago/Turabian StyleChe Nordin, Muhamad Alif, and Sin-Yeang Teow. 2018. "Review of Current Cell-Penetrating Antibody Developments for HIV-1 Therapy" Molecules 23, no. 2: 335. https://doi.org/10.3390/molecules23020335





